Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops
Abstract
:1. Introduction
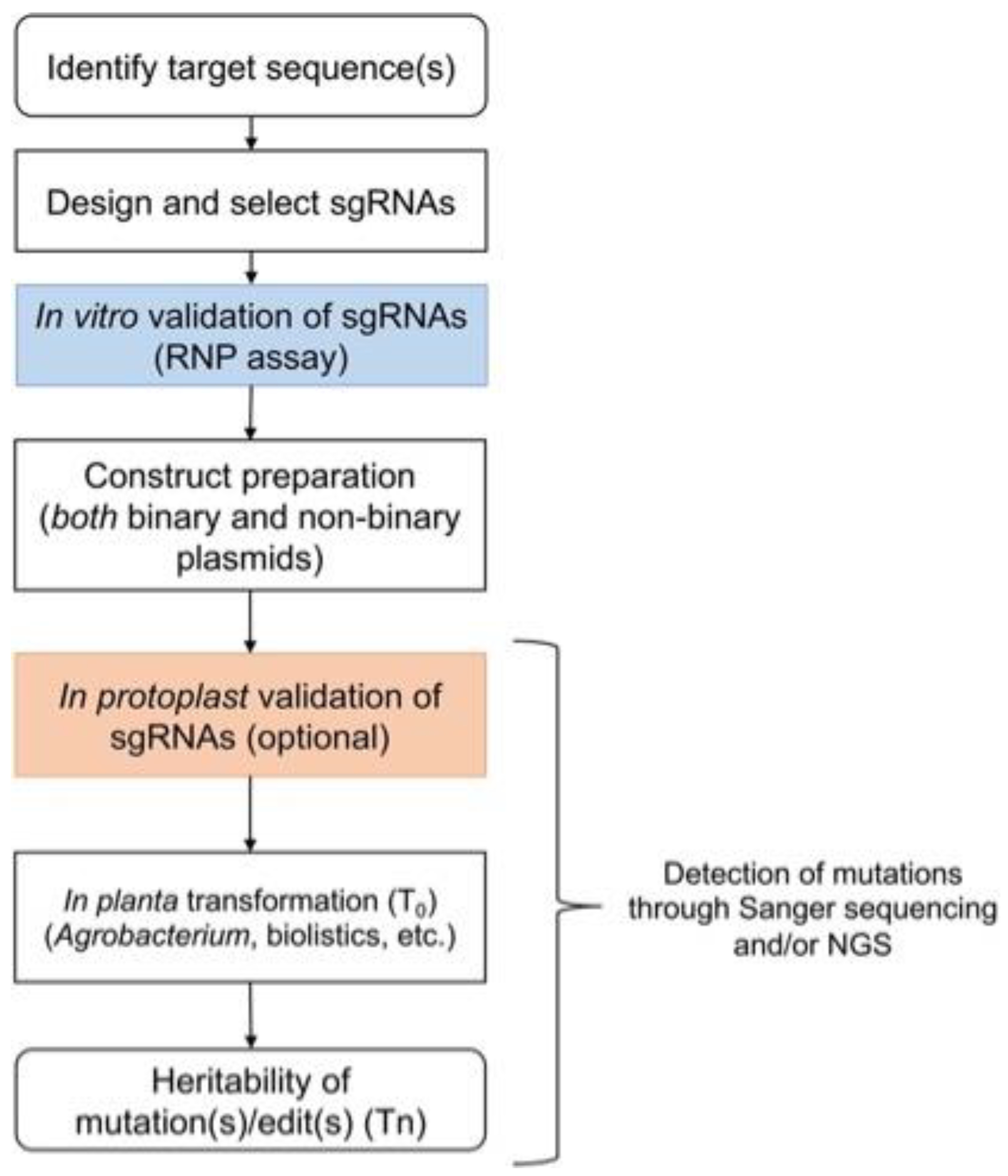
2. Procedure
2.1. In Silico Sequence Analysis
- Identify the target sequences of the crop species of interest from their respective databases (Table 1).
- Download the genomic sequence of interest and the respective transcript and coding sequences.
- Note: Ensure that the transcript and coding sequences of all the predicted splicing variants are downloaded.
- 3.
- Map the obtained transcript and coding sequences to the genomic sequence to prepare the gene structure for the respective gene, including intron/exon locations for all transcript variants.
- Note: In our case, we used MAFFT Multiple Sequence Alignment Software Version 7 [18] through Benchling (https://www.benchling.com/ (accessed on 1 September 2023)).
- 4.
- Annotate the target genomic sequence manually or confirm the gene annotation found in various sequence databases.
