Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai
Abstract
:1. Introduction
2. Results
2.1. Optimization of Parameters in the UHPLC-MS/MS Conditions
2.2. Validation of the Method
2.2.1. Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
2.2.2. Precision
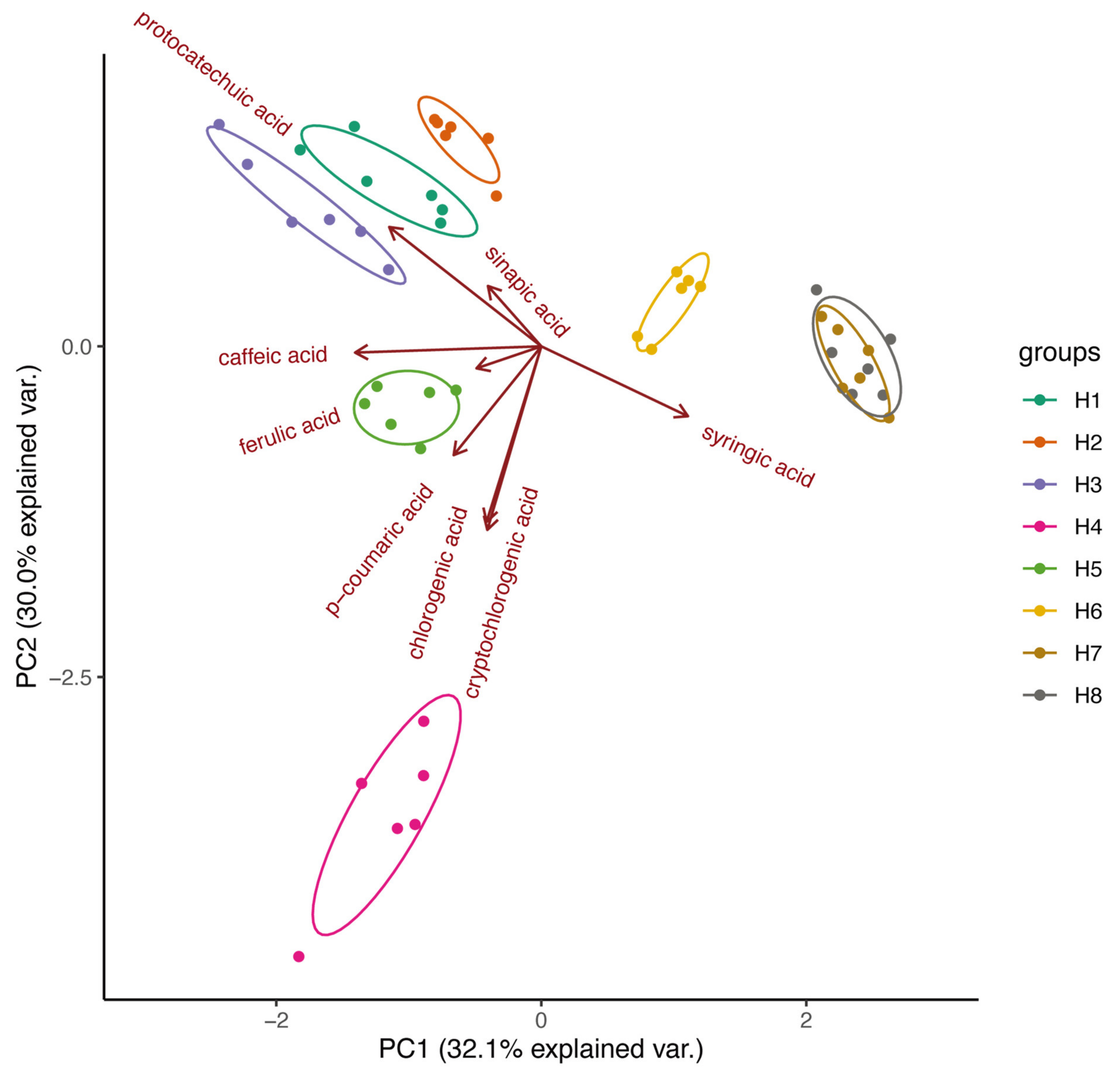
2.3. Quantitative Phenolic Acids Analysis in Fruiting Bodies of S. baumii
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals and Reagents
4.3. Instruments
4.4. Preparation of Standard Solutions
4.5. Sample Preparation
4.6. UHPLC Conditions
4.7. MS/MS Conditions
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [PubMed]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Physicochemical stability of blood orange juice during frozen storage. Int. J. Food Prop. 2017, 20, 1930–1943. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Rong, L.; Wu, Z.; Sun, W. Polyphenol Composition and Antioxidant Activity of Japonica Rice Cultivars and Intake Status. Foods 2022, 11, 3788. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible Med. Mushrooms; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Thakur, M.P. Advances in mushroom production: Key to food, nutritional and employment security: A review. Indian Phytopathol. 2020, 73, 377–395. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Wu, S.H.; Chang, C.C.; Wei, C.L.; Jiang, G.Z.; Cui, B.K. Sanghuangporus toxicodendri sp. nov. (Hymenochaetales, Basidiomycota) from China. MycoKeys 2019, 57, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, J.; Zhou, J.L.; Si, J.; Cui, B.K. Species Diversity, Phylogeny, Divergence Time, and Biogeography of the Genus Sanghuangporus (Basidiomycota). Front. Microbiol. 2019, 10, 812. [Google Scholar]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Zeng, P.; Li, X.T.; Shi, L.G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Tang, K.; Liang, Y.; Xu, Y.; Sheng, K.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Purification and antioxidant and anti-Inflammatory activity of extracellular polysaccharopeptide from sanghuang mushroom, Sanghuangporus lonicericola. J. Sci. Food Agric. 2021, 101, 1009–1020. [Google Scholar] [CrossRef]
- Zheng, N.; Ming, Y.; Chu, J.; Yang, S.; Wu, G.; Li, W.; Zhang, R.; Cheng, X. Optimization of Extraction Process and the Antioxidant Activity of Phenolics from Sanghuangporus baumii. Molecules 2021, 26, 3850. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.X.; Wu, D.M.; Gao, N.; Si, J.; Cui, B.K. Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi. J. Fungi 2023, 9, 242. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef]
