Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum
Abstract
1. Introduction
2. Results
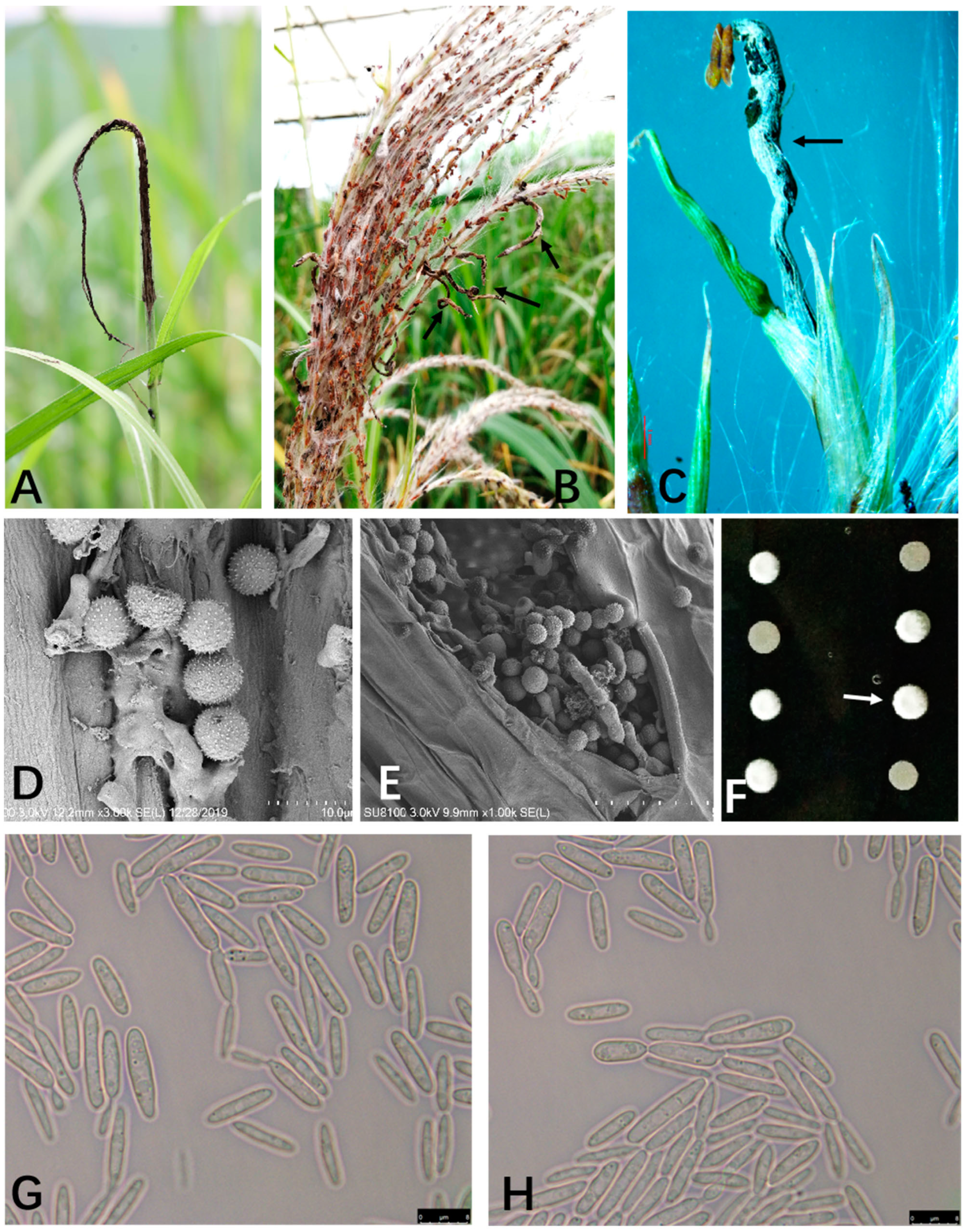
2.1. Diseased Symptoms and Morphological Characteristics of Smut Fungus
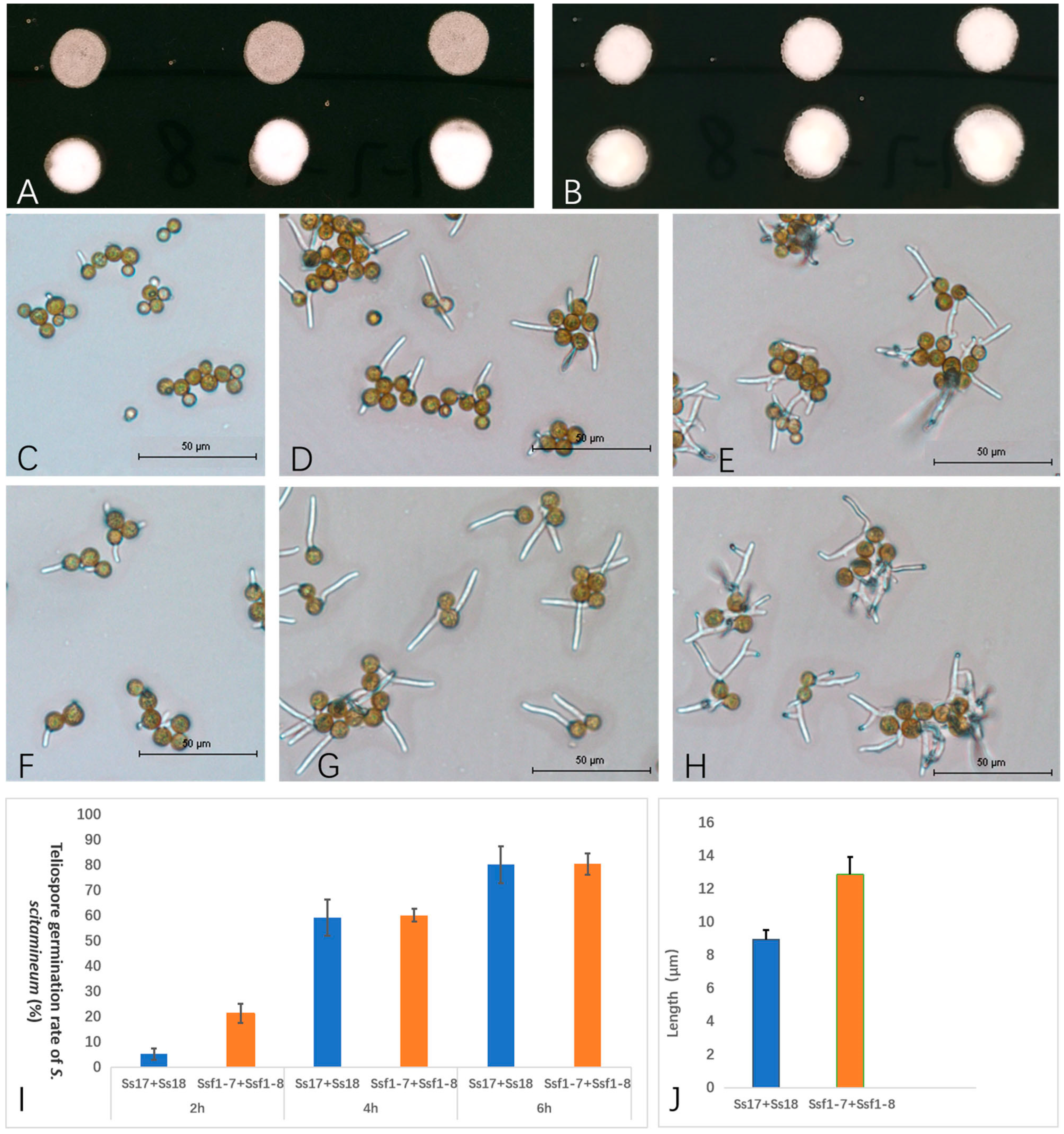
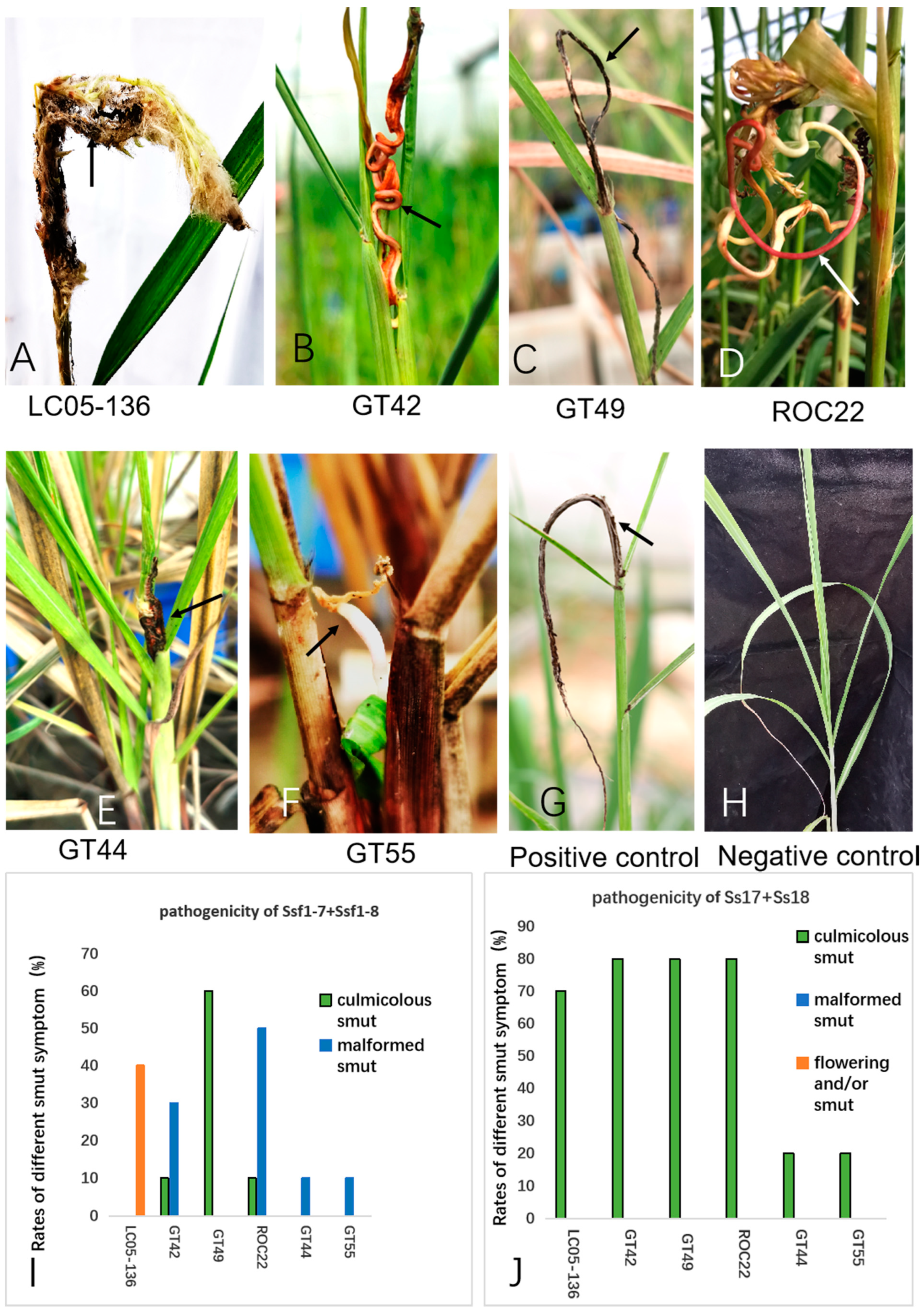
2.2. Pathogenicity of Smut Fungus Isolated from Sugarcane Inflorescence
2.3. ITS Identification
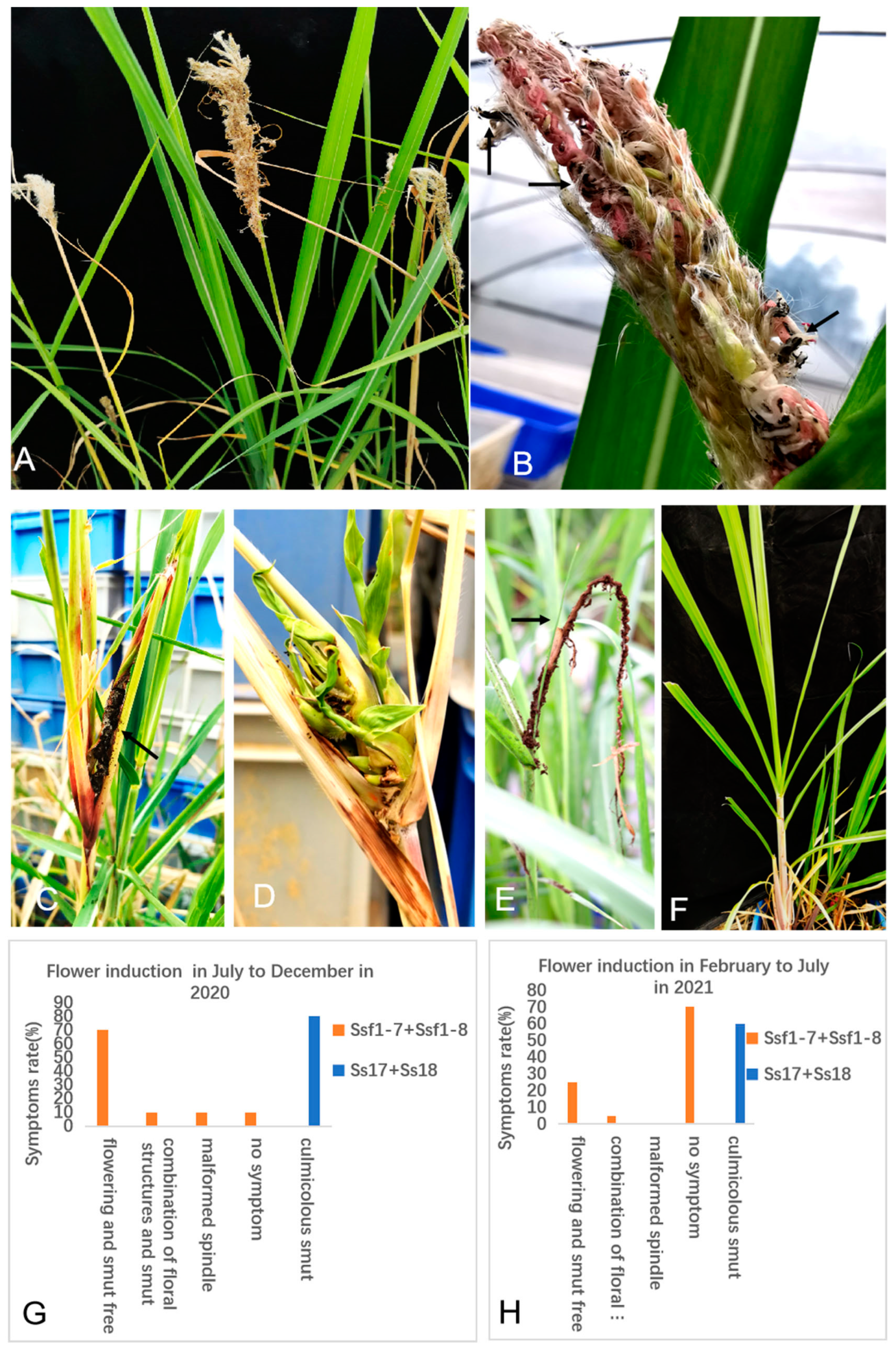
2.4. Flowering Induction by S. scitamineum in Sugarcane Variety LC05-136
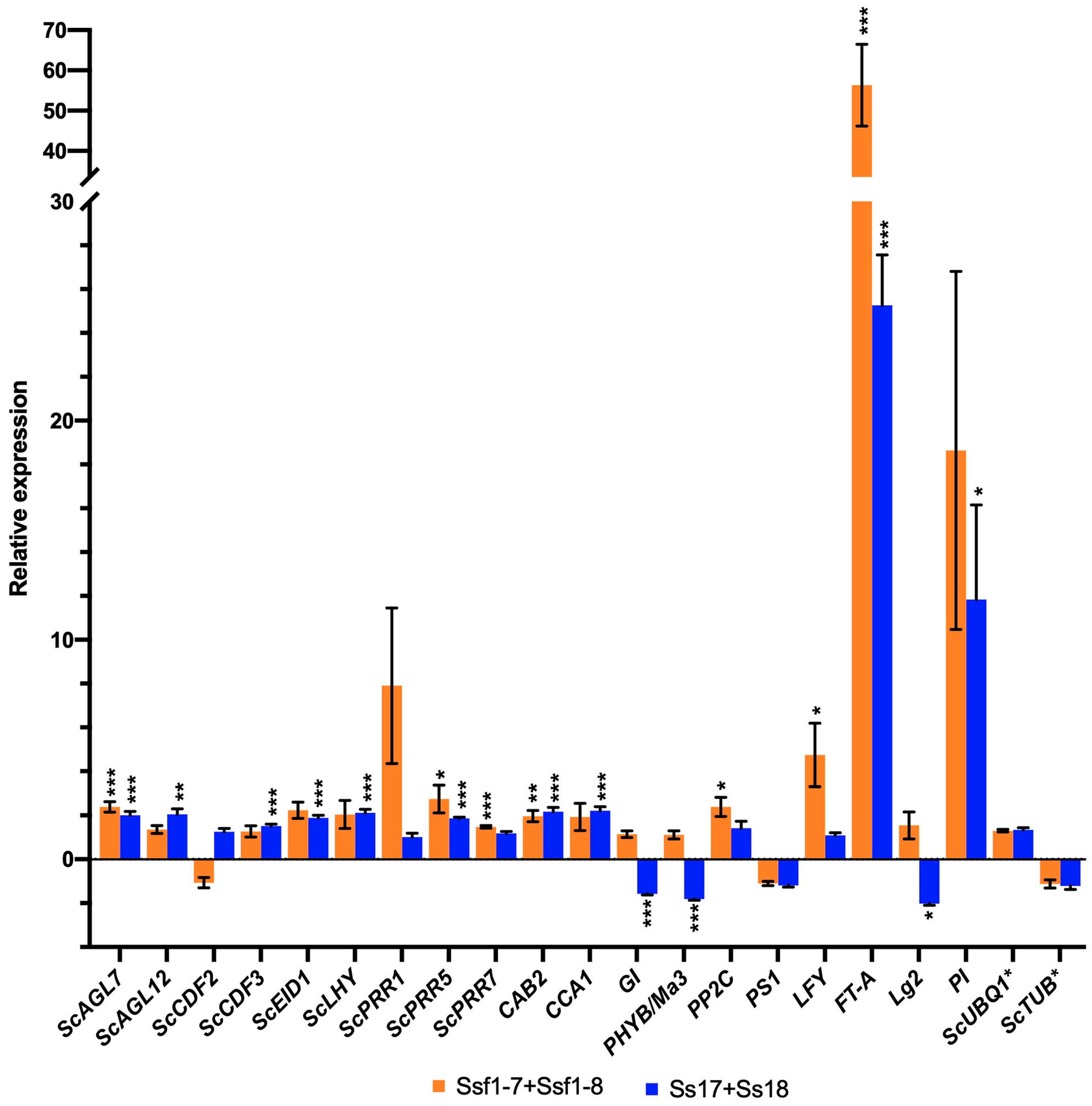
2.5. Assessment of Comparative Expression of Flowering Related Genes by RT-qPCR
3. Materials and Methods
3.1. Isolation of the Causal Agent of Smut from Inflorescence of Sugarcane
3.2. Microscopic Observations
3.3. Scanning Electron Microscope (SEM) Examination
3.4. Pathogenicity Test
3.5. Molecular Identification
3.6. Flowering Induction of Sugarcane Variety LC05-136 with Smut Fungus Isolates
3.7. Quantitative Real-Time PCR (RT-qPCR) Assessment of Flowering-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, X.P.; Tian, D.D.; Chen, M.H.; Qin, Z.Q.; Wei, J.J.; Wei, C.Y.; Zhang, X.Q.; Li, D.W.; Yang, L.T.; Li, Y.R. Cloning and identification of differentially expressed genes associated with smut in sugarcane. Sugar Tech 2018, 20, 717–724. [Google Scholar] [CrossRef]