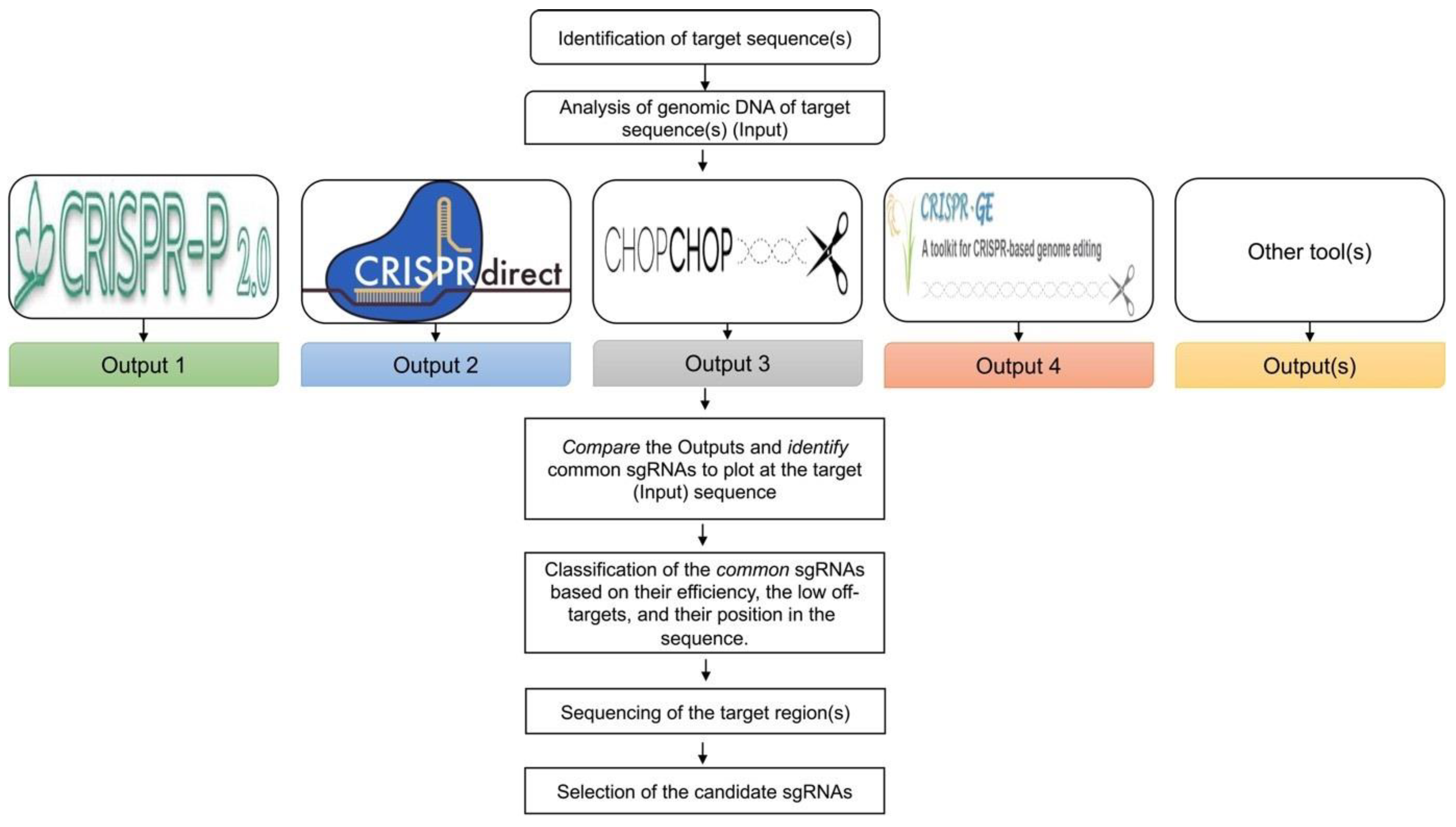
2.2. Design of Guide RNAs
- 5.
- Use the genomic sequence of the target region as an “input sequence” for the analysis in online databases commonly used for the design of sgRNAs in plants (Table 2), such as CRISPR-P 2.0 [20], CRISPR-direct [21], CHOPCHOP [22], target design [23], CRISPR-PLANT v2 [24], and/or other available web tools [25], focusing on the design of sgRNAs.
- Note: In our experiments, we observed that the output of this analysis from the respective databases differed substantially for the same target region(s).
- 6.
- Identify the “common” sgRNAs that are present in the output of most, if not all, databases.
- Note: In our case, we found that most of the common sgRNAs were often clustered at certain, potentially conserved, regions of the target sequence.
- 7.
- Map the selected sgRNAs on the gene structure alignments and classify them based on the following criteria:
- i.
- Targeting all the transcript variants (pay attention to alternative splicing);
- ii.
- Predicted high efficiency;
- iii.
- Fewer off-targets;
- iv.