- Huang, Z.R.; Huang, Q.Z.; Chen, K.W.; Huang, Z.F.; Liu, Y.; Jia, R.B.; Liu, B. Sanghuangporus vaninii fruit body polysaccharide alleviates hyperglycemia and hyperlipidemia via modulating intestinal microflora in type 2 diabetic mice. Front. Nutr. 2022, 9, 1013466. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.H.; Dai, Y.C.; Zhou, L.W.; Cai, W.M.; Guo, L.D.; Cui, B.K.; Li, N.; Lei, P.; Li, C.T.; et al. Sanghuang industry in China: Current status, challenges and perspectives: The Qiandao Lake declaration for sanghuang industry development. Mycosystema 2023, 42, 855–873. [Google Scholar]
- Wang, S.; Liu, Z.; Wang, X.; Liu, R.; Zou, L. Mushrooms Do Produce Flavonoids: Metabolite Profiling and Transcriptome Analysis of Flavonoid Synthesis in the Medicinal Mushroom Sanghuangporus baumii. J. Fungi 2022, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chen, C.C.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomed. Pharmacother. 2022, 153, 113434. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Khamis, M.M.; Mohammed Saeid, W.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Yang, J.; Yao, L.; Gong, K.; Li, K.; Sun, L.; Cai, W. Identification and Quantification of Chlorogenic Acids from the Root Bark of Acanthopanax gracilistylus by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. ACS Omega 2022, 7, 25675–25685. [Google Scholar] [CrossRef]
- Song, J.L.; Zhou, Z.F.; Yan, J.; Lu, N.; Cheng, J.W.; Yuan, W.D.; Wang, W.K. Study of effects of bran on the metabolism of Sanghuangporus vaninii based on metabolomics of UPLC-MS/MS. Mycosystema 2021, 40, 641–655. [Google Scholar]
- Zhao, X.Q.; Guo, S.; Yan, H.; Lu, Y.Y.; Zhang, F.; Qian, D.W.; Wang, H.Q.; Duan, J.A. Analysis of phenolic acids and flavonoids in leaves of Lycium barbarum from different habitats by ultra-high-performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4552. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, X.; Xu, Y.; Liu, X.; Zhang, J.; He, Z. Simultaneous determination of 49 amino acids.; B vitamins.; flavonoids.; and phenolic acids in commonly consumed vegetables by ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2021, 344, 128712. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Deng, Z.P.; Liang, S.; Zou, X.W.; Teng, Y.; Wang, W.K.; Fu, L.Z. Quantitative Analysis of Flavonoids in Fruiting Bodies of Sanghuangporus Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry. Molecules 2023, 28, 5166. [Google Scholar] [CrossRef]