- Liu, S.Y.; Lin, N.Q.; Chen, Y.M.; Liang, Z.B.; Liao, L.S.; Lv, M.F.; Chen, Y.F.; Tang, Y.X.; He, F.; Chen, S.H.; et al. Biocontrol of Sugarcane Smut Disease by Interference of Fungal Sexual Mating and Hyphal Growth Using a Bacterial Isolate. Front. Microbiol. 2017, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Z.; Zhang, B.; Chang, C.; Wang, Y.; Lu, S.; Sun, S.; Zhang, X.; Chen, B.; Jiang, Z. The MAP kinase SsKpp2 is required for mating/filamentation in Sporisorium scitamineum. Front. Microbiol. 2018, 9, 2555. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Sun, S.Q.; Deng, Y.Z.; Huang, P.S.; Sun, X.; Wang, Y.T.; Chang, C.Q.; Jiang, Z.D. Histidine Kinase Sln1 and cAMP/PKA Signaling Pathways Antagonistically Regulate Sporisorium scitamineum Mating and Virulence via Transcription Factor Prf1. J. Fungi 2021, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Deng, Y.; Cai, E.; Yan, M.; Cui, G.; Wang, Z.; Zou, C.; Zhang, B.; Xi, P.; Chang, C.; et al. Identification and functional analysis of the pheromone response factor gene of Sporisorium scitamineum. Front. Microbiol. 