Understanding the Technical-Scientific Gaps of Underutilized Tropical Species: The Case of Bactris gasipaes Kunth
Abstract
:1. Introduction
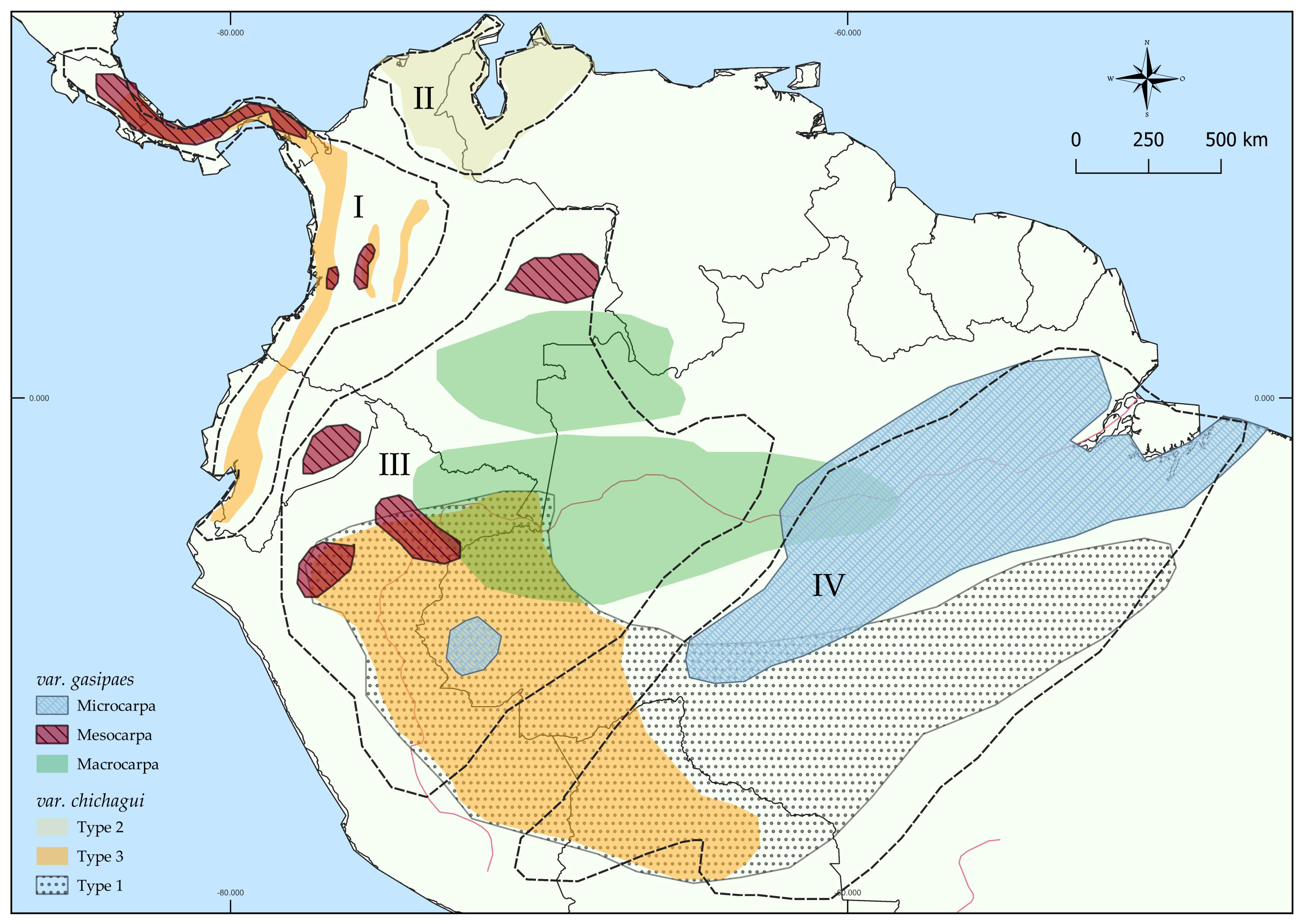
2. Peach Palm Is a Domesticated Palm in Neotropics
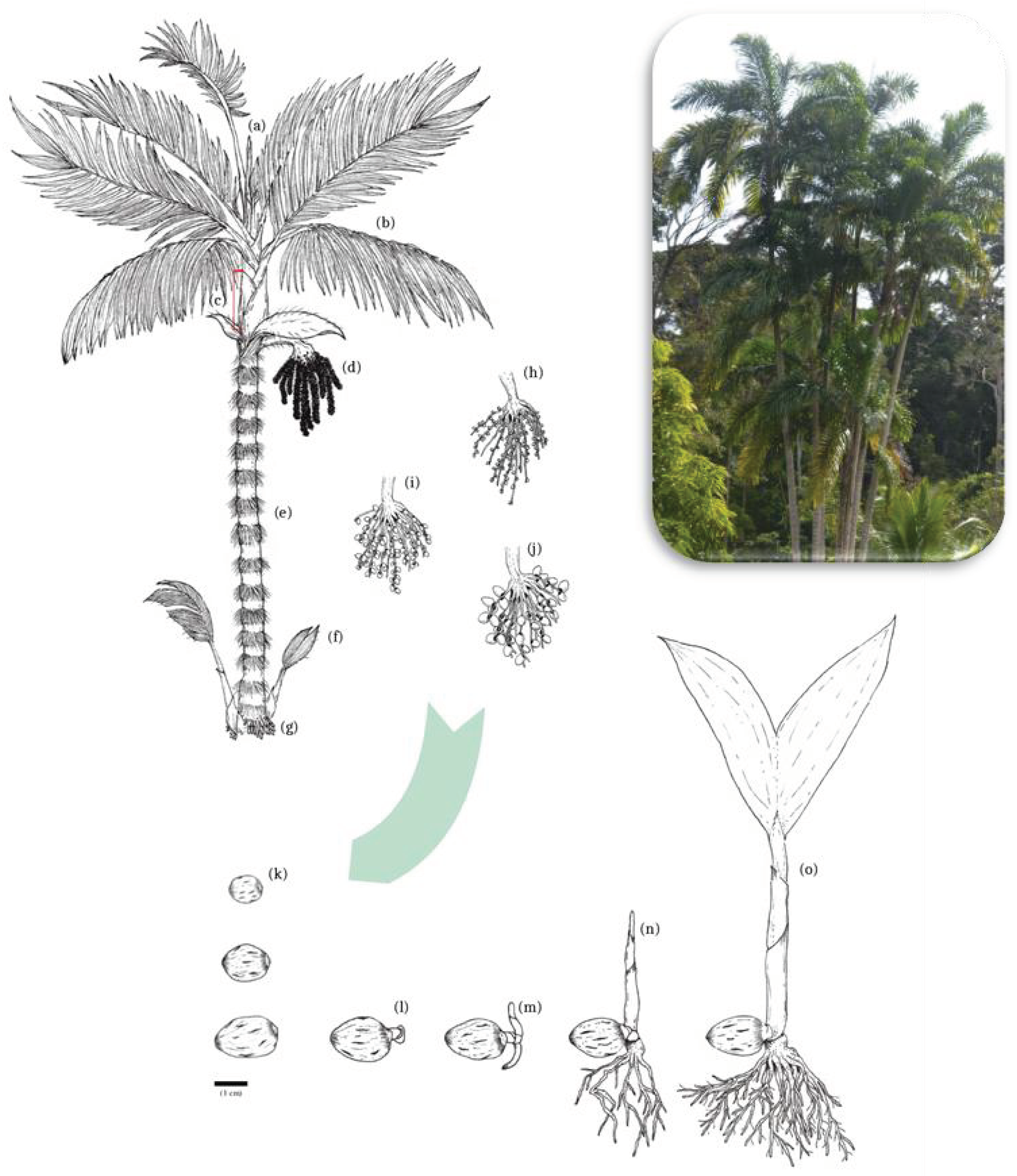
2.1. General Morphology of Peach Palm
2.2. Ecological Relationships
3. Agronomic and Physiological Aspects of Peach Palm
3.1. Practices in B. gasipaes Cultivation
3.2. Abiotic Stress
3.3. Pests and Diseases
4. Peach Palm Products: Diversity in Consumption, Chemical Composition and Biotechnological Application
4.1. Palm Heart Is Present in International Market, but Fruits and By-Products Consumption Is Associated with Basal Market
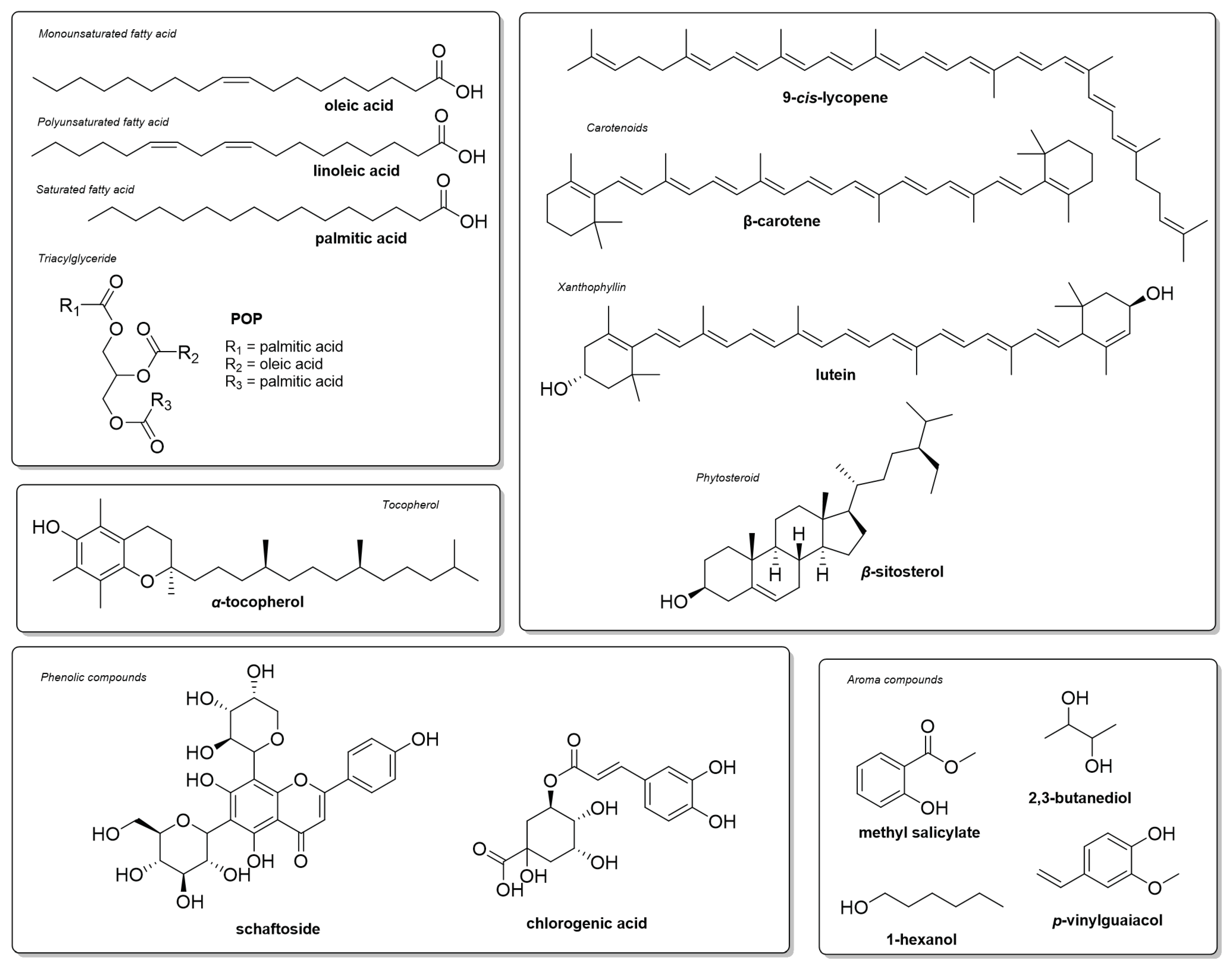
4.2. Fruit Cheminal Composition
Specialized Metabolites
4.3. Beside the Fruit, Peach Palm Agro-Industrial Residues Has Been Focus of Research
5. Genetic Resources: Conservation, Breeding and In Vitro Culture
5.1. Breeding and Implications for Genetic Erosion and Conservation
5.2. In Vitro Culture as a Perspective for Advances in Breeding
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clement, C.R.; Weber, J.C.; van Leeuwen, J.; Astorga Domian, C.; Cole, D.M.; Arévalo Lopez, L.A.; Argüello, H. Why Extensive Research and Development Did Not Promote Use of Peach Palm Fruit in Latin America. Agrofor. Syst. 2004, 61–62, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Patiño, V.M. An Ethnobotanical Sketch of the Palm Bactris (Guilielma) Gasipaes. Principes 1992, 35, 143–147. [Google Scholar]
- Henderson, A. Bactris (Palmae). Flora Neotrop. 2000, 79, 181. [Google Scholar]
- Steinmacher, D.A.; Guerra, M.P.; Saare-Surminski, K.; Lieberei, R. A Temporary Immersion System Improves in Vitro Regeneration of Peach Palm through Secondary Somatic Embryogenesis. Ann. Bot. 2011, 108, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, J.; Pérez, L.; Clement, C.R. Field Genebanks May Impede Instead of Promote Crop Development: Lessons of Failed Genebanks of “Promising” Brazilian Palms. Agrociencia 2005, 9, 61–66. [Google Scholar]
- Cornelius, J.P.; Clement, C.R.; Weber, J.C.; Sotelo-Montes, C.; van Leeuwen, J.; Ugarte-Guerra, L.J.; Ricse-Tembladera, A.; Arévalo-López, L. The Trade off between Genetic Gain and Conservation in a Participatory Improvement Programme: The Case of Peach Palm (Bactris gasipaes Kunth). For. Trees Livelihoods 2006, 16, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.R.; Santos, R.P.; Desmouliere, S.J.M.; Ferreira, E.J.L.; Neto, J.T.F. Ecological Adaptation of Wild Peach Palm, Its In Situ Conservation and Deforestation-Mediated Extinction in Southern Brazilian Amazonia. PLoS ONE 2009, 4, e4564. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.R. Domestication of the Pejibaye Palm (Bactris gasipaes): Past and Present. Adv. Econ. Bot. 1988, 6, 155–174. [Google Scholar]
- Couvreur, T.L.P.; Hahn, W.J.; de Granville, J.-J.; Pham, J.-L.; Ludeña, B.; Pintaud, J.-C. Phylogenetic Relationships of the Cultivated Neotropical Palm Bactris gasipaes (Arecaceae) with Its Wild Relatives Inferred from Chloroplast and Nuclear DNA Polymorphisms. Syst. Bot. 2007, 32, 519–530. [Google Scholar] [CrossRef]
- Patiño, V.M. Datos Etnobotánicos Adicionales Sobre El Cachipay o Pijibay (Bactris gasipaes Kunth), Arecaceae, y Especies Afines En América Intertropical. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 1995, 19, 661–671. [Google Scholar]
- Silva, J.B.F.; Clement, C.R. Wild Pejibaye (Bactris gasipaes Kunth var. chichagui) in Southeastern Amazonia. Acta Bot. Bras. 2005, 19, 281–284. [Google Scholar] [CrossRef]
- Mora-Urpí, J.; Clement, C.R. Races and Populations of Peach Palm Found in the Amazon Bacin. In Final Report (revised): Peach Palm (Bactris gasipaes H.B.K.) Germplasm Bank; Clement, C.R., Coradin, L., Eds.; Instituto Nacional de Pesquisas da Amazônia/Centro Nacional de Recursos Genéticos: Manaus, Brasil, 1988; pp. 78–94. [Google Scholar]
- Harlan, J.R.; Wet, J.M.J. Toward a Rational Classification of Cultivated Plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Clement, C.R.; Aradhya, M.K.; Manshardt, R.M. Allozyme Variation in Spineless Pejibaye (Bactris gasipaes Palmae). Econ. Bot. 1997, 51, 149–157. [Google Scholar] [CrossRef]
