Natural Active Ingredients and TRPV1 Modulation: Focus on Key Chemical Moieties Involved in Ligand–Target Interaction
Abstract
1. Introduction
2. The Structure of TRPV1 and Key Features of Its Interaction with Endogenous Ligands
3. Natural Modulators of TRPV1 Channel
3.1. TRPV1 Agonists
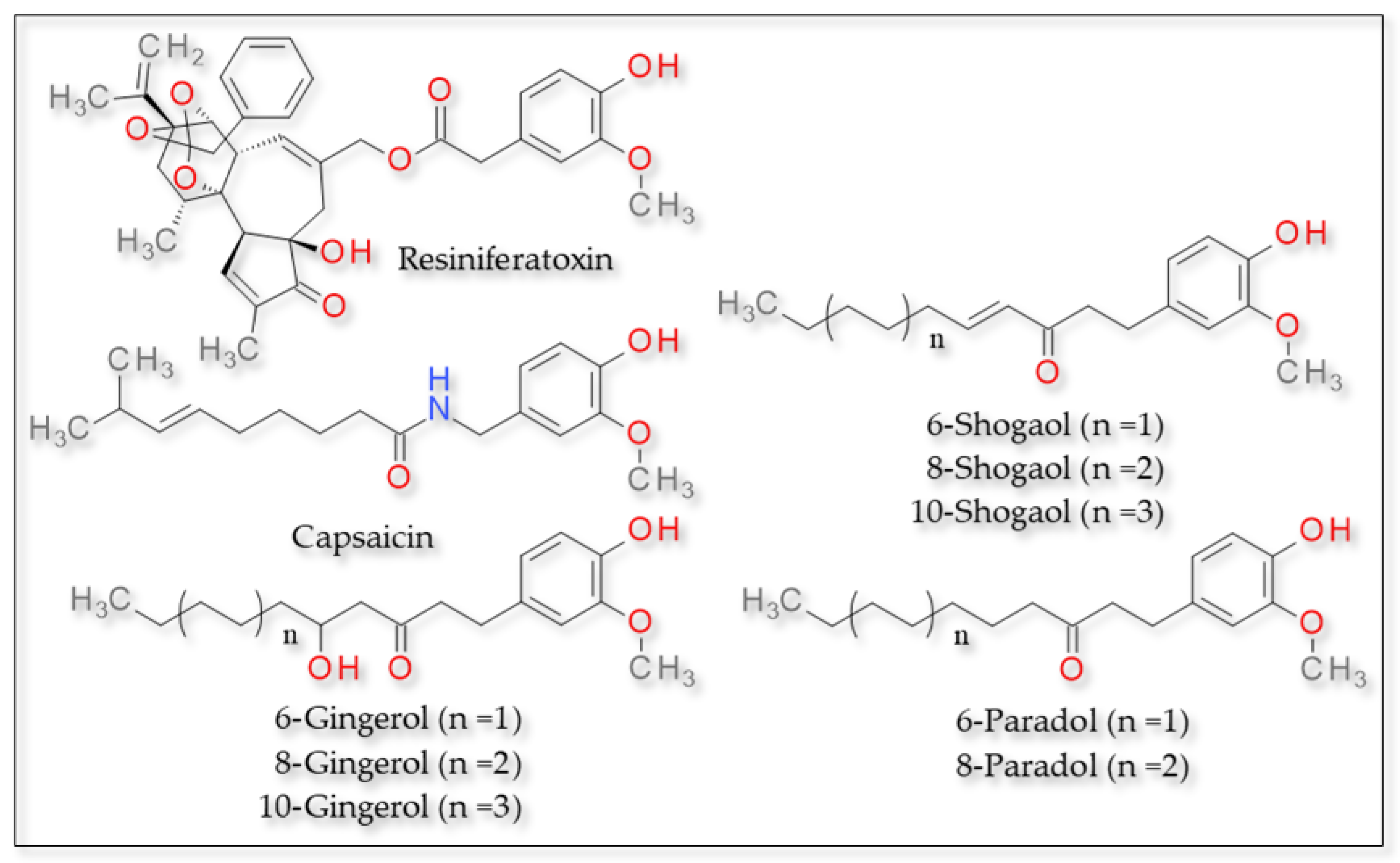
3.1.1. Capsaicin and Related Compounds (Vanilloid Derivatives)
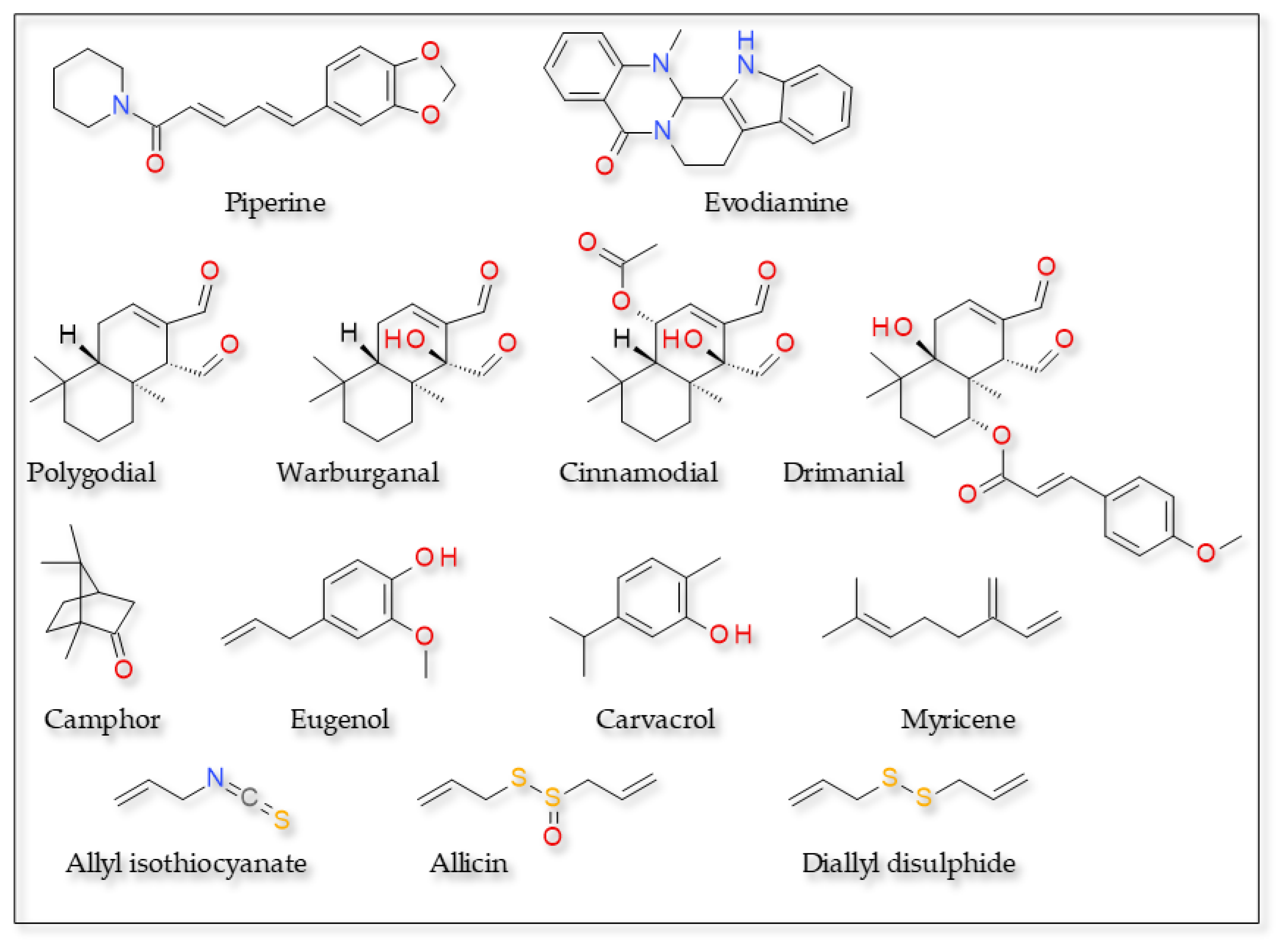
3.1.2. Agonists with Various Structures
Alkaloids
Unsaturated Dialdehyde Terpenes
Substances with Pronounced Electrophilic Character
Monoterpenes
Phytocannabinoids
3.2. Antagonists
3.2.1. Compounds Whose Effect on Tb Is Not Reported
3.2.2. Compounds Increasing Tb
3.2.3. Compounds Not Influencing Tb
4. Discussions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Balunas, M.J.; Kinghorn, A.D. Drug Discovery from Medicinal Plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Maude, R.J.; Woodrow, C.J.; White, L.J. Artemisinin Antimalarials: Preserving the “Magic Bullet”. Drug Dev. Res. 2010, 71, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, M.; García, A.G.; Marco-Contelles, J.; López, M.G. An Update on the Pharmacology of Galantamine. Expert Opin. Investig. Drugs 2007, 16, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What Has Been Done and the Challenges Remain Ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Rahman, M.A.; Faizi, M.S.H.; Khan, M.S. Next Generation Antineoplastic Agents: A Review on Structurally Modified Vinblastine (VBL) Analogues. Curr. Med. Chem. 2018, 25, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Omari, S.A.; Adams, M.J.; Geraghty, D.P. TRPV1 Channels in Immune Cells and Hematological Malignancies. Adv. Pharmacol. 2017, 79, 173–198. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Suri, A.; Szallasi, A. The Emerging Role of TRPV1 in Diabetes and Obesity. Trends Pharmacol. Sci. 2008, 29, 29–36. [Google Scholar] [CrossRef]
- Birder, L.A.; Kanai, A.J.; De Groat, W.C.; Kiss, S.; Nealen, M.L.; Burke, N.E.; Dineley, K.E.; Watkins, S.; Reynolds, I.J.; Caterina, M.J. Vanilloid Receptor Expression Suggests a Sensory Role for Urinary Bladder Epithelial Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13396–13401. [Google Scholar] [CrossRef]
- Tang, L.; Gao, J.; Cao, X.; Chen, L.; Wang, H.; Ding, H. TRPV1 Mediates Itch-Associated Scratching and Skin Barrier Dysfunction in DNFB-Induced Atopic Dermatitis Mice. Exp. Dermatol. 2022, 31, 398–405. [Google Scholar] [CrossRef]
- Yun, J.; Seo, J.A.; Jeong, Y.S.; Bae, I.; Jang, W.; Lee, J.; Kim, S.; Shin, S.; Woo, B.; Lee, K.; et al. TRPV1 Antagonist Can Suppress the Atopic Dermatitis-like Symptoms by Accelerating Skin Barrier Recovery. J. Dermatol. Sci. 2011, 62, 8–15. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.S.; Huang, W.C.; Chang, W.Y.; Krueger, J.G.; Tsai, T.F. Capsaicin Attenuates Imiquimod-Induced Epidermal Hyperplasia and Cutaneous Inflammation in a Murine Model of Psoriasis. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef]
- Bonchak, J.G.; Swerlick, R.A. Emerging Therapies for Atopic Dermatitis: TRPV1 Antagonists. J. Am. Acad. Dermatol. 2018, 78, S63–S66. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.C.; Durand, D.M. TRPV1 Antagonist Capsazepine Suppresses 4-AP-Induced Epileptiform Activity in Vitro and Electrographic Seizures in Vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef]
- Cho, S.J.; Vaca, M.A.; Miranda, C.J.; N’Gouemo, P. Inhibition of Transient Potential Receptor Vanilloid Type 1 Suppresses Seizure Susceptibility in the Genetically Epilepsy-Prone Rat. CNS Neurosci. Ther. 2018, 24, 18–28. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, D.; Pieróg, M.; Wlaź, P. α-Spinasterol, a TRPV1 Receptor Antagonist, Elevates the Seizure Threshold in Three Acute Seizure Tests in Mice. J. Neural Transm. 2015, 122, 1239–1247. [Google Scholar] [CrossRef]
- Shirazi, M.; Izadi, M.; Amin, M.; Rezvani, M.E.; Roohbakhsh, A.; Shamsizadeh, A. Involvement of Central TRPV1 Receptors in Pentylenetetrazole and Amygdala-Induced Kindling in Male Rats. Neurol. Sci. 2014, 35, 1235–1241. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, J.G.; Yon, J.M.; Oh, K.W.; Baek, I.J.; Nahm, S.S.; Lee, B.J.; Yun, Y.W.; Nam, S.Y. Capsaicin Prevents Kainic Acid-Induced Epileptogenesis in Mice. Neurochem. Int. 2011, 58, 634–640. [Google Scholar] [CrossRef]
- Socała, K.; Wlaź, P. Evaluation of the Antidepressant- and Anxiolytic-like Activity of α-Spinasterol, a Plant Derivative with TRPV1 Antagonistic Effects, in Mice. Behav. Brain Res. 2016, 303, 19–25. [Google Scholar] [CrossRef]
- Sartim, A.G.; Brito, B.M.; Gobira, P.H.; Joca, S.R.L. Attenuation of Glutamatergic and Nitrergic System Contributes to the Antidepressant-like Effect Induced by Capsazepine in the Forced Swimming Test. Behav. Pharmacol. 2019, 30, 59–66. [Google Scholar] [CrossRef]
- Navarria, A.; Tamburella, A.; Iannotti, F.A.; Micale, V.; Camillieri, G.; Gozzo, L.; Verde, R.; Imperatore, R.; Leggio, G.M.; Drago, F.; et al. The Dual Blocker of FAAH/TRPV1 N-Arachidonoylserotonin Reverses the Behavioral Despair Induced by Stress in Rats and Modulates the HPA-Axis. Pharmacol. Res. 2014, 87, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.-E. TRP Channels as Lower Urinary Tract Sensory Targets. Med. Sci. 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Charrua, A.; Cruz, C.D.; Narayanan, S.; Gharat, L.; Gullapalli, S.; Cruz, F.; Avelino, A. GRC-6211, a New Oral Specific TRPV1 Antagonist, Decreases Bladder Overactivity and Noxious Bladder Input in Cystitis Animal Models. J. Urol. 2009, 181, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.; Charrua, A.; Cruz, C.D.; Gharat, L.; Avelino, A.; Cruz, F. Rat Detrusor Overactivity Induced by Chronic Spinalization Can Be Abolished by a Transient Receptor Potential Vanilloid 1 (TRPV1) Antagonist. Auton. Neurosci. Basic Clin. 2012, 166, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Wada, M.; Kanehisa, T.; Miyai, A.; Usui, K.; Maekawa, M.; Sakata, M.; Matsuo, A.; Hayashi, M.; Matsushita, M. JTS-653 Blocks Afferent Nerve Firing and Attenuates Bladder Overactivity without Affecting Normal Voiding Function. J. Urol. 2013, 189, 1137–1146. [Google Scholar] [CrossRef]