2019, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Deng, Y.; Cai, E.; Yan, M.; Li, L.; Chen, B.; Chang, C.; Jiang, Z. The farnesyltransferase β-subunit ram1 regulates Sporisorium scitamineum mating, pathogenicity and cell wall integrity. Front. Microbiol. 2019, 10, 976. [Google Scholar] [CrossRef]
- Mc Martin, A. Sugarcane smut: Reappearance in Natal. S. Afr. Sugar J. 1945, 29, 55–57. [Google Scholar]
- Sundar, A.R.; Barnabas, E.L.; Malathi, P.; Viswanathan, R. A mini-review on smut disease of sugarcane caused by Sporisorium scitamineum. In Botany; Mworia, J.K., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Yan, M.; Cai, E.; Zhou, J.; Chang, C.; Xi, P.; Shen, W.; Li, L.; Jiang, Z.; Deng, Y.; Zhang, L. A dual-color imaging system for sugarcane smut fungus Sporisorium scitamineum. Plant Dis. 2016, 100, 2357–2362. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Deng, Q.; Bao, H.; Chen, J.; Shen, W. Cytochrome P450 Sterol 14 Alpha-Demethylase Gene SsCI72380 Is Required for Mating/Filamentation and Pathogenicity in Sporisorium scitamineum. Front. Microbiol. 2021, 12, 696117. [Google Scholar] [CrossRef]
- Xu, L.; Que, Y.; Chen, R. Genetic diversity of Ustilago scitaminea in Mainland China. Sugar Tech 2004, 6, 267–271. [Google Scholar] [CrossRef]