- Sawazaki, H.E.; Bovi, M.L.A.; Sodek, L.; Colombo, C.A. Diversidade Genética em Palmeiras Através de Isoenzimas e RAPD. Rev. Bras. Biol. 1998, 58, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.P.; Astolfi Filho, S.; Clement, C.R. Molecular Marker-Mediated Validation of Morphologically Defined Landraces of Pejibaye (Bactris gasipaes) and Their Phylogenetic Relationships. Genet. Resour. Crop Evol. 2005, 51, 871–882. [Google Scholar] [CrossRef]
- Araújo, M.C.; Rodrigues, D.P.; Astolfi Filho, S.; Clement, C.R. Genetic Variability in the Peach Palm Genebank with RAPD Markers. Crop Breed. Appl. Biotechnol. 2010, 10, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.R.; de Cristo-Araújo, M.; Coppens d’Eeckenbrugge, G.; dos Reis, V.M.; Lehnebach, R.; Picanço-Rodrigues, D. Origin and Dispersal of Domesticated Peach Palm. Front. Ecol. Evol. 2017, 5, 148. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, G.; Dufour, D.; Thomas, E.; van Zonneveld, M.; Escobar Salamanca, A.F.; Giraldo Toro, A.; Rivera, A.; Salazar Duque, H.; Suárez Baron, H.; Gallego, G.; et al. An Integrated Hypothesis on the Domestication of Bactris gasipaes. PLoS ONE 2015, 10, e0144644. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ugalde, J.A.; Mora-Urpí, J.; Rocha, O.J. Genetic Relationships among Wild and Cultivated Populations of Peach Palm (Bactris gasipaes Kunth, Palmae): Evidence for Multiple Independent Domestication Events. Genet. Resour. Crop Evol. 2011, 58, 571–583. [Google Scholar] [CrossRef]
- Buitrago Acosta, M.C.; Montúfar, R.; Guyot, R.; Mariac, C.; Tranbarger, T.J.; Restrepo, S.; Couvreur, T.L.P. Bactris gasipaes Kunth var. gasipaes Complete Plastome and Phylogenetic Analysis. Mitochondrial DNA Part B 2022, 7, 1540–1544. [Google Scholar] [CrossRef]
- Silva, R.S.; Clement, C.R.; Balsanelli, E.; de Baura, V.A.; Souza, E.M.; Fraga, H.P.F.; Vieira, L.N. The Plastome Sequence of Bactris gasipaes and Evolutionary Analysis in Tribe Cocoseae (Arecaceae). PLoS ONE 2021, 16, e0256373. [Google Scholar] [CrossRef]
- Cymerys, M.; Clement, C.R. Bactris gasipaes Kunth. In Frutíferas e Plantas Úteis na Vida Amazônica; Imazon: Belém, Brazil, 2005; pp. 203–208. [Google Scholar]
- Silva, V.L.; Môro, F.V.; Damião Filho, C.F.; Môro, J.R.; Silva, B.M.d.S.e.; Charlo, H.C.d.O. Morfologia e Avaliação do Crescimento Inicial de Plântulas de Bactris gasipaes Kunth. (Arecaceae) em Diferentes Substratos. Rev. Bras. Frutic. 2006, 28, 477–480. [Google Scholar] [CrossRef]
- Dos Santos, O.V.; Soares, S.D.; Dias, P.C.S.; Nascimento, F.C.A.; Conceição, L.R.V.; da Costa, R.S.; Pena, R.S. White Peach Palm (Pupunha) a New Bactris gasipaes Kunth. Variety from the Amazon: Nutritional Composition, Bioactive Lipid Profile, Thermogravimetric and Morphological Characteristics. J. Food Compos. Anal. 2022, 112, 104684. [Google Scholar] [CrossRef]
- Nazário, P.; Ferreira, S.A.d.N.; Borges, E.E.d.L.; Genovese-Marcomini, P.R.; Mendonça, M.S.d. Anatomical and Histochemical Aspects of the Peach Palm (Bactris gasipaes Kunth) Seed. J. Seed Sci. 2013, 35, 171–178. [Google Scholar] [CrossRef]
- Ferreira, S.A.d.N.; dos Santos, L.A. Viabilidade de Sementes de Pupunha (Bactris gasipaes Kunth.). Acta Amaz. 1992, 22, 303–307. [Google Scholar] [CrossRef]
- Nazário, P.; Ferreira, S.A.d.N.; Borges, E.E.d.L. Embryonic Dormancy in Seeds of Bactris gasipaes Kunth (Peach-Palm). J. Seed Sci. 2017, 39, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Edelman, S.M.; Richards, J.H. Review of Vegetative Branching in the Palms (Arecaceae). Bot. Rev. 2019, 85, 40–77. [Google Scholar] [CrossRef]
- De Carvalho, C.J.R.; Ishida, F.Y. Respostas de Pupunheiras (Bactris gasipaes Kunth) Jovens ao Alagamento. Pesqui. Agropecuária Bras. 2002, 37, 1231–1238. [Google Scholar] [CrossRef]
- Ferreira, S.A.d.Ν.; Clement, C.R.; Ranzani, G.; Costa, S.d.S. Contribuição ao Conhecimento do Sistema Radicular da Pupunheira (Bactris gasipaes Kunth, Palmae). II. Solo Latossolo Amarelo, Textura Argilosa. Acta Amaz. 1995, 25, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Armani, M.; Charles-Dominique, T.; Barton, K.E.; Tomlinson, K.W. Developmental Constraints and Resource Environment Shape Early Emergence and Investment in Spines in Saplings. Ann. Bot. 2019, 124, 1133–1142. [Google Scholar] [CrossRef]