- Cefalu, J.S.; Guillon, M.A.; Burbach, L.R.; Zhu, Q.M.; Hu, D.Q.; Ho, M.J.; Ford, A.P.D.W.; Nunn, P.A.; Cockayne, D.A. Selective Pharmacological Blockade of the TRPV1 Receptor Suppresses Sensory Reflexes of the Rodent Bladder. J. Urol. 2009, 182, 776–785. [Google Scholar] [CrossRef]
- Abdelhamid, R.E.; Kovács, K.J.; Nunez, M.G.; Larson, A.A. Depressive Behavior in the Forced Swim Test Can Be Induced by TRPV1 Receptor Activity and Is Dependent on NMDA Receptors. Pharmacol. Res. 2014, 79, 21–27. [Google Scholar] [CrossRef]
- Reyes-Mendez, M.E.; Castro-Sánchez, L.A.; Dagnino-Acosta, A.; Aguilar-Martínez, I.; Pérez-Burgos, A.; Vázquez-Jiménez, C.; Moreno-Galindo, E.G.; Álvarez-Cervera, F.J.; Góngora-Alfaro, J.L.; Navarro-Polanco, R.A.; et al. Capsaicin Produces Antidepressant-like Effects in the Forced Swimming Test and Enhances the Response of a Sub-Effective Dose of Amitriptyline in Rats. Physiol. Behav. 2018, 195, 158–166. [Google Scholar] [CrossRef]
- Kasckow, J.W.; Mulchahey, J.J.; Geracioti, T.D. Effects of the Vanilloid Agonist Olvanil and Antagonist Capsazepine on Rat Behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 291–295. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The Vanilloid Receptor TRPV1: 10 Years from Channel Cloning to Antagonist Proof-of-Concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Immke, D.C.; Gavva, N.R. The TRPV1 Receptor and Nociception. Semin. Cell Dev. Biol. 2006, 17, 582–591. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective Blockade of the Capsaicin Receptor TRPV1 Attenuates Bone Cancer Pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef]
- Szallasi, A.; Sheta, M. Targeting TRPV1 for Pain Relief: Limits, Losers and Laurels. Expert Opin. Investig. Drugs 2012, 21, 1351–1369. [Google Scholar] [CrossRef]
- Maher, M.P.; Bhattacharya, A.; Ao, H.; Swanson, N.; Wu, N.T.; Freedman, J.; Kansagara, M.; Scott, B.; Li, D.H.; Eckert, W.A.; et al. Characterization of 2-(2,6-Dichloro-Benzyl)-Thiazolo[5,4-d]Pyrimidin-7-Yl]- (4-Trifluoromethyl-Phenyl)-Amine (JNJ-39729209) as a Novel TRPV1 Antagonist. Eur. J. Pharmacol. 2011, 663, 40–50. [Google Scholar] [CrossRef]
- Honore, P.; Chandran, P.; Hernandez, G.; Gauvin, D.M.; Mikusa, J.P.; Zhong, C.; Joshi, S.K.; Ghilardi, J.R.; Sevcik, M.A.; Fryer, R.M.; et al. Repeated Dosing of ABT-102, a Potent and Selective TRPV1 Antagonist, Enhances TRPV1-Mediated Analgesic Activity in Rodents, but Attenuates Antagonist-Induced Hyperthermia. Pain 2009, 142, 27–35. [Google Scholar] [CrossRef]
- Watabiki, T.; Kiso, T.; Kuramochi, T.; Yonezawa, K.; Tsuji, N.; Kohara, A.; Kakimoto, S.; Aoki, T.; Matsuoka, N. Amelioration of Neuropathic Pain by Novel Transient Receptor Potential Vanilloid 1 Antagonist AS1928370 in Rats without Hyperthermic Effect. J. Pharmacol. Exp. Ther. 2011, 336, 743–750. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Faltynek, C.R.; Jarvis, M.F. Systemic and Site-Specific Effects of A-425619, a Selective TRPV1 Receptor Antagonist, on Wide Dynamic Range Neurons in CFA-Treated and Uninjured Rats. J. Neurophysiol. 2006, 95, 18–25. [Google Scholar] [CrossRef]
- Puttfarcken, P.S.; Han, P.; Joshi, S.K.; Neelands, T.R.; Gauvin, D.M.; Baker, S.J.; Lewis, L.G.R.; Bianchi, B.R.; Mikusa, J.P.; Koenig, J.R.; et al. A-995662 [(R)-8-(4-Methyl-5-(4-(Trifluoromethyl)Phenyl)Oxazol-2-Ylamino)-1, 2,3,4-Tetrahydronaphthalen-2-Ol], a Novel, Selective TRPV1 Receptor Antagonist, Reduces Spinal Release of Glutamate and CGRP in a Rat Knee Joint Pain Model. Pain 2010, 150, 319–326. [Google Scholar] [CrossRef]
- Mills, C.D.; Nguyen, T.; Tanga, F.Y.; Zhong, C.; Gauvin, D.M.; Mikusa, J.; Gomez, E.J.; Salyers, A.K.; Bannon, A.W. Characterization of Nerve Growth Factor-Induced Mechanical and Thermal Hypersensitivity in Rats. Eur. J. Pain 2013, 17, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Pomonis, J.D.; Harrison, J.E.; Mark, L.; Bristol, D.R.; Valenzano, K.J.; Walker, K. N-(4-Tertiarybutylphenyl)-4-(3-Cholorphyridin-2-Yl)Tetrahydropyrazine -1(2H)-Carbox-Amide (BCTC), a Novel, Orally Effective Vanilloid Receptor 1 Antagonist with Analgesic Properties: II. in Vivo Characterization in Rat Models of Inflammatory and Neuropathic Pain. J. Pharmacol. Exp. Ther. 2003, 306, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Saper, J.R.; Klapper, J.; Mathew, N.T.; Rapoport, A.; Phillips, S.B.; Bernstein, J.E. Intranasal Civamide for the Treatment of Episodic Cluster Headaches. Arch. Neurol. 2002, 59, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Bourne, N.; Bernstein, D.I.; Stanberry, L.R. Civamide (Cis-Capsaicin) for Treatment of Primary or Recurrent Experimental Genital Herpes. Antimicrob. Agents Chemother. 1999, 43, 2685–2688. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Jakubowska, A.; Kulig, K. Zucapsaicin for the Treatment of Neuropathic Pain. Expert Opin. Investig. Drugs 2014, 23, 1433–1440. [Google Scholar] [CrossRef]
- SB-705498 Dental Pain Study After Tooth Extraction—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00281684 (accessed on 5 November 2022).