- Shen, W.K.; Xu, G.H.; Luo, M.Z.; Jiang, Z.D. Genetic diversity of Sporisorium scitamineum in Mainland China assessed by SCoT analysis. Trop. Plant Pathol. 2016, 41, 288–296. [Google Scholar] [CrossRef]
- Grisham, M.P. An international project on genetic variability within sugarcane smut. Proc. Int. Soc. Sugar Cane Technol. 2001, 24, 459–461. [Google Scholar]
- Schenck, S.; Pearl, H.M.; Liu, Z.; Moore, P.H.; Ming, R. Genetic variation of Ustilago scitaminea pathotypes in Hawaii evaluated by host range and AFLP markers. Sugarcane Int. 2005, 23, 15–19. [Google Scholar]
- Da Silva, W.; Sanguino, A. Evaluating reaction of American cane varieties to Sporisorium scitamineum in Brazil. Sugarcane Pathol. Newsl. 1978, 21, 10–11. [Google Scholar] [CrossRef]
- Muhammad, S.; Kausar, A.G. Preliminary studies on the genetics of sugarcane smut, Ustilago scitaminea Sydow. Biologia 1962, 8, 64–74. [Google Scholar]
- Lee, C.S.; Yuan, C.H.; Liang, Y.G. Occurrence of a new pathogenic race of culmicolous smut of sugarcane in Taiwan. Proc. Aust. Soc. Sugar Cane Technol. 1999, 23, 406–407. [Google Scholar]
- Shen, W.; Xi, P.; Li, M.; Liu, R.; Sun, L.; Jiang, Z.; Zhang, L. Genetic diversity of Ustilago scitaminea Syd. in Southern China revealed by combined ISSR and RAPD analysis. Afr. J. Biotechnol. 2012, 11, 11693–11703. [Google Scholar] [CrossRef]