- For knockout mutations, create the intended mutations near the beginning of the coding sequence to generate premature stop codons and truncated peptides.
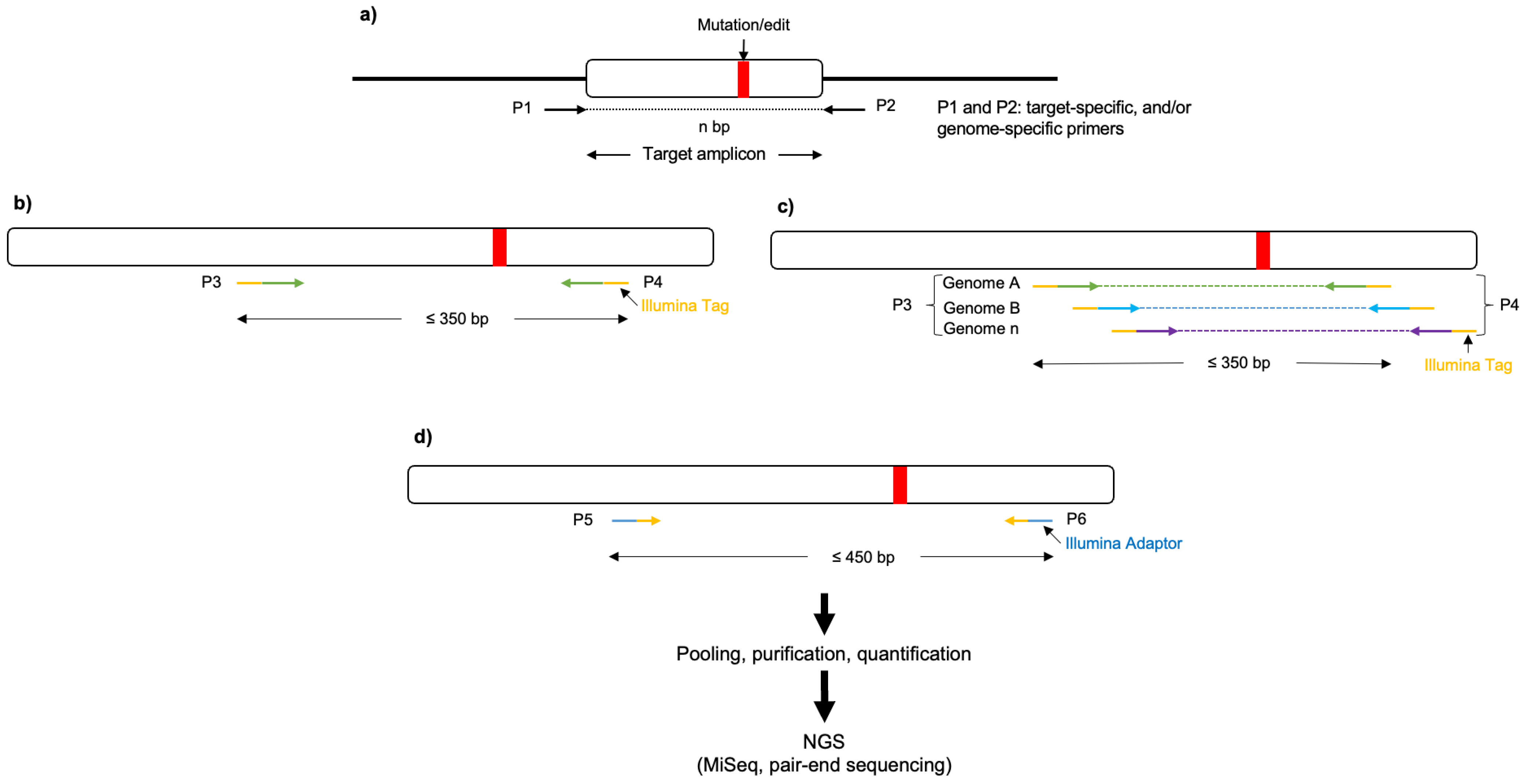
2.3. Primer Design and Sequencing of Target Regions
- 8.
- Design primers that flank the target regions using NCBI Primer Blast [27] and Oligo Analyzer (IDT) (https://www.idtdna.com/calc/Analyzer/Home/Instructions (accessed on 1 September 2023)).
- Note: The ideal target amplicon size should fall between 500 bp and 1200 bp to yield the best results from Sanger sequencing. However, the size of the amplicon may vary, especially in cases of polyploid crops, where it is necessary to design genome-specific primers.
- 9.
- Amplify the target region using proofreading Taq polymerase.
- Note: Various providers have different enzymes with proofreading activities. Some of the commonly used enzymes include Phusion High-Fidelity DNA Polymerase (2 U/µL) (Thermo Fisher Scientific, Waltham, MA, USA), Q5 High-Fidelity DNA Polymerase (NEB), and KAPA HiFi DNA Polymerase (Roche, Indianapolis, IN, USA).