- Study to Investigate the Analgesic Efficacy of a Single Dose of AZD1386—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00672646 (accessed on 5 November 2022).
- Study of SAF312 as an Eye Drop for Treatment of Eye Pain Following Photorefractive Keratectomy (PRK) Surgery—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02961062 (accessed on 5 November 2022).
- The Facing Pain Study—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04010019?term=%28diabetes+OR+diabetic%29+AND+pain+AND+%28+cannabis+OR+cbd+OR+cannabidiol+OR+thc+OR+delta-9-tetrahydrocannabinol+OR+tetrahydrocannabinol+%29&draw=3&rank=15 (accessed on 5 November 2022).
- Use Of SB-705498 In The Acute Treatment Of Migraine—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00269022 (accessed on 5 November 2022).
- A Study to Evaluate the Efficacy and Safety of CC-90001 in Subjects with Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03142191 (accessed on 5 November 2022).
- Sorrento Therapeutics Study to Evaluate Intra-Articular Resiniferatoxin to Treat Moderate to Severe Pain From Knee Osteoarthritis—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04885972 (accessed on 5 November 2022).
- Miller, F.; Björnsson, M.; Svensson, O.; Karlsten, R. Experiences with an Adaptive Design for a Dose-Finding Study in Patients with Osteoarthritis. Contemp. Clin. Trials 2014, 37, 189–199. [Google Scholar] [CrossRef]
- Arsenault, P.; Chiche, D.; Brown, W.; Miller, J.; Treister, R.; Leff, R.; Walker, P.; Katz, N. NEO6860, Modality-Selective TRPV1 Antagonist: A Randomized, Controlled, Proof-of-Concept Trial in Patients with Osteoarthritis Knee Pain. Pain Reports 2018, 3, e696. [Google Scholar] [CrossRef]
- NCT01688947 Analgesic Efficacy and Safety of V116517 in Subjects with Moderate to Severe Chronic Pain Due to Osteoarthritis (OA) of the Knee—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01688934 (accessed on 5 November 2022).
- Ghouri, A.; Conaghan, P.G. Treating Osteoarthritis Pain: Recent Approaches Using Pharmacological Therapies. Clin. Exp. Rheumatol. 2019, 37, 124–129. [Google Scholar]
- Wong, G.Y.; Gavva, N.R. Therapeutic Potential of Vanilloid Receptor TRPV1 Agonists and Antagonists as Analgesics: Recent Advances and Setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef]
- Dumitrache, M.-D.; Jieanu, A.; Scheau, C.; Badarau, I.; Popescu, G.; Caruntu, A.; Costache, D.; Costache, R.; Constantin, C.; Neagu, M.; et al. Comparative Effects of Capsaicin in Chronic Obstructive Pulmonary Disease and Asthma (Review). Exp. Ther. Med. 2021, 22. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Sándor, Z. Multisteric TRPV1 Nocisensor: A Target for Analgesics. Trends Pharmacol. Sci. 2012, 33, 646–655. [Google Scholar] [CrossRef]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia Induced by Transient Receptor Potential Vanilloid-1 (TRPV1) Antagonists in Human Clinical Trials: Insights from Mathematical Modeling and Meta-Analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, X.; Lee, B.H.; Vu, S.; Yang, W.; Yarov-Yarovoy, V.; Zheng, J. The Conformational Wave in Capsaicin Activation of Transient Receptor Potential Vanilloid 1 Ion Channel. Nat. Commun. 2018, 9, 2879. [Google Scholar] [CrossRef]
- Boukalova, S.; Teisinger, J.; Vlachova, V. Protons Stabilize the Closed Conformation of Gain-of-Function Mutants of the TRPV1 Channel. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 520–528. [Google Scholar] [CrossRef]
- Thangavel, N.; Albratty, M. Pharmacophore Model-Aided Virtual Screening Combined with Comparative Molecular Docking and Molecular Dynamics for Identification of Marine Natural Products as SARS-CoV-2 Papain-like Protease Inhibitors. Arab. J. Chem. 2022, 15, 104334. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Nitulescu, G.; Mihai, D.P.; Nitulescu, G.M. Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals 2022, 15, 38. [Google Scholar] [CrossRef]
- Carnevale, V.; Rohacs, T. TRPV1: A Target for Rational Drug Design. Pharmaceuticals 2016, 9, 52. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 Ion Channel Determined by Electron Cryo-Microscopy. Nature 2013, 504, 107. [Google Scholar] [CrossRef]
- Moiseenkova-bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 Channel Revealed by Electron Cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef]
- Bagood, M.D.; Isseroff, R.R. TRPV1: Role in Skin and Skin Diseases and Potential Target for Improving Wound Healing. Int. J. Mol. Sci. 2021, 22, 6135. [Google Scholar] [CrossRef]
- Bevan, S.; Quallo, T.; Andersson, D.A. Trpv1. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, ISBN 9783642542152. [Google Scholar]
- Tominaga, M.; Tominaga, T. Structure and Function of TRPV1. Pflugers Arch. Eur. J. Physiol. 2005, 451, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Jara-Oseguera, A.; Huffer, K.E.; Swartz, K.J. The Ion Selectivity Filter Is Not an Activation Gate in TRPV1-3 Channels. eLife 2019, 8, e51212. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 Activation Power Can Switch an Action Mode for Its Polypeptide Ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid Potentiation of the Capsaicin Receptor Determined by a Key Extracellular Site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Yang, F.; Wang, K.W.; Zheng, J. Divalent Cations Potentiate TRPV1 Channel by Lowering the Heat Activation Threshold. J. Gen. Physiol. 2014, 143, 75–90. [Google Scholar] [CrossRef]