- Xu, G.H.; Deng, Q.Q.; Shen, W.K.; Chen, S.; Wu, X.M. Assessment of genetic diversity and structure of Sporisorium scitamineum from China using ISSR markers. Afr. J. Biotechnol. 2017, 16, 727–737. [Google Scholar] [CrossRef]
- Deng, Q.Q.; Xu, G.H.; Dou, Z.M.; Shen, W.K. Identification of three Sporisorium scitamineum pathogenic races in mainland China. Int. J. Agric. Biol. 2018, 20, 799–802. [Google Scholar] [CrossRef]
- Glassop, D.; Rae, A.L. Expression of sugarcane genes associated with perception of photoperiod and floral induction reveals cycling over a 24-hour period. Funct. Plant Biol. 2019, 46, 314–327. [Google Scholar] [CrossRef]
- Manechini, J.; Santos, P.; Romanel, E.; Brito, M.; Scarpari, M.; Jackson, S.; Pinto, L.; Vicentini, R. Transcriptomic Analysis of Changes in Gene Expression During Flowering Induction in Sugarcane Under Controlled Photoperiodic Conditions. Front. Plant Sci. 2021, 12, 635784. [Google Scholar] [CrossRef]
- Glassop, D.; Bonnett, G.; Croft, B.; Bhuiyan, S.; Aitken, K.; Rae, A. Flowering-related genes are not involved in the development of smut whip. Proc. Aust. Soc. Sugar Cane Technol. 2014, 36, 244–253. [Google Scholar]