- Clement, C.R.; Manshardt, R.M. A Review of the Importance of Spines for Pejibaye Heart-of-Palm Production. Sci. Hortic. 2000, 83, 11–23. [Google Scholar] [CrossRef]
- Rickson, F.R.; Cresti, M.; Beach, J.H. Plant Cells Which Aid in Pollen Digestion within a Beetle’s Gut. Oecologia 1990, 82, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.A.; Soliman, E.P.; Pavarini, R.; Zorzenon, F.J.; Nomura, E.S.; Rodrigues, D.S. A Survey of the Entomofauna Associated with the Inflorescences of Pejibaye (Arecaceae: Bactris gasipaes Kunth) in the Ribeira Valley, SP, Brazil. Arq. Inst. Biológico 2013, 80, 111–115. [Google Scholar] [CrossRef]
- Hurtado, F.H.M.; Mosquera-Espinosa, A.T.; Gómez-Carabalí, A.; Otero, J.T. Temporal Variation in Arbuscular Mycorrhizal Fungi Colonization of Bactris gasipaes Kunth in Buenaventura, Colombia. Acta Agron. 2013, 62, 344–351. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Clement, C.R.; Habte, M. Genotypic Variation in Vesicular-arbuscular Mycorrhizal Dependence of the Pejibaye Palm. J. Plant Nutr. 1995, 18, 1907–1916. [Google Scholar] [CrossRef]
- Chalita, P.B.; Farias, E.d.N.C.; da Costa, I.B.; Sousa, B.F.; dos Santos, M.A.O.; de Albuquerque, T.C.S.; Vital, M.J.S.; da Silva, K. Characterization of Bacterial Endophytes from the Roots of Native and Cultivated Brazil Nut Trees (Bertholletia excelsa). Acta Amaz. 2019, 49, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef] [Green Version]
- Rana, K.L.; Kour, D.; Yadav, A.N.; Yadav, N.; Saxena, A.K. Agriculturally Important Microbial Biofilms: Biodiversity, Ecological Significances, and Biotechnological Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Yadav, M.K., Singh, B.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–265. [Google Scholar] [CrossRef]
- Belniaki, A.C.; Panobianco, M. Sementes de Pupunha: Da Colheita Ao Armazenamento; Comunicado Técnico; EMBRAPA: Colombo, Brasil, 2020; p. 11. [Google Scholar]
- Bovi, M.L.A. Expansão do Cultivo da Pupunheira Para Palmito no Brasil; Palestra. Suplemento; Horticultura Brasileira: Brasília, Brazil, 1997; Volume 15, pp. 183–185. [Google Scholar]
- Ellis, R.H. Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regul. 1992, 11, 249–255. [Google Scholar] [CrossRef]
- Modolo, V.A. Palmito (Pupunha). In Instruções Agrícolas para as Principais Culturas Econômicas; Instituto Agronômico de Campinas: Campinas, Brasil, 2014; pp. 329–333. [Google Scholar]
- Clement, C.R.; UrpÍ, J.E.M. Pejibaye Palm (Bactris gasipaes, Arecaceae): Multi-Use Potential for the Lowland Humid Tropics. Econ. Bot. 1987, 41, 302–311. [Google Scholar] [CrossRef]
- Graefe, S.; Dufour, D.; van Zonneveld, M.; Rodriguez, F.; Gonzalez, A. Peach Palm (Bactris gasipaes) in Tropical Latin America: Implications for Biodiversity Conservation, Natural Resource Management and Human Nutrition. Biodivers. Conserv. 2013, 22, 269–300. [Google Scholar] [CrossRef] [Green Version]
- Flores, W.B.; Yuyama, K.; da Silva, R.G. Asexual Propagation of Peach Palm by Division of the Clump and Extraction of the Off-Shoots. Hortic. Bras. 2012, 30, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Vieira, T.A.; Rosa, L.S.; Vasconcelos, P.C.S.; dos Santos, M.M.; Modesto, R.d.S. Sistemas Agroflorestais em Áreas de Agricultores Familiares em Igarapé-Açu, Pará: Caracterização Florística, Implantação e Manejo. Acta Amaz. 2007, 37, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Moraes, L.A.C.; Fageria, N.K. Nutritional Limitations in Multi-Strata Agroforestry System with Native Amazonian Plants. J. Plant Nutr. 2012, 35, 1791–1805. [Google Scholar] [CrossRef]
- Yuyama, K.; Silva, F.M.S. Desenvolvimento Inicial da Pupunheira em Monocultivo e Intercalado com Culturas Anuais. Hortic. Bras. 2003, 21, 15–19. [Google Scholar] [CrossRef]
- De Souza, G.S.; Alves, D.I.; Dan, M.L.; Lima, J.S.d.S.; da Fonseca, A.L.C.C.; Araújo, J.B.S.; Guimarães, L.A.d.O.P. Soil Physico-Hydraulic Properties Under Organic Conilon Coffee Intercropped with Tree and Fruit Species. Pesqui. Agropecuária Bras. 2017, 52, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Alegre, J.; Lao, C.; Silva, C.; Schrevens, E. Recovering Degraded Lands in the Peruvian Amazon by Cover Crops and Sustainable Agroforestry Systems. Peruvian J. Agron. 2017, 1, 7. [Google Scholar] [CrossRef]