- Yang, F.; Ma, L.; Cao, X.; Wang, K.W.; Zheng, J. Divalent Cations Activate TRPV1 through Promoting Conformational Change of the Extracellular Region. J. Gen. Physiol. 2014, 143, 91–103. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Moff, I.; Sudanagunta, S.P.; Levine, J.D. Molecular Cloning of an N-Terminal Splice Variant of the Capsaicin Receptor. J. Biol. Chem. 2000, 275, 2756–2762. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Zheng, J. Molecular Mechanism of TRP Channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and Pharmacology of Endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef]
- Van Der Stelt, M.; Di Marzo, V. Endovanilloids: Putative Endogenous Ligands of Transient Receptor Potential Vanilloid 1 Channels. Eur. J. Biochem. 2004, 271, 1827–1834. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Nie, Y.; Tian, Y.; Xiao, X.; Yang, F. Endocannabinoid Activation of the TRPV1 Ion Channel Is Distinct from Activation by Capsaicin. J. Biol. Chem. 2021, 297, 101022. [Google Scholar] [CrossRef]
- Muller, C.; Lynch, D.L.; Hurst, D.P.; Reggio, P.H. A Closer Look at Anandamide Interaction with TRPV1. Front. Mol. Biosci. 2020, 7, 144. [Google Scholar] [CrossRef]
- Nieto-Posadas, A.; Picazo-Juárez, G.; Llorente, I.; Jara-Oseguera, A.; Morales-Lázaro, S.; Escalante-Alcalde, D.; Islas, L.D.; Rosenbaum, T. Lysophosphatidic Acid Directly Activates TRPV1 through a C-Terminal Binding Site. Nat. Chem. Biol. 2012, 8, 78–85. [Google Scholar] [CrossRef]
- Knotkova, H.; Pappagallo, M.; Szallasi, A. Capsaicin (TRPV1 Agonist) Therapy for Pain Relief: Farewell or Revival? Clin. J. Pain 2008, 24, 142–154. [Google Scholar] [CrossRef]
- Darré, L.; Domene, C. Binding of Capsaicin to the TRPV1 Ion Channel. Mol. Pharm. 2015, 12, 4454–4465. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural Mechanism Underlying Capsaicin Binding and Activation of the TRPV1 Ion Channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef]
- Jara-Oseguera, A.; Simon, S.; Rosenbaum, T. TRPV1: On the Road to Pain Relief. Curr. Mol. Pharmacol. 2010, 1, 255–269. [Google Scholar] [CrossRef]
- Touska, F.; Marsakova, L.; Teisinger, J.; Vlachova, V. A “Cute” Desensitization of TRPV1. Curr. Pharm. Biotechnol. 2010, 12, 122–129. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Takahashi, K.; Ohta, T. The Effects of Vanilloid Analogues Structurally Related to Capsaicin on the Transient Receptor Potential Vanilloid 1 Channel. Biochem. Biophys. Rep. 2022, 30, 101243. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a Phorbol-Related Diterpene, Acts as an Ultrapotent Analog of Capsaicin, the Irritant Constituent in Red Pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Z.; Mtui, T.; Gao, Y.D.; Kohler, M.; Middleton, R.E. Resiniferatoxin Binds to the Capsaicin Receptor (TRPV1) near the Extracellular Side of the S4 Transmembrane Domain. Biochemistry 2004, 43, 2501–2511. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Pagano, E.; Souto, E.B.; Durazzo, A.; Sharifi-Rad, J.; Lucarini, M.; Souto, S.B.; Salehi, B.; Zam, W.; Montanaro, V.; Lucariello, G.; et al. Ginger (Zingiber Officinale Roscoe) as a Nutraceutical: Focus on the Metabolic, Analgesic, and Antiinflammatory Effects. Phyther. Res. 2021, 35, 2403–2417. [Google Scholar] [CrossRef]
- Fajrin, F.A.; Rahmayanti, F.; Pratoko, D.K. The Binding Prediction of 6-Paradol and Its Derivatives on TRPV1 Agonist as a New Compound for Treating Painful Diabetic Neuropathy. J. ILMU DASAR 2020, 21, 133. [Google Scholar] [CrossRef]
- Yin, Y.; Dong, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. Structural Mechanisms Underlying Activation of TRPV1 Channels by Pungent Compounds in Gingers. Br. J. Pharmacol. 2019, 176, 3364–3377. [Google Scholar] [CrossRef]
- Ohbuchi, K.; Mori, Y.; Ogawa, K.; Warabi, E.; Yamamoto, M.; Hirokawa, T. Detailed Analysis of the Binding Mode of Vanilloids to Transient Receptor Potential Vanilloid Type I (TRPV1) by a Mutational and Computational Study. PLoS ONE 2016, 11, e0162543. [Google Scholar] [CrossRef]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.S.; Lee, Y.J.; Kozlov, S. Marine Cyclic Guanidine Alkaloids Monanchomycalin B and Urupocidin a Act as Inhibitors of TRPV1, TRPV2 and TRPV3, but Not TRPA1 Receptors. Mar. Drugs 2017, 15, 87. [Google Scholar] [CrossRef]
- Pearce, L.V.; Petukhov, P.A.; Szabo, T.; Kedei, N.; Bizik, F.; Kozikowski, A.P.; Blumberg, P.M. Evodiamine Functions as an Agonist for the Vanilloid Receptor TRPV1. Org. Biomol. Chem. 2004, 2, 2281–2286. [Google Scholar] [CrossRef]
- Wang, S.; Yamamoto, S.; Kogure, Y.; Zhang, W.; Noguchi, K.; Dai, Y. Partial Activation and Inhibition of TRPV1 Channels by Evodiamine and Rutaecarpine, Two Major Components of the Fruits of Evodia Rutaecarpa. J. Nat. Prod. 2016, 79, 1225–1230. [Google Scholar] [CrossRef]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of Piperine, the Pungent Component of Black Pepper, at the Human Vanilloid Receptor (TRPV1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, W.; Zhang, Z.S.; Yang, T.; Grant, A.; Oxford, G.; Simon, S.A. Nicotine Inhibits Voltage-Dependent Sodium Channels and Sensitizes Vanilloid Receptors. J. Neurophysiol. 2004, 91, 1482–1491. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A Distinct Structural Mechanism Underlies TRPV1 Activation by Piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Yu, H.; Zhang, Y.; Gong, W.; Jin, H.; Zhang, L.; Liang, H. Binding Mode Prediction of Evodiamine within Vanilloid Receptor TRPV1. Int. J. Mol. Sci. 2012, 13, 8958–8969. [Google Scholar] [CrossRef]
- Szallasi, A.; Bíró, T.; Modarres, S.; Garlaschelli, L.; Petersen, M.; Klusch, A.; Vidari, G.; Jonassohn, M.; De Rosa, S.; Sterner, O.; et al. Dialdehyde Sesquiterpenes and Other Terpenoids as Vanilloids. Eur. J. Pharmacol. 1998, 356, 81–89. [Google Scholar] [CrossRef]