- 10.
- Agarose electrophoresis and excision of the target fragments from the gel, followed by purification with a gel extraction kit, enables the collection of clean amplicons with minimal impurities.
- 11.
- Sanger sequencing of the obtained amplicon can be performed at this point; however, TOPO-cloning (for example using the Zero Blunt™ TOPO™ PCR Cloning Kit, Thermo Fisher Scientific, Waltham, MA, USA) of the fragments and submission of 3–5 clones derived from each amplicon for sequencing is highly recommended, especially when sequencing is intended for genotyping the target region. This ensures that all possible sequence variants in the amplicon are detected.
2.4. In Vitro CRISPR/Cas9 Ribonucleoprotein (RNP) Assay Validation Step 1
- 12.
- Perform the assay using the amplicon that was eluted from step 11 as substrate DNA.
- Notes: In our experience ≥70 ng of substrate DNA is adequate for clear visualization in the agarose gel.
- 13.
- Visualization of the fragments can be performed using agarose electrophoresis (generally 1.5–2% (w/v)), at 100 V for 20–45 min, depending on the size of the expected fragments. A single uncut band indicates failure of the sgRNA with that target amplicon, whereas a larger uncut band combined with two smaller cut bands (often quite faint) indicates successful cleavage by Cas9 with those sgRNA designs.
2.5. Construct Preparation
- 14.
- Assemble binary and/or non-binary version(s) of the plasmids, utilizing the different components of the previously described modular systems.
- Note: Consider the use of codon-optimized Cas9 nucleases for monocot or dicot species, depending on the target crop.
2.6. Protoplast Transformation and Validation of sgRNAs (Validation step 2; Optional)
- 15.
- Isolate protoplasts from the respective crop species.
- Note: A comprehensive list of protoplast isolation protocols for different plant species can be found in Reed and Bargmann, 2021 [38].
- 16.
- Optimize the length of the viability of the isolated protoplasts [42] at different temperatures (e.g., 4 °C, 13 °C, and 25 °C).
- Note: To determine the viability of the protoplasts, we used the Plant Cell Viability Assay Kit (Sigma-Aldrich, Burlington, MA, USA, Cat. No.: PA0100-1KT).
- 17.
- 18.
- Optimize the plasmid concentration required for efficient protoplast transformation by testing different concentrations of the previously utilized GFP plasmid (see step 17).
- 19.
- Assess the GFP expression levels when driven by promoters that are used to drive the expression of different biological components of the designed genome editing cassette (if needed).
- 20.
- Transform protoplasts using the non-binary version of the cassette containing Cas9 nuclease and the sgRNAs.
2.7. Stable Transformation of Crop Species for Efficient Genome Editing
- 21.
- Stable transformation of the genome editing components using the desirable method.
- 22.
- Detection of mutations as described below (see Section 2.8).
2.8. Detection of Mutations
- 23.
- Amplify the target sequence using primers flanking the induced mutation site using a proofreading polymerase.
- Note: Perform agarose electrophoresis and elute the expected size amplicon from the gel using a gel extraction kit or perform a PCR clean-up if there are no other PCR byproducts.
- 24.
- Submit the eluted (or purified) amplicon(s) for Sanger sequencing.
- 25.
- Analyze the sequencing results using Synthego’s free bioinformatics tool, Inference of CRISPR Edits (ICE) [76], to easily assess the possibility of putative CRISPR-induced edits at the target sites.
- 26.
- Further verification of the putative edits can be performed by TOPO-cloning of the eluted (or pure) amplicon(s) and subsequent submission of at least five clones for Sanger sequencing. This ensures that all possible variants are properly characterized, including heterozygous and biallelic loci.
- Note: We recommend that submission of ≥ five clones per PCR amplicon suffice for the detection of potential edits using Sanger sequencing. For more accurate results, it is advisable to submit more clones [69] or to conduct multiplex target amplicon sequencing (see step 28).
- 27.
- Compare the sequencing results by mapping them to the template sequence (see step 3).
- 28.
- (Optional) Perform next-generation sequencing (NGS) of the target amplicons using the eluted (or pure) amplicon obtained previously (step 27) as the initial template (nested PCR) to determine the rate of induced mutations generated by CRISPR/Cas and potential off-target edits (Figure 3). To be cost-effective, this requires many amplicon samples to be pooled together using barcoded DNA indices to label each sample during the sequencing library preparation.
- 29.
- Analysis of the obtained Illumina MiSeq results using CRIS.py [83], a versatile and high-throughput analysis program for CRISPR-based genome editing.