- Mehareb, E.M.; Salem, E.S.R.; Ghonema, M.A. Temperature and relative humidity effects on sugarcane flowering under natural conditions in Egypt. Indian J. Sugarcane Technol. 2017, 32, 13–19. [Google Scholar]
- Abu-Ellail, F.F.B.; Mccord, P.H. Temperature and relative humidity effects on sugarcane flowering ability and pollen viability under natural and seminatural conditions. Sugar Tech 2019, 21, 83–92. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, G.; Lin, S.; Xian, X.; Chang, C.; Xi, P.; Shen, W.; Huang, W.; Cai, E.; Jiang, Z.; et al. The mating-type locus b of the sugarcane smut sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genet. Biol. 2015, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yan, M.; Jiang, J.; Zhang, Z.; Huang, J.; Zhang, L.; Deng, Y.; Zhou, X.; He, F. Mycophenolic Acid as a Promising Fungal Dimorphism Inhibitor to Control Sugarcane Disease Caused by Sporisorium scitamineum. J. Agric. Food Chem. 2019, 67, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.R.; Hoy, J.W.; Beatriz, A.D.G.; Viveros, A.F.G.; Vieira, M.L.C.; Baisakh, N. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef]
- Ling, H.; Fu, X.; Huang, N.; Zhong, Z.; Su, W.; Lin, W.; Cui, H.; Que, Y. A sugarcane smut fungus effector simulates the host endogenous elicitor peptide to suppress plant immunity. New Phytol. 2022, 233, 919–933. [Google Scholar] [CrossRef]
- Brefort, T.; Doehlemann, G.; Mendoza-mendoza, A.; Reissmann, S.; Djamel, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef]
- Ghareeb, H.; Becker, A.; Iven, T.; Feussner, I.; Schirawski, J. Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiol. 2011, 156, 2037–2052. [Google Scholar] [CrossRef]
- Chandra, A.; Huff, D.R. A fungal parasite regulates a putative female -suppressor gene homologous to maize tasselseed2 and causes inducedhermaphroditism in male buffalograss. Mol. Plant Microbe Interact. 2010, 23, 239–250. [Google Scholar] [CrossRef]
- Schaker, P.; Palhares, A.C.; Taniguti, L.M.; Peters, L.P.; Monteiro-Vitorello, C.B. Rnaseq transcriptional profiling following whip development in sugarcane smut disease. PLoS ONE 2016, 11, e0162237. [Google Scholar] [CrossRef]
- Eckardt, N.A. Dissecting cis-regulation of flowering locus t. Plant Cell 2010, 22, 1422–1422. [Google Scholar] [CrossRef]
- Hale, A.L.; White, P.M.; Webber, C.L.; Todd, J.R.; Fang, D.D. Effect of growing media and fertilization on sugarcane flowering under artificial photoperiod. PLoS ONE 2017, 12, e0181639. [Google Scholar] [CrossRef]