- Li, X.; Xing, B.; Liu, X.; Jiang, X.-W.; Lu, H.-Y.; Xu, Z.-H.; Yang, Y.; Wu, Q.; Yao, D.; Zhang, Y.S.; et al. Network Pharmacology-Based Research Uncovers Cold Resistance and Thermogenesis Mechanism of Cinnamomum Cassia. Fitoterapia 2021, 149, 104824. [Google Scholar] [CrossRef]
- Sterner, O.; Szallasi, A. Novel Natural Vanilloid Receptor Agonists: New Therapeutic Targets for Drug Development. Trends Pharmacol. Sci. 1999, 20, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, C.; Santos, A.R.S.; Ferreira, J.; Malheiros, A.; Cechinel-Filho, V.; Yunes, R.A.; Calixto, J.B. Evidence for the Involvement of Glutamatergic Receptors in the Antinociception Caused in Mice by the Sesquiterpene Drimanial. Neuropharmacology 2002, 43, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.L.; Santos, A.R.S.; Malheiros, A.; Cechinel Filho, V.; Yunes, R.A.; Calixto, J.B. Assessment of Mechanisms Involved in Antinociception Caused by Sesquiterpene Polygodial. J. Pharmacol. Exp. Ther. 2000, 292, 164–172. [Google Scholar] [PubMed]
- Da Cunha, F.M.; Fröde, T.S.; Mendes, G.L.; Malheiros, A.; Filho, V.C.; Yunes, R.A.; Calixto, J.B. Additional Evidence for the Anti-Inflammatory and Anti-Allergic Properties of the Sesquiterpene Polygodial. Life Sci. 2001, 70, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Jonassohn, M.; Ács, G.; Bíró, T.; Ács, P.; Blumberg, P.M.; Sterner, O. The Stimulation of Capsaicin-Sensitive Neurones in a Vanilloid Receptor-Mediated Fashion by Pungent Terpenoids Possessing an Unsaturated 1,4-Dialdehyde Moiety. Br. J. Pharmacol. 1996, 119, 283–290. [Google Scholar] [CrossRef]
- André, E.; Campi, B.; Trevisani, M.; Ferreira, J.; Malheiros, Â.; Yunes, R.A.; Calixto, J.B.; Geppetti, P. Pharmacological Characterisation of the Plant Sesquiterpenes Polygodial and Drimanial as Vanilloid Receptor Agonists. Biochem. Pharmacol. 2006, 71, 1248–1254. [Google Scholar] [CrossRef]
- Alpizar, Y.A.; Boonen, B.; Gees, M.; Sanchez, A.; Nilius, B.; Voets, T.; Talavera, K. Allyl Isothiocyanate Sensitizes TRPV1 to Heat Stimulation. Pflugers Arch. Eur. J. Physiol. 2014, 466, 507–515. [Google Scholar] [CrossRef]
- Salazar, H.; Llorente, I.; Jara-Oseguera, A.; García-Villegas, R.; Munari, M.; Gordon, S.E.; Islas, L.D.; Rosenbaum, T. A Single N-Terminal Cysteine in TRPV1 Determines Activation by Pungent Compounds from Onion and Garlic. Nat. Neurosci. 2008, 11, 255–261. [Google Scholar] [CrossRef]
- Koizumi, K.; Iwasaki, Y.; Narukawa, M.; Iitsuka, Y.; Fukao, T.; Seki, T.; Ariga, T.; Watanabe, T. Diallyl Sulfides in Garlic Activate Both TRPA1 and TRPV1. Biochem. Biophys. Res. Commun. 2009, 382, 545–548. [Google Scholar] [CrossRef]
- Gees, M.; Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Everaerts, W.; Segal, A.; Xue, F.; Janssens, A.; Owsianik, G.; Nilius, B.; et al. Mechanisms of Transient Receptor Potential Vanilloid 1 Activation and Sensitization by Allyl Isothiocyanate. Mol. Pharmacol. 2013, 84, 325–334. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.W.; Patapoutian, A. The Pungency of Garlic: Activation of TRPA1 and TRPV1 in Response to Allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Klein, A.H.; Joe, C.L.; Davoodi, A.; Takechi, K.; Carstens, M.I.; Carstens, E. Eugenol and Carvacrol Excite First- and Second-Order Trigeminal Neurons and Enhance Their Heat-Evoked Responses. Neuroscience 2014, 271, 45–55. [Google Scholar] [CrossRef]
- Takaishi, M.; Uchida, K.; Suzuki, Y.; Matsui, H.; Shimada, T.; Fujita, F.; Tominaga, M. Reciprocal Effects of Capsaicin and Menthol on Thermosensation through Regulated Activities of TRPV1 and TRPM8. J. Physiol. Sci. 2016, 66, 143–155. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; La Marca, G.; Andr, E.; Preti, D.; Avonto, C.; et al. The “headache Tree” via Umbellulone and TRPA1 Activates the Trigeminovascular System. Brain 2012, 135, 376–390. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Marsakova, L.; Touska, F.; Krusek, J.; Vlachova, V. Pore Helix Domain Is Critical to Camphor Sensitivity of Transient Receptor Potential Vanilloid 1 Channel. Anesthesiology 2012, 116, 903–917. [Google Scholar] [CrossRef]
- Velisetty, P.; Stein, R.A.; Sierra-Valdez, F.J.; Vásquez, V.; Cordero-Morales, J.F. Expression and Purification of the Pain Receptor TRPV1 for Spectroscopic Analysis. Sci. Rep. 2017, 7, 9861. [Google Scholar] [CrossRef]
- Takahashi, K.; Yoshida, T.; Wakamori, M. Mode-Selective Inhibitory Effects of Eugenol on the Mouse TRPV1 Channel. Biochem. Biophys. Res. Commun. 2021, 556, 156–162. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and Terpene Regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current Application of Cannabidiol (CBD) in the Management and Treatment of Neurological Disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Machado Bergamaschi, M.; Helena Costa Queiroz, R.; Waldo Zuardi, A.; Alexandre, S.; Crippa, J. Safety and Side Effects of Cannabidiol, a Cannabis Sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Julius, D. Molecular Basis for Species-Specific Sensitivity to “Hot” Chili Peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- Xiao, S.; Song, P.; Bu, F.; Pang, G.; Zhou, A.; Zhang, Y.; Xie, J. The Investigation of Detection and Sensing Mechanism of Spicy Substance Based on Human TRPV1 Channel Protein-Cell Membrane Biosensor. Biosens. Bioelectron. 2021, 172, 112779. [Google Scholar] [CrossRef]
- Harb, A.A.; Bustanji, Y.K.; Almasri, I.M.; Abdalla, S.S. Eugenol Reduces LDL Cholesterol and Hepatic Steatosis in Hypercholesterolemic Rats by Modulating TRPV1 Receptor. Sci. Rep. 2019, 9, 14003. [Google Scholar] [CrossRef]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Molecular Docking Analysis of Ginger Active Compound on Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1). Indones. J. Chem. 2018, 18, 179–185. [Google Scholar] [CrossRef]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 Antagonists That Cause Hypothermia, Instead of Hyperthermia, in Rodents: Compounds’ Pharmacological Profiles, in Vivo Targets, Thermoeffectors Recruited and Implications for Drug Development. Acta Physiol. 2018, 223, 223. [Google Scholar] [CrossRef]