- Tucci, M.L.S.; Erismann, N.M.; Machado, E.C.; Ribeiro, R.V. Diurnal and Seasonal Variation in Photosynthesis of Peach Palms Grown under Subtropical Conditions. Photosynthetica 2010, 48, 421–429. [Google Scholar] [CrossRef]
- Tucci, M.L.S.; Machado, E.C.; Modolo, V.A.; de Magalhães Erismann, N. Photosynthesis and Water Relations of Peach Palms (Bactris gasipaes Kunth) under Soil Water Deficit. Theor. Exp. Plant Physiol. 2018, 30, 29–39. [Google Scholar] [CrossRef]
- Da Silva, J.R.A.; Falcão, N.P.d.S. Caracterização de Sintomas de Carências Nutricionais em Mudas de Pupunheira Cultivadas Em Solução Nutritiva. Acta Amaz. 2002, 32, 529. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.R.; de Matos, G.S.B.; de Carvalho, J.G. Deficiências Nutricionais de Macronutrientes e Sódio em Mudas de Pupunheira. Rev. Bras. Frutic. 2013, 35, 1178–1189. [Google Scholar] [CrossRef] [Green Version]
- Bovi, M.L.A.; Tucci, M.L.S.; Spiering, S.H.; Godoy, G., Jr.; Lambais, M.R. Biomass Accumulation and Arbuscular Mycorrhizal Colonization in Pejibaye (Bactris gasipaes Kunth) as a Function of NPK Fertilization. Acta Hortic. 1998, 513, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.R.; de Carvalho, J.G.; Curi, N.; Pinto, J.E.B.P.; Guimarães, P.d.T.G. Nutrição Mineral de Mudas de Pupunheira Sob Diferentes Níveis de Salinidade. Pesqui. Agropecuária Bras. 2002, 37, 1613–1619. [Google Scholar] [CrossRef]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; FAO Soils Bulletin; Food and Agriculture Organization of The United Nations: Rome, Italy, 1988; pp. 153–168. [Google Scholar]
- Gondim, M.G.C., Jr.; de Moraes, G.J. Life Cycle of Retracrus johnstoni Keifer (Acari: Phytoptidae). Neotrop. Entomol. 2003, 32, 197–201. [Google Scholar] [CrossRef]
- Vásquez-Ordóñez, A.A.; Löhr, B.L.; Marvaldi, A.E. Comparative Morphology of the Larvae of the Palm Weevils Dynamis borassi (Fabricius) and Rhynchophorus palmarum (Linnaeus) (Curculionidae: Dryophthorinae): Two Major Pests of Peach Palms in the Neotropics. Papéis Avulsos Zool. 2020, 60, 14. [Google Scholar] [CrossRef]
- Cuellar-Palacios, C.M.; Gaviria-Vega, J.; Montoya-Lerma, J. Life Cycle and Larval Growth of Dynamis borassi (Coleoptera: Dryophthoridae), an Emerging Pest to the Peach Palm. Ann. Agric. Sci. 2020, 65, 218–224. [Google Scholar] [CrossRef]
- Thomazini, M.J. Ocorrência de Herminodes sp. (Lepidoptera: Noctuidae) em Pupunheira nos Estados do Acre e Rondônia, Brasil. Acta Amaz. 2004, 34, 505–506. [Google Scholar] [CrossRef] [Green Version]
- Couturier, G. Conocimiento y Manejo de Los Insectos y Plagas de Los Frutales de La Amazonia. Folia Amaz. 2006, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Gaviria, J.; Montoya-Lerma, J.; Armbrecht, I.; Löhr, B.; Vásquez-Ordóñez, A.A. Dynamis Borassi (Coleoptera: Curculionidae), a New Potential Pest to the Palms (Arecaceae): An Early Warning for the Palm Producers. Fla. Entomol. 2021, 104, 107–116. [Google Scholar] [CrossRef]
- Pava, J.; Gonzáles, A.; Patiño, H. Aspectos de Interés Fitosanitario de La Palma de Chontaduro Bactris gasipaes H.B.K. En Algunas Regiones Del Valle Y Choco. Acta Agron. 1983, 33, 25–35. [Google Scholar]
- Santos, Á.F.; Tessmann, D.J.; Nunes, W.M.C.; Vida, J.B.; Jaccoud Filho, D.S. Doenças Foliares da Pupunheira (Bactris gasipaes) No Estado Do Pará. Bol. Pesqui. Florest. 2001, 42, 125–129. [Google Scholar]
- Mafacioli, R.; Santos, A.F.; Tessmann, D.J.; Vida, J.B. Etiologia e Manejo das Doenças da Pupunheira no Brasil. Pesq. Flor. Bras. 2009, 58, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Oquendo, C.; Bogantes-Arias, A.; Mora-Urpí, J. La Deshoja En El Manejo de La Bacteriosis del Palmito de Pejibaye (Bactris gasipaes). Agron. Mesoam. 2005, 18, 129. [Google Scholar] [CrossRef] [Green Version]
- Mora-Urpi, J.; Arroyo-Oquendo, C.; Mexzón-Vargas, R.; Bogantes-Arias, A. Diseminación de La “Bacteriosis del Palmito” de Pejibaye (Bactris gasipaes Kunth). Agron. Mesoam. 2007, 19, 155. [Google Scholar] [CrossRef] [Green Version]
- Lopes, H.V.; dos Santos, Á.F.; Luz, E.D.M.N.; Tessmann, D.J. Phytophthora Palmivora: Agente Causal da Podridão da Base do Estipe da Pupunheira no Brasil. Summa Phytopathol. 2019, 45, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Patiño, V.M. Plantas Cultivadas y Animales Domésticos En América Equinoccial I: Frutales; Imprenta Departamental: Cali, Colombia, 1963; p. 379. [Google Scholar]