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barabaschi, D.; Tondelli, A.; Desiderio, F.; Volante, A.; Vaccino, P.; Valè, G.; Cattivelli, L. Next Generation Breeding. Plant Sci. 2016, 242, 3–13. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 28.1–28.31. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Doudna, J.A. CRISPR Technology: A Decade of Genome Editing Is Only the Beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Genome Engineering for Crop Improvement and Future Agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Dobert, R.; Atanassova, A.; Pavely, C. Impacts of the Regulatory Environment for Gene Editing on Delivering Beneficial Products. In Vitro Cell. Dev. Biol. Plant 2021, 57, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Buchholzer, M.; Frommer, W.B. An Increasing Number of Countries Regulate Genome Editing in Crops. New Phytol. 2023, 237, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.F.; Grabowski, M.; Lener, M.; Engelhard, M.; Simon, S.; Dolezel, M.; Heissenberger, A.; Lüthi, C. Biosafety of Genome Editing Applications in Plant Breeding: Considerations for a Focused Case-Specific Risk Assessment in the EU. Biotech 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Kawall, K.; Cotter, J.; Then, C. Broadening the GMO Risk Assessment in the EU for Genome Editing Technologies in Agriculture. Env. Sci. Eur. 2020, 32, 106. [Google Scholar] [CrossRef]
- Agapito-Tenfen, S.Z.; Okoli, A.S.; Bernstein, M.J.; Wikmark, O.-G.; Myhr, A.I. Revisiting Risk Governance of GM Plants: The Need to Consider New and Emerging Gene-Editing Techniques. Front. Plant Sci. 2018, 9, 1874. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.J.; Biswas, S.; Tsakirpaloglou, N.; Septiningsih, E.M. Functional Allele Validation by Gene Editing to Leverage the Wealth of Genetic Resources for Crop Improvement. Int. J. Mol. Sci. 2022, 23, 6565. [Google Scholar] [CrossRef] [PubMed]
- Cardi, T.; Murovec, J.; Bakhsh, A.; Boniecka, J.; Bruegmann, T.; Bull, S.E.; Eeckhaut, T.; Fladung, M.; Galovic, V.; Linkiewicz, A.; et al. CRISPR/Cas-Mediated Plant Genome Editing: Outstanding Challenges a Decade after Implementation. Trends Plant Sci. 2023, 28, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in Agriculture and Plant Biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Tsakirpaloglou, N.; Trijatmiko, K.R.; Septiningsih, E.; Thomson, M.J. Gene and Base Editing Tools for the Improvement of Cereal Traits. In Developing Sustainable and Health Promoting Cereals and Pseudocereals: Conventional and Molecular Breeding; Rakszegi, M., Papageorgiou, M., Rocha, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Lema, M. Regulatory Assessment of Off-Target Changes and Spurious DNA Insertions in Gene-Edited Organisms for Agri-Food Use. J. Regul. Sci. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Zhao, H.; Wolt, J.D. Risk Associated with Off-Target Plant Genome Editing and Methods for Its Limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Naim, F.; Shand, K.; Hayashi, S.; O’Brien, M.; McGree, J.; Johnson, A.A.T.; Dugdale, B.; Waterhouse, P.M. Are the Current GRNA Ranking Prediction Algorithms Useful for Genome Editing in Plants? PLoS ONE 2020, 15, e0227994. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.-L. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol. Plant 2017, 10, 530–532. [Google Scholar] [CrossRef]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for Designing CRISPR/Cas Guide RNA with Reduced off-Target Sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR Web Toolbox beyond Genome Editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.-G. CRISPR-GE: A Convenient Software Toolkit for CRISPR-Based Genome Editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Minkenberg, B.; Zhang, J.; Xie, K.; Yang, Y. CRISPR-PLANT v2: An Online Resource for Highly Specific Guide RNA Spacers Based on Improved off-Target Analysis. Plant Biotechnol. J. 2019, 17, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.P.; Mansotra, K.; Yadav, S.K.; Kumar, V. An Overview of Designing and Selection of SgRNAs for Precise Genome Editing by the CRISPR-Cas9 System in Plants. 3 Biotech 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, J.; Aggarwal, R.; Buyyarapu, R.; Kumpatla, S. SNP Markers and Their Impact on Plant Breeding. Int. J. Plant Genom. 2012, 2012, 728398. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Mehravar, M.; Shirazi, A.; Mehrazar, M.M.; Nazari, M. In Vitro Pre-Validation of Gene Editing by CRISPR/Cas9 Ribonucleoprotein. Avicenna J. Med. Biotechnol. 2019, 11, 259–263. [Google Scholar] [PubMed]