- Szelényi, Z.; Hummel, Z.; Szolcsányi, J.; Davis, J.B. Daily Body Temperature Rhythm and Heat Tolerance in TRPV1 Knockout and Capsaicin Pretreated Mice. Eur. J. Neurosci. 2004, 19, 1421–1424. [Google Scholar] [CrossRef]
- Steiner, A.A.; Turek, V.F.; Almeida, M.C.; Burmeister, J.J.; Oliveira, D.L.; Roberts, J.L.; Bannon, A.W.; Norman, M.H.; Louis, J.C.; Treanor, J.J.S.; et al. Nonthermal Activation of Transient Receptor Potential Vanilloid-1 Channels in Abdominal Viscera Tonically Inhibits Autonomic Cold-Defense Effectors. J. Neurosci. 2007, 27, 7459–7468. [Google Scholar] [CrossRef]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-Butylphenyl)-N-(2,3-Dihydrobenzo[b][1,4] Dioxin-6-Yl)Acrylamide], a Novel Vanilloid Receptor 1 (TRPV1) Antagonist with Antihyperalgesic Properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef]
- Yoshida, A.; Furube, E.; Mannari, T.; Takayama, Y.; Kittaka, H.; Tominaga, M.; Miyata, S. TRPV1 Is Crucial for Proinflammatory STAT3 Signaling and Thermoregulation-Associated Pathways in the Brain during Inflammation. Sci. Rep. 2016, 6, 26088. [Google Scholar] [CrossRef]
- McGaraughty, S.; Segreti, J.A.; Fryer, R.M.; Brown, B.S.; Faltynek, C.R.; Kym, P.R. Antagonism of TRPV1 Receptors Indirectly Modulates Activity of Thermoregulatory Neurons in the Medial Preoptic Area of Rats. Brain Res. 2009, 1268, 58–67. [Google Scholar] [CrossRef]
- Rowbotham, M.C.; Nothaft, W.; Duan, W.R.; Wang, Y.; Faltynek, C.; McGaraughty, S.; Chu, K.L.; Svensson, P. Oral and Cutaneous Thermosensory Profile of Selective TRPV1 Inhibition by ABT-102 in a Randomized Healthy Volunteer Trial. Pain 2011, 152, 1192–1200. [Google Scholar] [CrossRef]
- Quiding, H.; Jonzon, B.; Svensson, O.; Webster, L.; Reimfelt, A.; Karin, A.; Karlsten, R.; Segerdahl, M. TRPV1 Antagonistic Analgesic Effect: A Randomized Study of AZD1386 in Pain after Third Molar Extraction. Pain 2013, 154, 808–812. [Google Scholar] [CrossRef]
- Tafesse, L.; Kanemasa, T.; Kurose, N.; Yu, J.; Asaki, T.; Wu, G.; Iwamoto, Y.; Yamaguchi, Y.; Ni, C.; Engel, J.; et al. Structure-Activity Relationship Studies and Discovery of a Potent Transient Receptor Potential Vanilloid (TRPV1) Antagonist 4-[3-Chloro-5-[(1 S)-1,2-Dihydroxyethyl]-2-Pyridyl]-N-[5-(Trifluoromethyl)-2-Pyridyl]-3, 6-Dihydro-2 H-Pyridine-1-Carboxamide (V116). J. Med. Chem. 2014, 57, 6781–6794. [Google Scholar] [CrossRef]
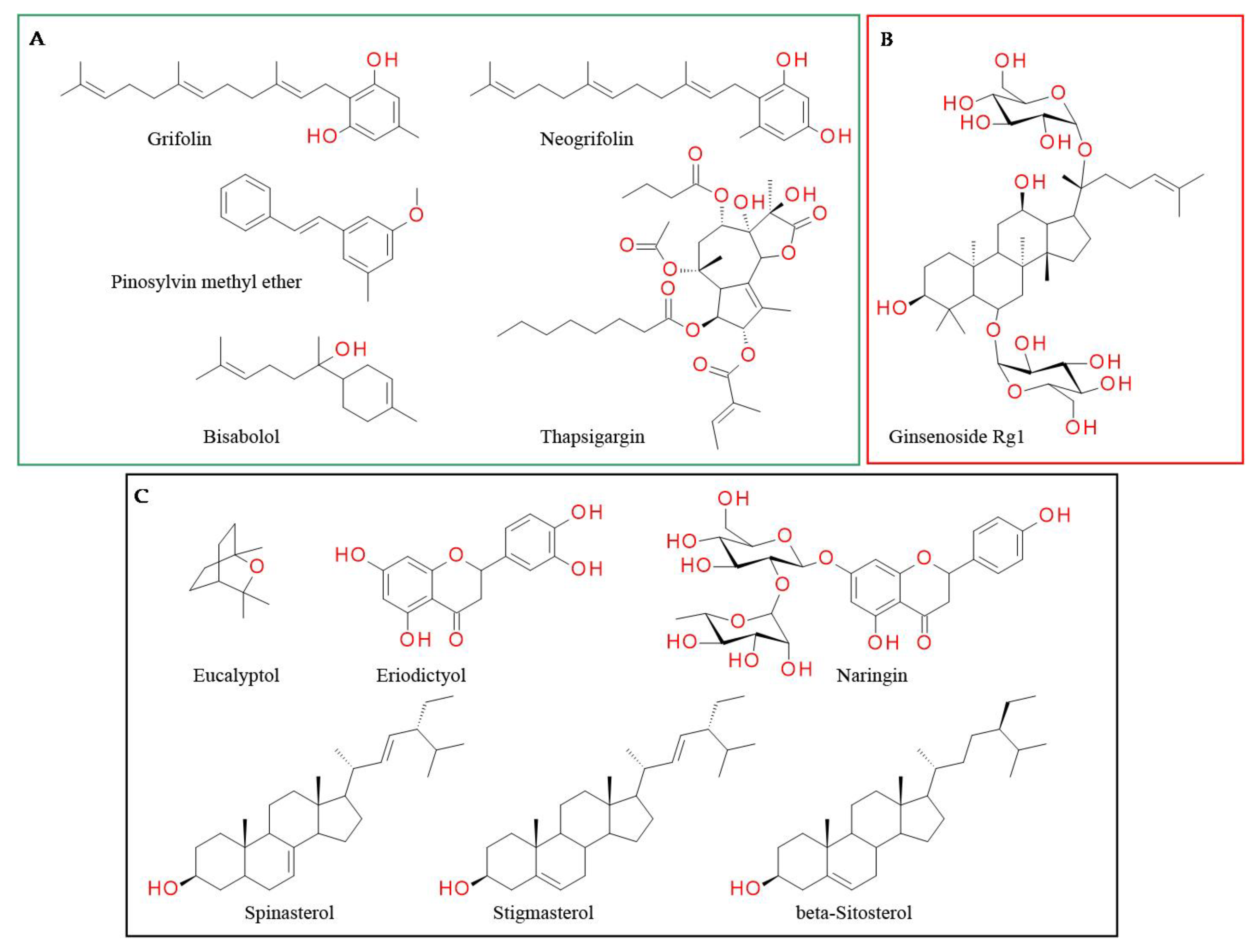
- Hettwer, S.; Bänziger, S.; Suter, B.; Obermayer, B. Grifolin Derivatives from Albatrellus Ovinus as TRPV1 Receptor Blockers for Cosmetic Applications. Int. J. Cosmet. Sci. 2017, 39, 379–385. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Kogure, Y.; Yamamoto, S.; Noguchi, K.; Dai, Y. Modulation of TRP Channels by Resveratrol and Other Stilbenoids. Mol. Pain 2013, 9, 1744–8069. [Google Scholar] [CrossRef]
- Jaffal, S.M.; Oran, S.A.; Alsalem, M. Anti-Nociceptive Effect of Arbutus Andrachne L. Methanolic Leaf Extract Mediated by CB1, TRPV1 and PPARs in Mouse Pain Models. Inflammopharmacology 2020, 28, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Horie, S.; Takayama, H.; Uchida, K.; Tominaga, M.; Watanabe, T. Activation and Inhibition of Thermosensitive TRP Channels by Voacangine, an Alkaloid Present in Voacanga Africana, an African Tree. J. Nat. Prod. 2014, 77, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Oláh, Z.; Rédei, D.; Pecze, L.; Vizler, C.; Jósvay, K.; Forgó, P.; Winter, Z.; Dombi, G.; Szakonyi, G.; Hohmann, J. Pellitorine, an Extract of Tetradium Daniellii, Is an Antagonist of the Ion Channel TRPV1. Phytomedicine 2017, 34, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmaher, K.M.; Denisenko, V.A.; Dmitrenok, P.S.; Ogurtsova, E.K.; Antonov, A.S.; et al. Pulchranin A, Isolated from the Far-Eastern Marine Sponge, Monanchora Pulchra: The First Marine Non-Peptide Inhibitor of TRPV-1 Channels. Tetrahedron Lett. 2013, 54, 1247–1250. [Google Scholar] [CrossRef]
- Matsushita, Y.; Manabe, M.; Kitamura, N.; Shibuya, I. Adrenergic Receptors Inhibit TRPV1 Activity in the Dorsal Root Ganglion Neurons of Rats. PLoS ONE 2018, 13, e0191032. [Google Scholar] [CrossRef]
- Papeschi, R.; Sourkes, T.L.; Youdim, M.B.H. The Effect of Yohimbine on Brain Serotonin Metabolism, Motor Behavior and Body Temperature of the Rat. Eur. J. Pharmacol. 1971, 15, 318–326. [Google Scholar] [CrossRef]
- Tóth, A.; Kedei, N.; Szabó, T.; Wang, Y.; Blumberg, P.M. Thapsigargin Binds to and Inhibits the Cloned Vanilloid Receptor-1. Biochem. Biophys. Res. Commun. 2002, 293, 777–782. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Huang, S.; Zamponi, G.W. The Terpenes Camphene and Alpha-Bisabolol Inhibit Inflammatory and Neuropathic Pain via Cav3.2 T-Type Calcium Channels. Mol. Brain 2021, 14, 166. [Google Scholar] [CrossRef]
- Teixeira, G.F.D.; Vieira-Neto, A.E.; da Costa, F.N.; e Silva, A.R.A.; Campos, A.R. Antinociceptive Effect of (-)-α-Bisabolol in Nanocapsules. Biomed. Pharmacother. 2017, 91, 946–950. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, L.; Ding, L.; Wang, S.; Wang, J.; Zhu, Q.; Song, F.; Hu, J. Ginsenoside Rb1 and Paeoniflorin Inhibit Transient Receptor Potential Vanilloid-1-Activated IL-8 and PGE2 Production in a Human Keratinocyte Cell Line HaCaT. Int. Immunopharmacol. 2010, 10, 1279–1283. [Google Scholar] [CrossRef]