- González-Jaramillo, N.; Bailon-Moscoso, N.; Duarte-Casar, R.; Romero-Benavides, J.C. Peach Palm (Bactris gasipaes Kunth.): Ancestral Tropical Staple with Future Potential. Plants 2022, 11, 3134. [Google Scholar] [CrossRef]
- Stevanato, N.; Ribeiro, T.H.; Giombelli, C.; Cardoso, T.; Wojeicchowski, J.P.; Danesi, E.D.G.; Bolanho Barros, B.C. Effect of Canning on the Antioxidant Activity, Fiber Content, and Mechanical Properties of Different Parts of Peach Palm Heart. J. Food Process. Preserv. 2020, 44, e14554. [Google Scholar] [CrossRef]
- De Cássia Spacki, K.d.C.; Corrêa, R.C.G.; Uber, T.M.; Barros, L.; Ferreira, I.C.F.R.; Peralta, R.A.; de Fátima Peralta Muniz Moreira, R.; Helm, C.V.; de Lima, E.A.; Bracht, A.; et al. Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives. Plants 2022, 11, 3175. [Google Scholar] [CrossRef]
- World Bank. World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2021. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2021/tradeflow/Exports/partner/WLD/product/200891# (accessed on 25 December 2022).
- World Bank. World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2006. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2006/tradeflow/Exports/partner/WLD/product/200891 (accessed on 25 December 2022).
- World Bank. World Trade Organization Palm Hearts; Prepared or Preserved, Whether or Not Containing Added Sugar, Other Sweetening Matter or Spirit Exports by Country in 2017. Available online: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2017/tradeflow/Exports/partner/WLD/product/200891 (accessed on 25 December 2022).
- Hernández, I.; Cely, N.; González, F.; Muñoz, E.; Prieto, I. The Discovery of New Export Products in Ecuador. SSRN Electron. J. 2010, 47, 122. [Google Scholar] [CrossRef] [Green Version]
- Santos, Á.F.; Corrêa Júnior, C.; Neves, E.J.M. (Eds.) Palmeiras para Produção de Palmito: Juçara, Pupunheira e Palmeira Real; Embrapa Florestas: Colombo, Brasil, 2008; p. 190. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Tabela 816—Produção, Venda e Valores Da Produção e Da Venda Na Extração Vegetal Por Produtos Da Extração Vegetal, Condição Do Produtor Em Relação Às Terras, Destino Da Produção, Grupos de Atividade Econômica e Grupos de Área Total. Available online: https://sidra.ibge.gov.br/tabela/816#/n1/all/n2/all/n3/all/v/1981,1983/p/all/c229/111573,111575/c218/0/c12763/0/c12764/0/c12517/113601/c220/0/d/v1981%200,v1983%200/l/v,p+c229+c218+c12763,t+c12764+c12517+c220/resultado (accessed on 26 December 2022).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Tabela 1689—Produção, Valor da Produção, Venda, Valor da Venda, Colheita, Área Plantada e Efetivos das Plantações da Lavoura Permanente nos Estabelecimentos Agropecuários com Mais de 50 Pés Existentes por Produtos da Lavoura Permanente, Grupos de Atividade Econômica e Grupos de Área Total. Available online: https://sidra.ibge.gov.br/tabela/1689#/n1/all/n2/all/n3/all/v/2382,2390/p/all/c227/4976,111653/c12517/0/c220/0/d/v2382%200,v2390%200/l/v,p+c227+c12517,t+c220/resultado (accessed on 26 December 2022).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Tabela 6950—Número de Estabelecimentos Agropecuários com Produtos da Extração Vegetal, Quantidade Produzida na Extração Vegetal, Quantidade Vendida de Produtos da Extração Vegetal, Valor da Produção na Extração Vegetal e Valor da Venda de Produtos da Extração Vegetal, por Tipologia, Produtos da Extração Vegetal e Grupos de Área Total. Available online: https://sidra.ibge.gov.br/tabela/6950#/n1/all/n2/all/n3/all/v/144,10073/p/all/c829/46302/c229/111573,111575/c220/110085/d/v144%200,v10073%200/l/v,p+c829+c229,t+c220/resultado (accessed on 26 December 2022).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Tabela 6956—Produção, Valor da Produção, Venda, Valor da Venda, Colheita, Área Plantada e Efetivos das Plantações da Lavoura Permanente nos Estabelecimentos Agropecuários, por Tipologia, Produtos Da Lavoura Permanente e Grupos de Área Total. Available online: https://sidra.ibge.gov.br/tabela/6956#/n1/all/n2/all/n3/all/v/9506,10077/p/all/c829/46302/c227/4976,111653/c220/110085/d/v9506%200,v10077%200/l/v,p+c829+c227,t+c220/resultado (accessed on 26 December 2022).