- Liu, Y.; Tao, W.; Wen, S.; Li, Z.; Yang, A.; Deng, Z.; Sun, Y. In Vitro CRISPR/Cas9 System for Efficient Targeted DNA Editing. mBio 2015, 6, e1714–e1715. [Google Scholar] [CrossRef]
- Hahn, F.; Korolev, A.; Sanjurjo Loures, L.; Nekrasov, V. A Modular Cloning Toolkit for Genome Editing in Plants. BMC Plant Biol. 2020, 20, 179. [Google Scholar] [CrossRef]
- Čermák, T.; Curtin, S.J.; Gil-Humanes, J.; Čegan, R.; Kono, T.J.Y.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W.; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.-F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 Toolkit for Multiplex Genome Editing in Plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Wyvekens, N.; Khayter, C.; Foden, J.A.; Thapar, V.; Reyon, D.; Goodwin, M.J.; Aryee, M.J.; Joung, J.K. Dimeric CRISPR RNA-Guided FokI Nucleases for Highly Specific Genome Editing. Nat. Biotechnol. 2014, 32, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 Multiplex Editing Capability with the Endogenous TRNA-Processing System. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant Protoplast Technology: Current Status. Acta Physiol. Plant 2005, 27, 117–130. [Google Scholar] [CrossRef]
- Yue, J.-J.; Yuan, J.-L.; Wu, F.-H.; Yuan, Y.-H.; Cheng, Q.-W.; Hsu, C.-T.; Lin, C.-S. Protoplasts: From Isolation to CRISPR/Cas Genome Editing Application. Front. Genome Ed. 2021, 3, 717017. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M.; Bargmann, B.O.R. Protoplast Regeneration and Its Use in New Plant Breeding Technologies. Front. Genome Ed. 2021, 3, 734951. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Wahl, N.J.; Thomson, M.J.; Cason, J.M.; McCutchen, B.F.; Septiningsih, E.M. Optimization of Protoplast Isolation and Transformation for a Pilot Study of Genome Editing in Peanut by Targeting the Allergen Gene Ara h 2. Int. J. Mol. Sci. 2022, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bridgeland, A.; Irum, S.; Thomson, M.J.; Septiningsih, E.M. Optimization of Prime Editing in Rice, Peanut, Chickpea, and Cowpea Protoplasts by Restoration of GFP Activity. Int. J. Mol. Sci. 2022, 23, 9809. [Google Scholar] [CrossRef]
- Larkin, P.J. Purification and Viability Determinations of Plant Protoplasts. Planta 1976, 128, 213–216. [Google Scholar] [CrossRef]
- Krens, F.A.; Molendijk, L.; Wullems, G.J.; Schilperoort, R.A. In Vitro Transformation of Plant Protoplasts with Ti-Plasmid DNA. Nature 1982, 296, 72–74. [Google Scholar] [CrossRef]
- Mathur, J.; Koncz, C. PEG-Mediated Protoplast Transformation with Naked DNA. In Arabidopsis Protocols; Martinez-Zapater, J.M., Salinas, J., Eds.; Humana Press: Totowa, NJ, USA, 1998; Volume 82, pp. 267–276. [Google Scholar]
- Chen, Z.; Debernardi, J.M.; Dubcovsky, J.; Gallavotti, A. Recent Advances in Crop Transformation Technologies. Nat. Plants 2022, 8, 1343–1351. [Google Scholar] [CrossRef]
- Lee, K.; Wang, K. Strategies for Genotype-Flexible Plant Transformation. Curr. Opin. Biotechnol. 2023, 79, 102848. [Google Scholar] [CrossRef]
- Gordon-Kamm, W.; Barone, P.; Svitashev, S.; Sander, J.D.; Kumar, S.; Jones, T. Strategies for CRISPR/Cas9-Mediated Genome Editing: From Delivery to Production of Modified Plants; Burleigh Dodds Science Publishing: Cambridge, UK, 2021. [Google Scholar]
- Hamilton, C.M.; Frary, A.; Lewis, C.; Tanksley, S.D. Stable Transfer of Intact High Molecular Weight DNA into Plant Chromosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9975–9979. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome Editing in Maize Directed by CRISPR-Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Zhang, Y.; Liu, J.; Yin, K.; Qiu, J.-L.; Gao, C. Genome Editing of Bread Wheat Using Biolistic Delivery of CRISPR/Cas9 in Vitro Transcripts or Ribonucleoproteins. Nat. Protoc. 2018, 13, 413–430. [Google Scholar] [CrossRef]