- Leão, C.; Simões, M.; Borges, A. 12-Marine Phenolics: Classes, Antibacterial Properties, and Applications. In Marine Phenolic Compounds; Pérez-Correa, J.R., Mateos, R., Domínguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 371–392. [Google Scholar]
- Zekrumah, M.; Begua, P.; Razak, A.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.; Elrashied, H.; Zou, X.; Zhang, D. Role of dietary polyphenols in non-communicable chronic disease prevention and interactions in food systems: An overview. Nutrition 2023, 112, 112034. [Google Scholar] [CrossRef] [PubMed]
- Manivel, P.; Chen, X. Chlorogenic, Caffeic, and Ferulic Acids and Their Derivatives in Foods. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–31. [Google Scholar]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar]
- Bai, L.; Kee, H.J.; Han, X.; Zhao, T.; Kee, S.J.; Jeong, M.H. Protocatechuic acid attenuates isoproterenol-induced cardiac hypertrophy via downregulation of ROCK1–Sp1–PKCγ axis. Sci. Rep. 2021, 11, 17343. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nasiri, N.; Karizi, S.Z.; Nejati, F.; Haghi-Aminjan, H.; Norouzi, S.; Piri, P.; Estakhr, L.; Faradonbeh, D.R.; Kohandani, M.; et al. An Updated Review of Various Medicinal Applications of p-Co umaric Acid: From Antioxidative and Anti-Inflammatory Properties to Effects on Cell Cycle and Proliferation. Mini Rev. Med. Chem. 2021, 21, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.D.; Ioannou, I.; Allais, F. Sinapic Acid and Sinapate Esters in Brassica: Innate Accumulation, Biosynthesis, Accessibility via Chemical Synthesis or Recovery from Biomass, and Biological Activities. Front. Chem. 2021, 9, 664602. [Google Scholar]
- Albert, K. Liquid chromatography–nuclear magnetic resonance spectroscopy. J. Chromatogr. A 1999, 856, 199–211. [Google Scholar] [CrossRef]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid chromatography–tandem mass spectrometry for clinical diagnostics. Nat. Rev. Methods Primers 2022, 2, 96. [Google Scholar] [CrossRef]
- Medeiros, P.M. Gas Chromatography–Mass Spectrometry (GC–MS). In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 530–535. [Google Scholar]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.; Xie, D.; Peng, N.; Wang, W.; Wang, D.; Li, B. Influence of traditional Chinese medicines on the in vivo metabolism of lopinavir/ritonavir based on UHPLC-MS/MS analysis. J. Pharm. Anal. 2022, 12, 270–277. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
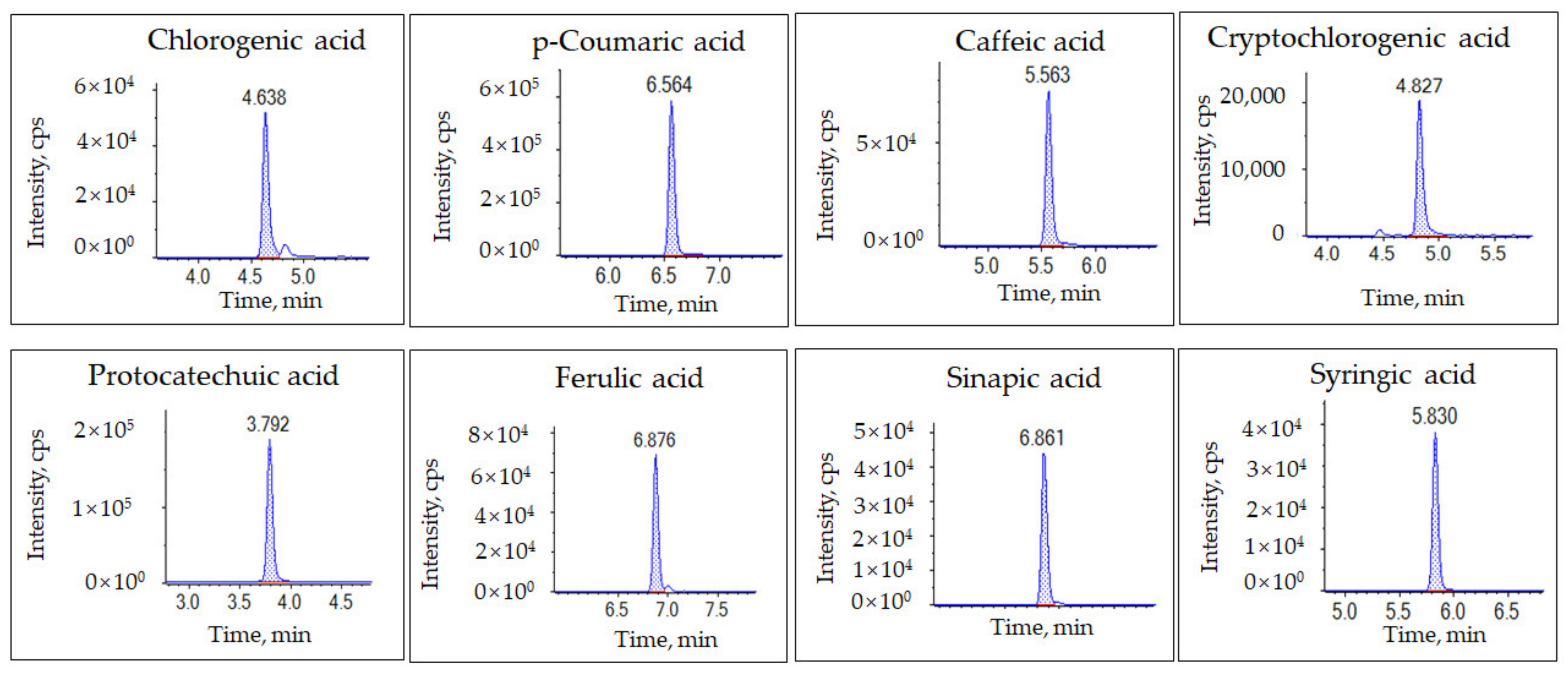

| Analyte | Q1 | Q3 | RT (min) | DP (V) | CE (V) |
|---|---|---|---|---|---|
| Chlorogenic acid 1 | 353.1 | 85.0 | 4.64 | −74 | −59 |