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of Tucumã (Astrocaryum Vulgare) and Peach Palm (Bactris gasipaes) Are by-Products Classified as Very High Carotenoid Sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Radice, M.; Viafara, D.; Neill, D.; Asanza, M.; Sacchetti, G.; Guerrini, A.; Maietti, S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth Seed Oils. J. Oleo Sci. 2014, 63, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkcoll, D.B.; Aguiar, J.P.L. Peach Palm (Bactris gasipaes H.B.K.), a New Source of Vegetable Oil from the Wet Tropics. J. Sci. Food Agric. 1984, 35, 520–526. [Google Scholar] [CrossRef]
- Yuyama, L.K.O.; Aguiar, J.P.L.; Yuyama, K.; Clement, C.R.; Macedo, S.H.M.; Fávaro, D.I.T.; Afonso, C.; Vasconcellos, M.B.A.; Pimentel, S.A.; Badolato, E.S.G.; et al. Chemical Composition of the Fruit Mesocarp of Three Peach Palm (Bactris gasipaes) Populations Grown in Central Amazonia, Brazil. Int. J. Food Sci. Nutr. 2003, 54, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Alves, B.S.F.; Pereira Junior, J.B.; Carvalho, F.I.M.; Dantas Filho, H.A.; Fernandes Dantas, K.G. Mineral Composition of Amazonian Fruits by Flame Atomic Absorption Spectrometry Using Multivariate Analysis. Biol. Trace Elem. Res. 2019, 189, 259–266. [Google Scholar] [CrossRef]
- Johannessen, C.L. Pejibaye Palm: Physical and Chemical Analysis of the Fruit. Econ. Bot. 1967, 21, 371–378. [Google Scholar] [CrossRef]
- Yuyama, L.K.O.; Aguiar, J.P.L.; Macedo, S.H.M.; Gioia, T.; Yuyama, K.; Fávaro, D.I.T.; Afonso, C.; Vasconcellos, M.B.A.; Cozzolino, S.M.F. Determinação dos Teores de Elementos Minerais em Alimentos Convencionais e Não Convencionais da Região Amazônica pela Técnica de Análise por Ativação com Nêutrons Instrumental. Acta Amaz. 1997, 27, 183–195. [Google Scholar] [CrossRef]
- Panchal, S.K.; Wanyonyi, S.; Brown, L. Selenium, Vanadium, and Chromium as Micronutrients to Improve Metabolic Syndrome. Curr. Hypertens. Rep. 2017, 19, 10. [Google Scholar] [CrossRef]
- Aguiar, J.P.L. Tabela de Composição de Alimentos Da Amazônia. Acta Amaz. 1996, 26, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.F.G.; Marmesat, S.; Brito, E.S.; Alves, R.E.; Dobarganes, M.C. Major Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites 2013, 64, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Schiassi, M.C.E.V.; de Souza, V.R.; Lago, A.M.T.; Campos, L.G.; Queiroz, F. Fruits from the Brazilian Cerrado Region: Physico-Chemical Characterization, Bioactive Compounds, Antioxidant Activities, and Sensory Evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Soriano Sancho, R.A.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian Wild Fruits: Nutrients, Bioactive Compounds, Health-Promotion Properties and Commercial Interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Darnet, S.H.; da Silva, L.H.M.; Rodrigues, A.M.d.C.; Lins, R.T. Nutritional Composition, Fatty Acid and Tocopherol Contents of Buriti (Mauritia flexuosa) and Patawa (Oenocarpus bataua) Fruit Pulp from the Amazon Region. Ciênc. E Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Vásquez-Ocmín, P.G.; Freitas Alvarado, L.; Sotero Solís, V.; Paván Torres, R.; Mancini-Filho, J. Chemical Characterization and Oxidative Stability of the Oils from Three Morphotypes of Mauritia flexuosa L.f., from the Peruvian Amazon. Grasas Aceites 2010, 61, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Zumbado, M.E.; Murillo, M.G. Composition and Nutritive Value of Pejibaye (Bactris gasipaes) in Animal Feeds. Rev. Biol. Trop. 1984, 32, 51–56. [Google Scholar]
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Rome, Italy, 1985. [Google Scholar]
- Cantu-Jungles, T.M.; Cipriani, T.R.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. A Pectic Polysaccharide from Peach Palm Fruits (Bactris gasipaes) and Its Fermentation Profile by the Human Gut Microbiota In Vitro. Bioact. Carbohydr. Diet. Fibre 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Ferrari Felisberto, M.H.; Costa, M.S.; Villas Boas, F.; Leivas, C.L.; Franco, C.M.L.; Souza, S.M.; Clerici, M.T.P.S.; Cordeiro, L.M.C. Characterization and Technological Properties of Peach Palm (Bactris gasipaes var. gasipaes) Fruit Starch. Food Res. Int. 2020, 136, 109569. [Google Scholar] [CrossRef]
- Zárate, R.; Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, By HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Dos Santos, M.; Mamede, R.; Rufino, M.; de Brito, E.; Alves, R. Amazonian Native Palm Fruits as Sources of Antioxidant Bioactive Compounds. Antioxidants 2015, 4, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.F.G.; Alves, R.E.; Ruíz-Méndez, M.V. Minor Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites 2013, 64, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.d.C.; Darnet, S.; Silva, L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia flexuosa), Patawa (Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis) and Inaja (Maximiliana maripa) Fruits. J. Braz. Chem. Soc. 2010, 21, 2000–2004. [Google Scholar] [CrossRef]
- Faria, J.V.; Valido, I.H.; Paz, W.H.P.; da Silva, F.M.A.; de Souza, A.D.L.; Acho, L.R.D.; Lima, E.S.; Boleti, A.P.A.; Marinho, J.V.N.; Salvador, M.J.; et al. Comparative Evaluation of Chemical Composition and Biological Activities of Tropical Fruits Consumed in Manaus, Central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836. [Google Scholar] [CrossRef] [PubMed]
- Bataglion, G.A.; da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Simultaneous Quantification of Phenolic Compounds in Buriti Fruit (Mauritia flexuosa L.f.) by Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Res. Int. 2014, 66, 396–400. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Chisté, R.C.; Costa, E.L.N.; Monteiro, S.F.; Mercadante, A.Z. Carotenoid and Phenolic Compound Profiles of Cooked Pulps of Orange and Yellow Peach Palm Fruits (Bactris gasipaes) from the Brazilian Amazonia. J. Food Compos. Anal. 2021, 99, 103873. [Google Scholar] [CrossRef]
- Santos, M.F.G.; Alves, R.E.; Roca, M. Carotenoid Composition in Oils Obtained from Palm Fruits from the Brazilian Amazon. Grasas Aceites 2015, 66, e086. [Google Scholar] [CrossRef] [Green Version]