- Wang, G.L.; He, Z.M.; Zhu, H.Y.; Gao, Y.G.; Zhao, Y.; Yang, H.; Zhang, L.X. Involvement of Serotonergic, Noradrenergic and Dopaminergic Systems in the Antidepressant-like Effect of Ginsenoside Rb1, a Major Active Ingredient of Panax Ginseng C.A. Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and Anti-Inflammatory Effects of Essential Oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Yin, C.; Liu, B.; Wang, P.; Li, X.; Li, Y.; Zheng, X.; Tai, Y.; Wang, C.; Liu, B. Eucalyptol Alleviates Inflammation and Pain Responses in a Mouse Model of Gout Arthritis. Br. J. Pharmacol. 2020, 177, 2042–2057. [Google Scholar] [CrossRef]
- de Maria de Albuquerque de Melo Júnior, J.; de Barros Mamede Vidal Damasceno, M.; Santos, S.A.A.R.; Barbosa, T.M.; Araújo, J.R.C.; Vieira-Neto, A.E.; Wong, D.V.T.; Lima-Júnior, R.C.P.; Campos, A.R. Acute and Neuropathic Orofacial Antinociceptive Effect of Eucalyptol. Inflammopharmacology 2017, 25, 247–254. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.N.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A. Vitexin Inhibits Inflammatory Pain in Mice by Targeting TRPV1, Oxidative Stress, and Cytokines. J. Nat. Prod. 2013, 76, 1141–1146. [Google Scholar] [CrossRef]
- Rossato, M.F.; Trevisan, G.; Walker, C.I.B.; Klafke, J.Z.; De Oliveira, A.P.; Villarinho, J.G.; Zanon, R.B.; Royes, L.F.F.; Athayde, M.L.; Gomez, M.V.; et al. Eriodictyol: A Flavonoid Antagonist of the TRPV1 Receptor with Antioxidant Activity. Biochem. Pharmacol. 2011, 81, 544–551. [Google Scholar] [CrossRef]
- Lv, G.; Zhu, G.; Xu, M.; Gao, X.; Xiao, Q. Inhibition of Carrageenan-Induced Dental Inflammatory Responses Owing to Decreased TRPV1 Activity by Dexmedetomidine. J. Inflamm. 2020, 17, 18. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.N.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.S.; Manchope, M.F.; Casagrande, R.; Verri, W.A. Protective Effects of the Flavonoid Hesperidin Methyl Chalcone in Inflammation and Pain in Mice: Role of TRPV1, Oxidative Stress, Cytokines and NF-ΚB. Chem. Biol. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef]
- Eom, S.; Lee, B.B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.H.; Nam, S.H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2022, 11, 64. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Pan, X.; Mo, X.; Gong, W.; Liu, X.; Chen, S. Inhibition on Acid-Sensing Ion Channels and Analgesic Activities of Flavonoids Isolated from Dragon’s Blood Resin. Phyther. Res. 2019, 33, 718–727. [Google Scholar] [CrossRef]
- Lee, S.B.; Noh, S.; Yeom, H.D.; Jo, H.; Eom, S.; Kim, Y.S.; Nam, S.; Bae, H.; Lee, J.H. A Molecular Basis for the Inhibition of Transient Receptor Potential Vanilloid Type 1 by Gomisin A. Evidence-based Complement. Altern. Med. 2017, 2017, 6451905. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zan, Y.; Wang, Z.J.J.; Hu, X.Y.; Huang, F. Quercetin Ameliorates Paclitaxel-Induced Neuropathic Pain by Stabilizing Mast Cells, and Subsequently Blocking PKCϵ-Dependent Activation of TRPV1. Acta Pharmacol. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hassan, S.; Rafiq, M.; Li, H.; He, Y.; Cai, Y.; Kang, X.; Liu, Z.; Yan, T. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evidence-based Complement. Altern. Med. 2020, 2020, 6681352. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Rossato, M.F.; Walker, C.I.B.; Klafke, J.Z.; Rosa, F.; Oliveira, S.M.; Tonello, R.; Guerra, G.P.; Boligon, A.A.; Zanon, R.B.; et al. Identification of the Plant Steroid α-Spinasterol as a Novel Transient Receptor Potential Vanilloid 1 Antagonist with Antinociceptive Properties. J. Pharmacol. Exp. Ther. 2012, 343, 258–269. [Google Scholar] [CrossRef]
- Jaffal, S.M.; Al-Najjar, B.O.; Abbas, M.A. Ononis Spinosa Alleviated Capsaicin-Induced Mechanical Allodynia in a Rat Model through Transient Receptor Potential Vanilloid 1 Modulation. Korean J. Pain 2021, 34, 262–270. [Google Scholar] [CrossRef]
- Şakul, A.A.; Okur, M.E. Beta-Sitosterol and Its Antinociceptive Mechanism Action. Ankara Univ. Eczac. Fak. Derg. 2022, 46, 238–252. [Google Scholar] [CrossRef]
- Jaffal, S.; Oran, S.; Alsalem, M.; Al-Najjar, B. Effect of Arbutus Andrachne L. Methanolic Leaf Extract on TRPV1 Function: Experimental and Molecular Docking Studies. J. Appl. Pharm. Sci. 2022, 12, 069–077. [Google Scholar] [CrossRef]
- Goldmann, D.; Pakfeifer, P.; Hering, S.; Ecker, G.F. Novel Scaffolds for Modulation of TRPV1 Identified with Pharmacophore Modeling and Virtual Screening. Future Med. Chem. 2015, 7, 243–256. [Google Scholar] [CrossRef]
- Kym, P.R.; Kort, M.E.; Hutchins, C.W. Analgesic Potential of TRPV1 Antagonists. Biochem. Pharmacol. 2009, 78, 211–216. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 Structures in Distinct Conformations Reveal Activation Mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Manwill, P.K.; Kalsi, M.; Wu, S.; Martinez-Rodriguez, E.J.; Cheng, X.; Piermarini, P.M.; Rakotondraibe, H.L. Semi-Synthetic Cinnamodial Analogues: Structural Insights into the Insecticidal and Antifeedant Activities of Drimane Sesquiterpenes against the Mosquito Aedes Aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008073. [Google Scholar] [CrossRef]
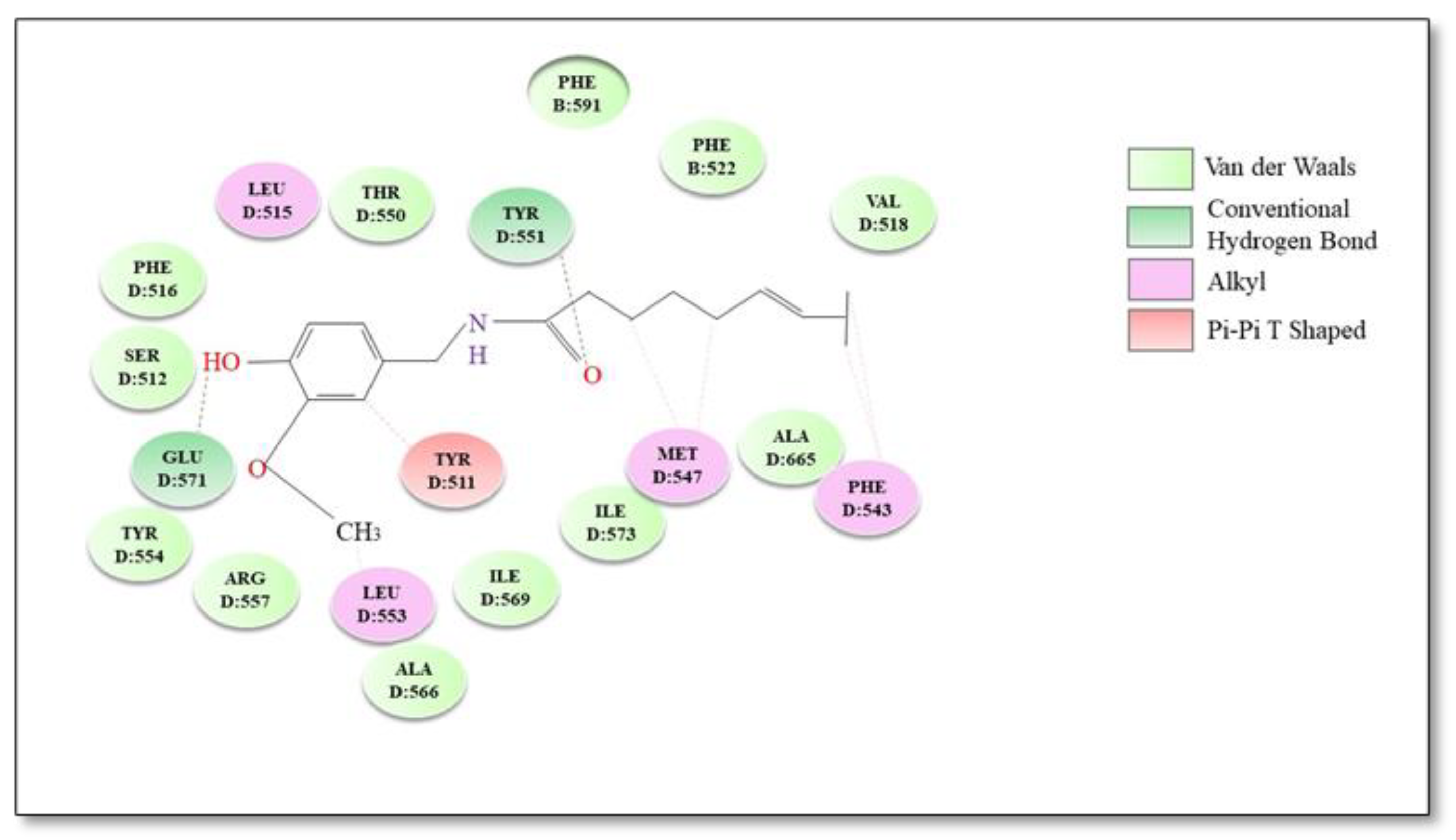
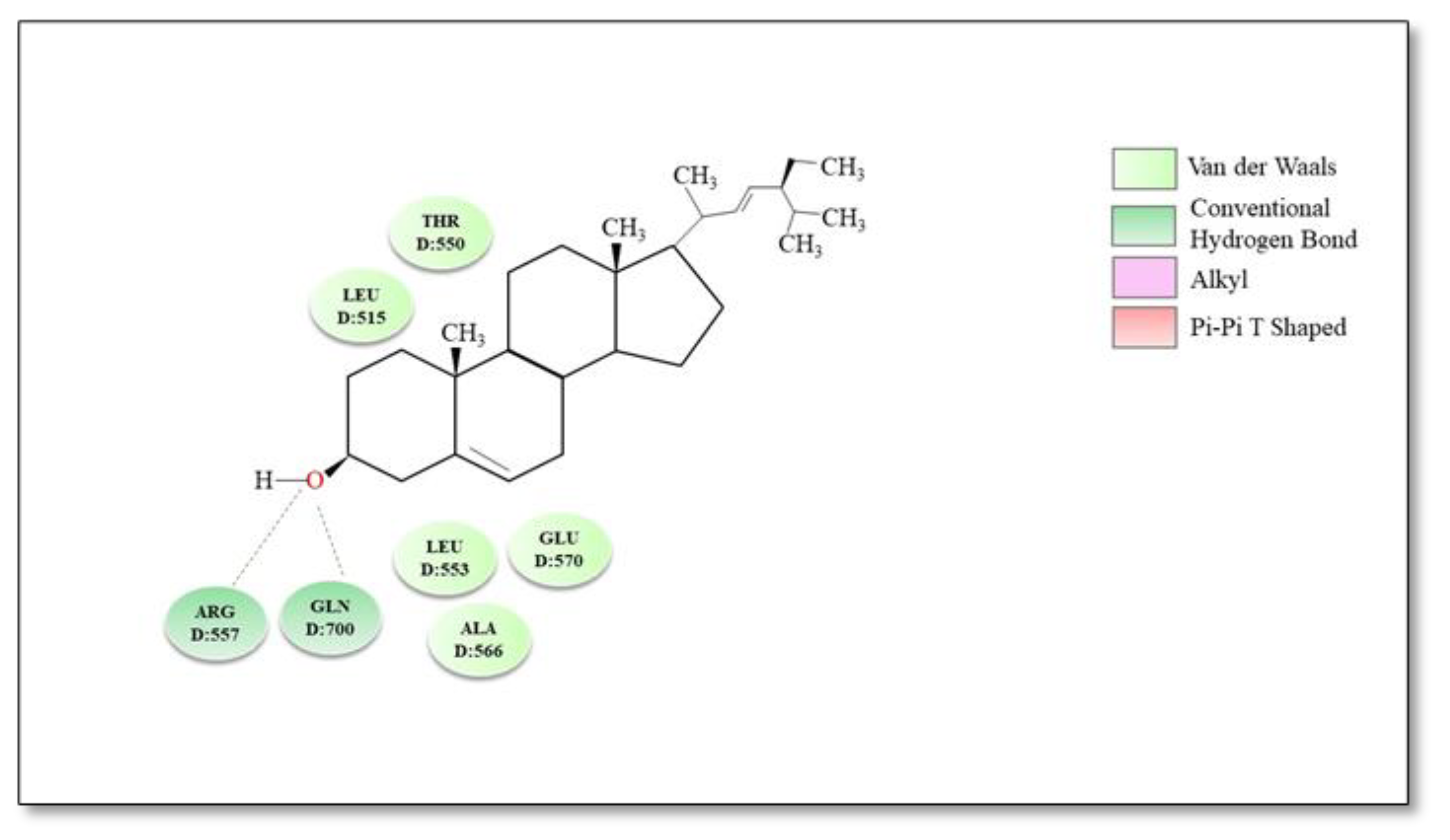
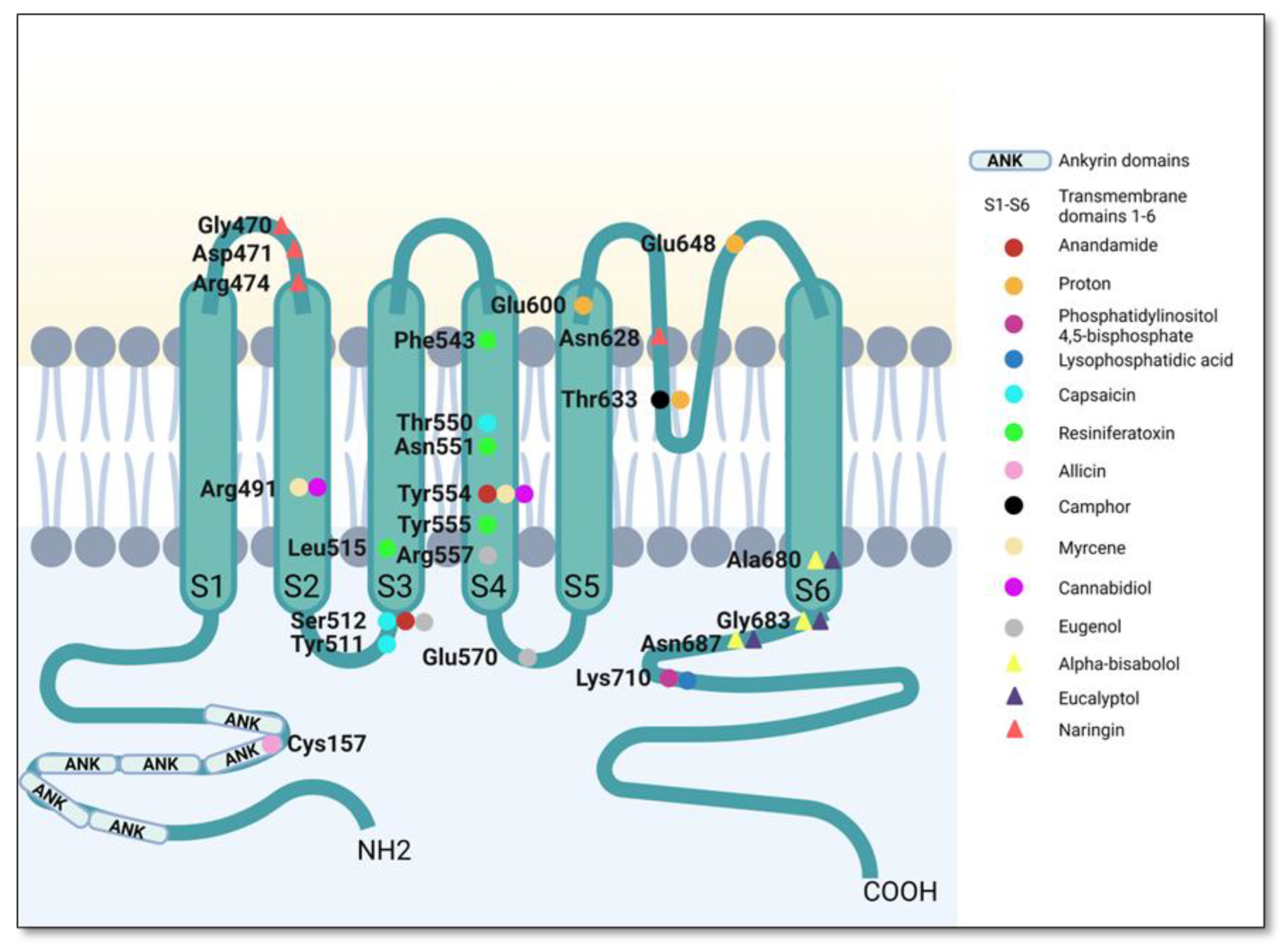
| Plants | Active Compounds | Chemical Class | Key Structures | TRPV1 Residues | Type of Bond | Reference |
|---|---|---|---|---|---|---|
| Capsicum spp. | Capsaicin | Vanilloids | Amide oxygen Aromatic hydroxyl | Tyr551 Glu 571 | Hydrogen bond | [134,135] |
| Vanillyl moiety | Leu515, Ile573, Phe587, Leu669 and Thr550 | Hydrophobic interactions | ||||