| Chlorogenic acid 2 | 353.1 | 191.1 | 4.64 | −56 | −19 |
| p-Coumaric acid 1 | 163.0 | 119.0 | 6.56 | −60 | −19 |
| p-Coumaric acid 2 | 163.0 | 117.1 | 6.56 | −71 | −43 |
| Caffeic acid 1 | 179.1 | 134.0 | 5.56 | −74 | −31 |
| Caffeic acid 2 | 179.1 | 89.1 | 5.56 | −60 | −42 |
| Cryptochlorogenic acid 1 | 353.1 | 173.0 | 4.83 | −50 | −23 |
| Protocatechuic acid 1 | 153.1 | 109.0 | 3.79 | −50 | −19 |
| Protocatechuic acid 2 | 153.1 | 91.0 | 3.79 | −57 | −34 |
| Ferulic Acid 1 | 193.0 | 134.1 | 6.88 | −50 | −20 |
| Ferulic Acid 2 | 193.0 | 178.0 | 6.88 | −50 | −16 |
| Sinapic acid 1 | 223.0 | 193.0 | 6.86 | −51 | −29 |
| Syringic acid 1 | 197.1 | 123.0 | 5.83 | −41 | −30 |
| Syringic acid 2 | 197.1 | 181.8 | 5.83 | −41 | −17 |
| Analyte | Regression Equation (Weighting: 1/x) | R2 | Linear Range (µg/L) | LOD (µg/L) | LOQ (µg/L) | Intra-Day (RSD, %) (n = 6) | Inter-Day (RSD, %) (n = 6) |
|---|---|---|---|---|---|---|---|
| Chlorogenic acid | y = 2453.62 x − 106.25 | 0.99957 | 0.10–100 | 0.429 | 1.429 | 5.2–9.8 | 1.4–6.5 |
| p-Coumaric acid | y = 20240.55 x + 1604.53 | 0.99932 | 0.10–100 | 0.566 | 1.887 | 3.9–9.9 | 1.6–2.4 |
| Caffeic acid | y = 2804.30 x + 769.49 | 0.99905 | 0.05–100 | 0.273 | 0.909 | 5.1–11.3 | 2.0–9.7 |
| Cryptochlorogenic acid | y = 879.37 x − 388.26 | 0.99910 | 1.00–100 | 0.333 | 1.111 | 3.4–5.9 | 1.9–6.9 |
| Protocatechuic acid | y = 5268.99 x + 3490.54 | 0.99929 | 1.00–100 | 0.857 | 2.857 | 4.5–10.9 | 2.1–5.0 |
| Ferulic Acid | y = 2811.47 x + 1720.03 | 0.99944 | 0.50–100 | 0.143 | 0.476 | 2.8–13.7 | 1.3–7.5 |
| Sinapic acid | y = 1697.38 x + 514.08 | 0.99955 | 0.05–100 | 0.333 | 1.111 | 1.9–15.7 | 1.7–11.7 |
| Syringic acid | y = 1427.33 x + 150.21 | 0.99955 | 0.50–100 | 0.353 | 1.176 | 4.8–11.8 | 4.4–10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Liang, S.; Zou, X.; Teng, Y.; Wang, W.; Fu, L. Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai. Plants 2023, 12, 3565. https://doi.org/10.3390/plants12203565
Zhou Z, Liang S, Zou X, Teng Y, Wang W, Fu L. Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai. Plants. 2023; 12(20):3565. https://doi.org/10.3390/plants12203565
Chicago/Turabian StyleZhou, Zhongjing, Shuang Liang, Xiaowei Zou, Yi Teng, Weike Wang, and Lizhong Fu. 2023. "Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai" Plants 12, no. 20: 3565. https://doi.org/10.3390/plants12203565
APA StyleZhou, Z., Liang, S., Zou, X., Teng, Y., Wang, W., & Fu, L. (2023). Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai. Plants, 12(20), 3565. https://doi.org/10.3390/plants12203565