- Yuyama, L.K.O.; Yonekura, L.; Aguiar, J.P.L.; Sousa, R.F.S. Biodisponibilidade de Vitamina A da Pupunha (Bactris gasipaes Kunth) Em Ratos. Acta Amaz. 1999, 29, 497. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Bacellar, R.S.; d’Almeida, J.R.M. Microstructural Characterization and Evaluation of Thermal, Mechanical and Wear Properties of Pupunha (Bactris gasipaes) Pseudostem. Polym. Renew. Resour. 2010, 1, 123–142. [Google Scholar] [CrossRef]
- Pinho Pinheiro, A.P.; Moraes d’Almeida, J.R. Peach Palm: Pseudo-Wood for Sustainable Jewelry Design. Mater. Today Proc. 2020, 33, 1869–1873. [Google Scholar] [CrossRef]
- Alhijazi, M.; Zeeshan, Q.; Safaei, B.; Asmael, M.; Qin, Z. Recent Developments in Palm Fibers Composites: A Review. J. Polym. Environ. 2020, 28, 3029–3054. [Google Scholar] [CrossRef]
- Quinaya, D.C.P.; da Silva, E.S.; d’Almeida, J.R.M. On the Use of Residues from the Sustainable Extraction of Heart of Palm in Agglomerated Panels. J. Nat. Fibers 2016, 13, 172–177. [Google Scholar] [CrossRef]
- Franco, T.S.; Potulski, D.C.; Viana, L.C.; Forville, E.; de Andrade, A.S.; de Muniz, G.I.B. Nanocellulose Obtained from Residues of Peach Palm Extraction (Bactris gasipaes). Carbohydr. Polym. 2019, 218, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; de Muñiz, G.I.B.; Masson, M.L. Application of Cellulose Nanofibrils Isolated from an Agroindustrial Residue of Peach Palm in Cassava Starch Films. Food Biophys. 2020, 15, 323–334. [Google Scholar] [CrossRef]
- Chicatto, J.A.; Rainert, K.T.; Gonçalves, M.J.; Helm, C.V.; Altmajer-Vaz, D.; Tavares, L.B.B. Decolorization of Textile Industry Wastewater in Solid State Fermentation with Peach-Palm (Bactris gasipaes) Residue. Braz. J. Biol. 2018, 78, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, E.A.; Nunes, L.V.; Goes, L.M.d.S.; Da Silva, E.G.P.; Franco, M.; Gross, E.; Uetanabaro, A.P.T.; da Costa, A.M. Peach-Palm (Bactris gasipaes Kunth.) Waste as Substrate for Xylanase Production by Trichoderma stromaticum AM7. Chem. Eng. Commun. 2018, 205, 975–985. [Google Scholar] [CrossRef]
- Vieira, T.F.; Corrêa, R.C.G.; de Fatima Peralta Muniz Moreira, R.; Peralta, R.A.; de Lima, E.A.; Helm, C.V.; Garcia, J.A.A.; Bracht, A.; Peralta, R.M. Valorization of Peach Palm (Bactris gasipaes Kunth) Waste: Production of Antioxidant Xylooligosaccharides. Waste Biomass Valorization 2021, 12, 6727–6740. [Google Scholar] [CrossRef]
- Clement, C.R.; Bovi, M.L.A. Melhoramento Genético da Pupunheira: Conhecimentos Atuais e Necessidades; Embrapa Rondônia: Porto Velho, Brazil, 1999; pp. 57–70. [Google Scholar]
- Clement, C.R. Pupunha: Recursos genéticos para a produção de palmito. Hort. Bras. 1997, 15, 186–191. [Google Scholar]
- Scheldeman, X.; Kanashiro, M.; Porro, R.; Dantas Medeiros, R. Amazon Initiative Workshop on Conservation and Use of Amazonian Fruits; IPGRI, EMBRAPA and the Amazon initiative: Boa Vista, Brasil, 2006. [Google Scholar]
- Engels, J.M.M.; Ebert, A.W. A Critical Review of the Current Global Ex Situ Conservation System for Plant Agrobiodiversity. I. History of the Development of the Global System in the Context of the Political/Legal Framework and Its Major Conservation Components. Plants 2021, 10, 1557. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.R.; Dulloo, E.; Sardos, J.; Thormann, I.; Burdon, J.J. In Situ Conservation-Harnessing Natural and Human-Derived Evolutionary Forces to Ensure Future Crop Adaptation. Evol. Appl. 2017, 10, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant Tissue Culture Media and Practices: An Overview. In Vitro Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant Stem Cells and De Novo Organogenesis. New Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, M.; Graner, É.M.; Brondani, G.E.; Artioli, A.; Almeida, L.V.; Leone, G.F.; Baccarin, F.J.B.; de Oliveira Antonelli, P.; Cordeiro, G.M.; Oberschelp, G.P.J.; et al. Plant Morphogenesis: Theorical Bases. Adv. For. Sci. 2015, 2, 13–22. [Google Scholar]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, O.; Huete, F. Propagacion Vegetativa In Vitro del Pejibaye (Bactris gasipaes H.B.K.). Turrialba 1983, 33, 103–108. [Google Scholar]
- Stein, M.; Stephens, C. Effect of 2,4-Dichlorophenoxyacetic Acid and Activated-Charcoal on Somatic Embryogenesis of Bactris gasipaes H.B.K. Turrialba 1991, 41, 196–201. [Google Scholar]
- Valverde, R.; Arias, O.; Thorpe, T.A. Picloram-Induced Somatic Embryogenesis in Pejibaye Palm (Bactris gasipaes H.B.K.). Plant Cell Tissue Organ Cult. 1987, 10, 149–156. [Google Scholar] [CrossRef]
- De Almeida, M.; Kerbauy, G.B. Micropropagation of Bactris gasipaes (Palmae) through Flower Bud Culture. Rev. Bras. Fisiol. Veg. 1996, 8, 215–217. [Google Scholar]
- Steinmacher, D.A.; Clement, C.R.; Guerra, M.P. Somatic Embryogenesis from Immature Peach Palm Inflorescence Explants: Towards Development of an Efficient Protocol. Plant Cell Tissue Organ Cult. 2007, 89, 15–22. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Cangahuala-Inocente, G.C.; Clement, C.R.; Guerra, M.P. Somatic Embryogenesis from Peach Palm Zygotic Embryos. Vitro Cell. Dev. Biol. Plant 2007, 43, 124–132. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Krohn, N.G.; Dantas, A.C.M.; Stefenon, V.M.; Clement, C.R.; Guerra, M.P. Somatic Embryogenesis in Peach Palm Using the Thin Cell Layer Technique: Induction, Morpho-Histological Aspects and AFLP Analysis of Somaclonal Variation. Ann. Bot. 2007, 100, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran Thanh Van, K.; Richard, L.; Gendy, C.A. An Experimental Model for the Analysis of Plant/Cell Differentiation: Thin Cell Layer Concept, Strategy, Methods, Records and Potential. In Plant Aging; Rodríguez, R., Tamés, R.S., Durzan, D.J., Eds.; Springer: Boston, MA, USA, 1990; pp. 215–224. ISBN 978-1-4684-5762-9. [Google Scholar]
- Heringer, A.S.; Steinmacher, D.A.; Fraga, H.P.F.; Vieira, L.N.; Montagna, T.; Quinga, L.A.P.; Quoirin, M.G.G.; Jiménez, V.M.; Guerra, M.P. Improved High-Efficiency Protocol for Somatic Embryogenesis in Peach Palm (Bactris gasipaes Kunth) Using RITA® Temporary Immersion System. Sci. Hortic. 2014, 179, 284–292. [Google Scholar] [CrossRef]
- Padilha, J.H.D.; Steinmacher, D.; Quoirin, M. Peach Palm Plantlet Growth in Different Culture Media in a Temporary Immersion System. Ciênc. Rural 2021, 51, e20190075. [Google Scholar] [CrossRef]