- Park, J.; Choe, S. DNA-Free Genome Editing with Preassembled CRISPR/Cas9 Ribonucleoproteins in Plants. Transgenic Res. 2019, 28, 61–64. [Google Scholar] [CrossRef]
- Lv, Z.; Jiang, R.; Chen, J.; Chen, W. Nanoparticle-Mediated Gene Transformation Strategies for Plant Genetic Engineering. Plant J. 2020, 104, 880–891. [Google Scholar] [CrossRef]
- Dunbar, T.; Tsakirpaloglou, N.; Septiningsih, E.M.; Thomson, M.J. Carbon Nanotube-Mediated Plasmid DNA Delivery in Rice Leaves and Seeds. Int. J. Mol. Sci. 2022, 23, 4081. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Zhang, Z.-B.; Zheng, D.-Y.; Zhang, T.-T.; Li, X.-L.; Zhang, C.; Yu, R.; Wei, J.-H.; Wu, Z.-Y. Efficient and Genotype Independent Maize Transformation Using Pollen Transfected by DNA-Coated Magnetic Nanoparticles. J. Integr. Plant Biol. 2022, 64, 1145–1156. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, Z.; Wang, Y.; Chen, W.; Sun, C.; Cui, B.; Cui, J.; Yu, M.; Zeng, Z.; Guo, S.; et al. Pollen Magnetofection for Genetic Modification with Magnetic Nanoparticles as Gene Carriers. Nat. Plants 2017, 3, 956–964. [Google Scholar] [CrossRef]
- Vejlupkova, Z.; Warman, C.; Sharma, R.; Scheller, H.V.; Mortimer, J.C.; Fowler, J.E. No Evidence for Transient Transformation via Pollen Magnetofection in Several Monocot Species. Nat. Plants 2020, 6, 1323–1324. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly Efficient DNA-Free Plant Genome Editing Using Virally Delivered CRISPR-Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Sun, Y.; Li, B.; Zhang, D.; Li, W.; Liu, J.; Li, D.; Gao, C.; Zhang, Y.; et al. Highly Efficient Heritable Genome Editing in Wheat Using an RNA Virus and Bypassing Tissue Culture. Mol. Plant 2021, 14, 1787–1798. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Li, Z.; Li, H.; Song, W.; Zhao, H.; Lai, J.; Xia, L.; Li, D.; Zhang, Y. A Barley Stripe Mosaic Virus-Based Guide RNA Delivery System for Targeted Mutagenesis in Wheat and Maize. Mol. Plant Pathol. 2019, 20, 1463–1474. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly Efficient, Genotype-Independent Transformation and Gene Editing in Watermelon (Citrullus lanatus) Using a Chimeric ClGRF4-GIF1 Gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Li, C.; Yang, Y.; Duan, Y.; Yang, Y.; Sun, X. Establishment of Agrobacterium-Mediated Genetic Transformation and Application of CRISPR/Cas9 Genome-Editing System to Brassica Rapa Var. Rapa. Plant Methods 2022, 18, 98. [Google Scholar] [CrossRef]
- Che, P.; Wu, E.; Simon, M.K.; Anand, A.; Lowe, K.; Gao, H.; Sigmund, A.L.; Yang, M.; Albertsen, M.C.; Gordon-Kamm, W.; et al. Wuschel2 Enables Highly Efficient CRISPR/Cas-Targeted Genome Editing during Rapid de Novo Shoot Regeneration in Sorghum. Commun. Biol. 2022, 5, 344. [Google Scholar] [CrossRef]
- Aesaert, S.; Impens, L.; Coussens, G.; van Lerberge, E.; Vanderhaeghen, R.; Desmet, L.; Vanhevel, Y.; Bossuyt, S.; Wambua, A.N.; van Lijsebettens, M.; et al. Optimized Transformation and Gene Editing of the B104 Public Maize Inbred by Improved Tissue Culture and Use of Morphogenic Regulators. Front. Plant Sci. 2022, 13, 883847. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-Mediated Genetic Transformation and CRISPR/Cas9-Mediated Targeted Mutagenesis in Hemp (Cannabis sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting Plant Genome Editing with a Versatile CRISPR-Combo System. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant Gene Editing through de Novo Induction of Meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef]
- Wang, N.; Ryan, L.; Sardesai, N.; Wu, E.; Lenderts, B.; Lowe, K.; Che, P.; Anand, A.; Worden, A.; van Dyk, D.; et al. Leaf Transformation for Efficient Random Integration and Targeted Genome Modification in Maize and Sorghum. Nat. Plants 2023, 9, 255–270. [Google Scholar] [CrossRef]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant Genome Editing Made Easy: Targeted Mutagenesis in Model and Crop Plants Using the CRISPR/Cas System. Plant Methods 2013, 9, 39. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/SgRNA-Mediated Targeted Gene Modification in Arabidopsis, Tobacco, Sorghum and Rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef]