| Aliphatic tail | Met547, Ala665, Phe543 and Tyr511 | |||||
| Euphorbia resinifera | Resiniferatoxin | Vanillyl moiety | Tyr555 | Hydrophobic interactions | [94,134] | |
| Side chains | Leu515, Phe543 and Asn551 | Hydrophobic interactions | ||||
| Cannabis sativa | Cannabidiol | Phytocannabinoids | Diverse C within the structure | Tyr554 and Arg491 | Hydrophobic interactions | [129] |
| Thymus vulgaris Cannabis sativa | Myrcene | Monoterpenes | Dimethyl group | Arg491, Tyr555, Phe434 and Asn437 | Hydrophobic interactions | |
| Diverse C within the structure | Ser512, Tyr554, Phe488, Phe516 and Glu513 | |||||
| Cinnamomum camphora | Camphor | Diverse C within the structure | Trp427, Lys432, Phe496, Phe490, Arg491, Tyr487, Leu638, Phe639 and Leu648 | Hydrophobic interactions | [125] | |
| Piper nigrum; Piper longum; Piper officinarum | Piperine | Alkaloids | Carbonyl group | Thr551 | Hydrogen bonds | [106] |
| Evodia rutaecarpa | Evodiamine | Ring 1 (the indole’s benzene ring) | Tyr511 (human) and Tyr514 (rabbit) | Hydrophobic interactions | [107] | |
| Ring 5 (the benzene of the quinazoline moiety) | Tyr555 (human) and Tyr558 (rabbit) | Hydrophobic and aromatic π–π interactions | ||||
| Indole nitrogen | Ile569 (human) and Ile572 (rabbit) | Hydrogen bond (donor) | ||||
| Formyl carbonyl oxygen | Lys571 (human) and Lys574 (rabbit) | Hydrogen bond (acceptor) | ||||
| Allium sativum | Allicin | Thiosulfinates | Thiosulfinate group | Tyr550 and Tyr554 | Hydrogen bond | [136] |
| Glu510 | Attractive charge | |||||
| Diverse C within the structure | Ile569, Ala566, Arg557, Leu553, Ser512, Asn551 and Leu515 | Van der Waals | ||||
| Eugenia caryophyllata; Ocimum gratissimum; Cinnamomum zeylanicum | Eugenol | Allylbenzenes | Hydroxyl group on vanillyl moiety | Glu570, Arg557 and Ser512 | Hydrogen bond | [137] |
| Allyl chain | Leu553, Ala566, Ile569 and Ile573 | Hydrophobic interactions | ||||
| Zingiber officinalis | 6-gingerol | Guaiacols | Carbon side chain | Phe49 and Phe93 | Hydrogen bond | [138] |
| Carbonyl group | Ile293 | |||||
| Hydroxyl group of aliphatic chain | Lys285 | |||||
| 8-gingerol | Diverse C within the side chain | Phe49, Phe58, Phe93 and | ||||
| Carbonyl group | Ile293 | |||||
| Hydroxyl group of aliphatic chain | Lys285 | |||||
| 10-gingerol | Diverse C within the side chain | Phe49, Phe58, Phe93 and Gly210 | ||||
| Carbonyl group | Ile293 | |||||
| Hydroxyl group of aliphatic chain | Lys285 | |||||
| 6-shogaol | Phenols | Diverse C within the side chain | Phe49, Phe93 | |||
| Carbonyl group | Ile293 | |||||
| 8-shogaol | Diverse C within the side chain | Phe49, Phe58 and Phe93 | ||||
| Carbonyl group | Ile293 | |||||
| 10-shogaol | Diverse C within the side chain | Phe49, Phe58 and Phe93 | ||||
| Carbonyl group | Ile293 | |||||
| Zingerone | Ketone group | Thr551 | Hydrogen bond | [98] | ||
| Vanillyl group | Glu571 and Thr671 | |||||
| 6-shogaol and 6-gingerol | Phenols and guaiacols | Ketone group | Thr551 | Hydrogen bond | ||
| Vanillyl group | Glu571 | |||||
| 2-paradol, 4-paradol and 8-paradol | Phenols | Hydroxyl group | Leu32 and Thr28 | Hydrogen bond | [97] | |
| 6-paradol | Glu140, Gln135 and Gln143 |
| Influence on Body Temperature | Plants | Active Compounds | Chemical Class | Key Structures | Residues TRPV1 | Observation | Reference |
|---|---|---|---|---|---|---|---|
| Unknown | Matricaria recutita and Myoporum crassifolium | Alpha-bisabolol | Sesquiterpenoid | Diverse C within the structure | Ala680 and Asn687 | Hydrophobic interactions | [158] |
| Hydroxyl group in position C-2 | Gly683 | Hydrogen bond | |||||
| Does not interfere | Vernonia tweedieana | Stigmasterol | Sterols | Hydroxyl group in position C-3 of the steroid skeleton | Arg557 and Gln700 | Hydrogen bond | [174] |
| Rings A and B of the steroid skeleton | Thr550, Leu515, Leu553, Ala566 and Glu570 | Hydrophobic interactions | |||||
| Arbutus andrachne | Beta-sitosterol | Sterols | Hydroxyl group in position C-3 | Phe543 | Hydrogen bond | [176] | |
| Sterolic nucleus | Leu515, Ala546, Met547, Leu553, Ala665 and Leu669 | Hydrophobic interactions | |||||
| Citrus peel | Naringin | Flavonoids and corresponding glycosides | Hydroxyl group on the phenyl moiety | Asn628 | Hydrogen bond | [168] | |
| Hydroxyl groups on the oses | Asp471 and Gly470 | ||||||
| Hydroxyl groups in position C-5, C-7 | Arg474 | ||||||
| Phenyl group in position C-2 | Val457 | Hydrophobic interactions | |||||
| Eucalyptus globulus | Eucalyptol | Monoterpenoids | Cineol ring | Ala680, Gly683 and Asn 687 | Hydrophobic interactions | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, C.; Zanfirescu, A.; Nițulescu, G.M.; Olaru, O.T.; Negreș, S. Natural Active Ingredients and TRPV1 Modulation: Focus on Key Chemical Moieties Involved in Ligand–Target Interaction. Plants 2023, 12, 339. https://doi.org/10.3390/plants12020339
Andrei C, Zanfirescu A, Nițulescu GM, Olaru OT, Negreș S. Natural Active Ingredients and TRPV1 Modulation: Focus on Key Chemical Moieties Involved in Ligand–Target Interaction. Plants. 2023; 12(2):339. https://doi.org/10.3390/plants12020339
Chicago/Turabian StyleAndrei, Corina, Anca Zanfirescu, George Mihai Nițulescu, Octavian Tudorel Olaru, and Simona Negreș. 2023. "Natural Active Ingredients and TRPV1 Modulation: Focus on Key Chemical Moieties Involved in Ligand–Target Interaction" Plants 12, no. 2: 339. https://doi.org/10.3390/plants12020339
APA StyleAndrei, C., Zanfirescu, A., Nițulescu, G. M., Olaru, O. T., & Negreș, S. (2023). Natural Active Ingredients and TRPV1 Modulation: Focus on Key Chemical Moieties Involved in Ligand–Target Interaction. Plants, 12(2), 339. https://doi.org/10.3390/plants12020339