- Campos-Boza, S.; Vinas, M.; Solórzano-Cascante, P.; Holst, A.; Steinmacher, D.A.; Guerra, M.P.; Jiménez, V.M. Somatic Embryogenesis and Plant Regeneration from Transverse Thin Cell Layers of Adult Peach Palm (Bactris gasipaes) Lateral Offshoots. Front. Plant Sci. 2022, 13, 995307. [Google Scholar] [CrossRef]
| Bactris gasipaes | Mauritia flexuosa | References | |
|---|---|---|---|
| Moisture (%) | 45–65.1 | 50.5–79.35 | [90,95,96,97] |
| Starch (%) | 27–59.5 | 7.28–36.3 | [89,95,98] |
| Protein (%) | 2.12–14.7 | 1.8–3.7 | [89,92,95,99] |
| Oils (%) | 5.57–27 | 11.20–19.0 | [89,95,96,99] |
| Total fiber (%) | 1.25–6.6 | 7.9–22.8 | [90,92,95,99] |
| Ashes (%) | 0.6–0.9 | 0.6–0.8 | |
| Energy (Kcal 100 g−1) | 351.4 | 189.6–1006 | |
| Minerals (100 g) | |||
| K (mg) | 206.4–289.3 | 183.55–919.6 | [90,93,97,100] |
| Ca (mg) | 10.2–24.7 | 35.4–132 | |
| Mg (mg) | 16.9–17.6 | 14.29–60.2 | |
| Na (mg) | 0.2–12.6 | 134.4 | [90,93,100] |
| Fe (mg) | 0.47–0.74 | 0.69–4 | [90,97,100] |
| Cu (mg) | - | 0.61 | [100] |
| Zn (mg) | 0.26–0.28 | 1.08 | [90,93,100] |
| Mn (mg) | 0.08–0.11 | 8.72 | |
| Cl (µg) | 7.6–30.7 | - | [90] |
| Cr (µg) | 8.2–13.9 | - | |
| Se (µg) | 3.3–11.4 | - | |
| Rb (µg) | 491.4–924.1 | - | |
| Br (µg) | 34.3–189.4 | - | |
| Ba (µg) | 103.9–164.5 | - | |
| Pa (µg) | 56.4–60.9 | - | |
| Ce (µg) | 1.3–2.1 | - | |
| La (ng) | 70.5–521.8 | - | |
| Sb (ng) | 31.0–99.0 | - | |
| Au (ng) | 30.3–57.8 | - | |
| Sc (ng) | 7–10.4 | - | |
| Fatty acids (%) | |||
| Palmitic (16:0) | 24.1–39.6 | 18.9 | [90,99] |
| Palmitoleic (16:1) | 5.2–7.4 | 0.3 | |
| Margaric (17:0) | - | - | |
| Stearic (18:0) | 0.8–1.7 | 1.3 | |
| Oleic (18:1) | 42.8–60.8 | 75.7 | |
| Linoleic (18:2) | 1.2–1.4 | 2.1 | |
| Linolenic (18:3) | 0–1.8 | - | |
| Arachidonic (20:0) | - | 1.7 | |
| Amino acids | |||
| Bactris gasipaes | FAO | ||
| Essential (mg g−1) | |||
| Histidine | 0.09 | 16 | [90,101,102] |
| Isoleucine | 0.16–1.70 | 13 | |
| Leucine | 0.28–3.14 | 19 | |
| Lysine | 0.21–1.67 | 16 | |
| Methionine | 0.08–0.8 | 17 a | |
| Phenylalanine | 0.14–2.04 | 19 b | |
| Threonine | 0.18–2.71 | 9 | |
| Valine | 0.19–2.83 | 13 | |
| Tryptophan | 0.45 | 5 | |
| Non-essential (ug g−1) | |||
| Alanine | 3.51 | - | [90,101] |
| Arginine | 0.29 | - | |
| Aspartate | 4.33 | - | |
| Serine | 2.72 | - | |
| Glutamate | 4.98 | - | |
| Glycine | 0.27–2.87 | - | |
| Tyrosine | 0.14 | - | |
| Proline | 2.57 | - | |
| Terpenoids (μg/g) | Bactris gasipaes | Mauritia flexuosa | References |
|---|---|---|---|
| cis-γ-Carotene 1 | 3.2 | - | [106] |
| cis-γ-Carotene 2 | 2.3 | 2.33 | |
| cis-γ-Carotene 3 | 2.1 | 9.88 | |
| cis-γ-Carotene 4 | 28.3 | - | |
| cis-γ-Carotene 5 | 0.13 | - | |
| cis-δ-Carotene 1 | 5.2 | 5.46 | |
| cis-δ-Carotene 2 | 2.1 | 3.67 | |
| cis-δ-Carotene 3 | 0.86 | 2.42 | |
| cis-β-Zeacarotene 1 | - | - | |
| cis-β-Zeacarotene 2 | - | - | |
| cis-Violaxanthin | - | - | |
| cis-Neoxanthin | - | - | |
| cis-Lutein | - | - | |
| 9-cis-Lycopene | 8.4 | - | |
| 9-cis-β-Carotene | 2.2 | 18.57 | |
| 13-cis-β-Carotene | 4.02 | 59.23 | |
| 15-cis-β-Carotene | 0.08 | 8.87 | |
| all-trans-α-Carotene | 1.8 | 3.23 | |
| all-trans-α-Cryptoxanthin | 0.12 | 1.28 | |
| all-trans-β-Carotene | 55.5 | 372.32 | |
| all-trans-β-Cryptoxanthin | - | - | |
| all-trans-β-Zeacarotene | - | - | |
| all-trans-δ-Carotene | 45.8 | 2.09 | |
| all-trans-γ-Carotene | 35.4 | 14.76 | |
| all-trans-ζ-Carotene | - | 0.08 | |
| all-trans-Neoxanthin | - | - | |
| all-trans-Zeaxanthin | - | - | |
| 5,6-epoxy-β-Carotene | - | 0.41 | |
| 5,6-epoxy-β-Cryptoxanthin | - | 0.1 | |
| 5,8-epoxy-β-carotene | 0.03 | 7.44 | |
| Phytoene | - | 0.34 | |
| Zeaxanthin | - | - | |
| all-trans-Lutein | - | 0.03 | |
| di-cis-α-Carotene | - | 1.25 | |
| Vitamins (100 g) | |||
| Thiamine (μg) | - | - | [92] |
| Riboflavin (μg) | - | - | |
| Niacin (mg) | 0.13 | - | |
| Ascorbic acid (mg) | 0.9–14 | 13 | [92,107] |
| α-Tocopherol (mg) | 11.7 | 110–197 | [108,109] |
| β + γ-Tocopherol (mg) | - | 476 | |
| δ-Tocopherol (mg) | - | 44.1 | |
| Phenolic compounds (μg g−1) | |||
| Apigenin | 0.002 | 102.48 | [110,111] |
| Caffeic acid | - | 895.53 | |
| Chlorogenic acid | 0.02 | 1154.15 | |
| Ferulic acid | 0,16 | 184.66 | |
| Kaempferol | - | 41.54 | |
| Luteolin | - | 1060.9 | |
| Myricetin | 0.02 | 145.11 | |
| Protocatechuic acid | 0.03 | 2175.93 | |
| p-Coumaric acid | 0.01 | 277.74 | |
| Quercetin | - | 83.27 | |
| Quinic acid (mg g−1) | - | 230.71 | |
| (+)-Catechin | - | 961.21 | |
| (−)-Epicatechin | - | 1109.93 | |
| Phytosterols (mg 100 g−1) | |||
| β-Sitosterol | 8.22 | 7.66 | [108] |
| Campesterol | 1.09 | 1.39 | |
| Stigmasterol | 0.42 | 0.81 | |
| Δ5-Avenasterol | 0.27 | 0.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, Y.V.; Clement, C.R.; de Carvalho, J.C.; Fernandes, A.V.; da Silva, C.V.A.; Koolen, H.H.F.; Aguiar, J.P.L.; Nunes-Nesi, A.; Ramos, M.V.; Araújo, W.L.; et al. Understanding the Technical-Scientific Gaps of Underutilized Tropical Species: The Case of Bactris gasipaes Kunth. Plants 2023, 12, 337. https://doi.org/10.3390/plants12020337
Kramer YV, Clement CR, de Carvalho JC, Fernandes AV, da Silva CVA, Koolen HHF, Aguiar JPL, Nunes-Nesi A, Ramos MV, Araújo WL, et al. Understanding the Technical-Scientific Gaps of Underutilized Tropical Species: The Case of Bactris gasipaes Kunth. Plants. 2023; 12(2):337. https://doi.org/10.3390/plants12020337
Chicago/Turabian StyleKramer, Yasmin Verçosa, Charles Roland Clement, Josiane Celerino de Carvalho, Andreia Varmes Fernandes, Carlos Vinicius Azevedo da Silva, Hector Henrique Ferreira Koolen, Jaime Paiva Lopes Aguiar, Adriano Nunes-Nesi, Marcio Viana Ramos, Wagner L. Araújo, and et al. 2023. "Understanding the Technical-Scientific Gaps of Underutilized Tropical Species: The Case of Bactris gasipaes Kunth" Plants 12, no. 2: 337. https://doi.org/10.3390/plants12020337
APA StyleKramer, Y. V., Clement, C. R., de Carvalho, J. C., Fernandes, A. V., da Silva, C. V. A., Koolen, H. H. F., Aguiar, J. P. L., Nunes-Nesi, A., Ramos, M. V., Araújo, W. L., & Gonçalves, J. F. d. C. (2023). Understanding the Technical-Scientific Gaps of Underutilized Tropical Species: The Case of Bactris gasipaes Kunth. Plants, 12(2), 337. https://doi.org/10.3390/plants12020337