- Xie, K.; Yang, Y. RNA-Guided Genome Editing in Plants Using a CRISPR-Cas System. Mol. Plant 2013, 6, 1975–1983. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, H.; Xu, N.; Zhang, B.; Gou, F.; Zhu, J.-K. Application of the CRISPR-Cas System for Efficient Genome Engineering in Plants. Mol. Plant 2013, 6, 2008–2011. [Google Scholar] [CrossRef]
- Voytas, D.F. Plant Genome Engineering with Sequence-Specific Nucleases. Annu. Rev. Plant Biol. 2013, 64, 327–350. [Google Scholar] [CrossRef]
- Bock, C.; Datlinger, P.; Chardon, F.; Coelho, M.A.; Dong, M.B.; Lawson, K.A.; Lu, T.; Maroc, L.; Norman, T.M.; Song, B.; et al. High-Content CRISPR Screening. Nat. Rev. Methods Primers 2022, 2, 8. [Google Scholar] [CrossRef]
- Hanna, R.E.; Doench, J.G. Design and Analysis of CRISPR-Cas Experiments. Nat. Biotechnol. 2020, 38, 813–823. [Google Scholar] [CrossRef]
- Conant, D.; Hsiau, T.; Rossi, N.; Oki, J.; Maures, T.; Waite, K.; Yang, J.; Joshi, S.; Kelso, R.; Holden, K.; et al. Inference of CRISPR Edits from Sanger Trace Data. CRISPR J. 2022, 5, 123–130. [Google Scholar] [CrossRef]
- de Muinck, E.J.; Trosvik, P.; Gilfillan, G.D.; Hov, J.R.; Sundaram, A.Y.M. A Novel Ultra High-Throughput 16S RRNA Gene Amplicon Sequencing Library Preparation Method for the Illumina HiSeq Platform. Microbiome 2017, 5, 68. [Google Scholar] [CrossRef]
- Illumina. Illumina 16S Metagenomic Sequencing Library Preparation (Illumina Technical Note 15044223). 2013. Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 5 August 2022).
- Oyola, S.O.; Otto, T.D.; Gu, Y.; Maslen, G.; Manske, M.; Campino, S.; Turner, D.J.; Macinnis, B.; Kwiatkowski, D.P.; Swerdlow, H.P.; et al. Optimizing Illumina Next-Generation Sequencing Library Preparation for Extremely AT-Biased Genomes. BMC Genom. 2012, 13, 1. [Google Scholar] [CrossRef]
- Quail, M.A.; Corton, C.; Uphill, J.; Keane, J.; Gu, Y. Identifying the Best PCR Enzyme for Library Amplification in NGS. bioRxiv, 2022; preprint. [Google Scholar] [CrossRef]
- Park, S.H.; Cao, M.; Bao, G. Detection and Quantification of Unintended Large On-Target Gene Modifications Due to CRISPR/Cas9 Editing. Curr. Opin. Biomed. Eng. 2023, 28, 100478. [Google Scholar] [CrossRef]
- Chu, P.; Agapito-Tenfen, S.Z. Unintended Genomic Outcomes in Current and Next Generation GM Techniques: A Systematic Review. Plants 2022, 11, 2997. [Google Scholar] [CrossRef]
- Connelly, J.P.; Pruett-Miller, S.M. CRIS.Py: A Versatile and High-Throughput Analysis Program for CRISPR-Based Genome Editing. Sci. Rep. 2019, 9, 4194. [Google Scholar] [CrossRef]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing Crop Transformation in the Era of Genome Editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
| Crop Species | Web Links (All Links Were Accessed on 1 September 2023) |
|---|---|
| Rice | |
| Wheat | |
| Barley | |
| Maize | |
| Sorghum | |
| Solanaceae | |
| Cotton | |
| Soybean | |
| Legumes |
| Online Databases | Web Links (All Links Were Accessed on 1 September 2023) |
|---|---|
| CRISPR-P 2.0 | http://crispr.hzau.edu.cn/CRISPR2/ |
| CRISPR-direct | https://crispr.dbcls.jp |
| Chopchop | https://chopchop.cbu.uib.no |
| Target design | http://skl.scau.edu.cn/targetdesign/ |
| CRISPR-PLANT v2 | http://omap.org/crispr2/ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakirpaloglou, N.; Septiningsih, E.M.; Thomson, M.J. Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops. Plants 2023, 12, 3564. https://doi.org/10.3390/plants12203564
Tsakirpaloglou N, Septiningsih EM, Thomson MJ. Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops. Plants. 2023; 12(20):3564. https://doi.org/10.3390/plants12203564
Chicago/Turabian StyleTsakirpaloglou, Nikolaos, Endang M. Septiningsih, and Michael J. Thomson. 2023. "Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops" Plants 12, no. 20: 3564. https://doi.org/10.3390/plants12203564
APA StyleTsakirpaloglou, N., Septiningsih, E. M., & Thomson, M. J. (2023). Guidelines for Performing CRISPR/Cas9 Genome Editing for Gene Validation and Trait Improvement in Crops. Plants, 12(20), 3564. https://doi.org/10.3390/plants12203564