| Gene | Gene Name | Forward Primer Reverse Primer | Forward Primer Reverse Primer |
|---|---|---|---|
| ScAGL7 | AGAMOUS LIKE-7 | GACGGTTCAGGCTCAGATT | GCTTTAATGAACGCACACCTC |
| ScAGL12 | AGAMOUS LIKE-12 | GAGATGGGCTATTCCTTCTGAC | CTCCTGAAGGGCTATGGTTTAT |
| ScCDF2 | CYCLING DOF FACTOR 2 | CTGTGATGGTGCCAGGTAAA | GCACAAGTGGGTATGGAAATG |
| ScCDF3 | CYCLING DOF FACTOR 3 | TCAGGTTTCGACTGGAATGG | AAGGAGATGAGAAGGCAGAAAG |
| ScEID1 | EID1-like1 | TTCTGAGGACACAAAGGAAGAG | CAAAGAGAAAGGCAGCTAGGA |
| ScLHY | LATE ELONGATED HYPOCOTYL | GTGTCTCTCCACACAGAGTTAAA | TTGTCCGCATCTACATCACTAC |
| ScPRR1 | PSEUDO-RESPONSE REGULATOR 1 | CTCAAGCACATACACCACCA | ATGCCGATGACCACACATT |
| ScPRR5 | PSEUDO-RESPONSE REGULATOR 5 | ACAGAAGCAGAAACTGACTCG | CCTTCAGTCTTACCAGTCCAAT |
| ScPRR7 | PSEUDO-RESPONSE REGULATOR 7 | CAGTGGCAGTGGAAGTGAAA | CATTGAGTCCGACACTGAAGTC |
| CAB2 | CHLOROPHYLL a/b BINDING PROTEIN | TGATACATGCATCTGTGCTGCTT | TGGTAGGCCGGCGTGTAG |
| CCA1 | CIRCADIAN CLOCK ASSOCIATED 1 | ATGAGAAGGTGAAGCAAGCCT | TGCTTCTAAATCTGCGGTGGT |
| GI | GIGANTEA | ACATGCCGAAGGAGTTGAAG | GTGCAGTGGCATCGATAGTG |
| PHYB/Ma3 | PHYTOCHROME B/MATURITY GENE 3 | GCCTATATTTGCCAGGAGA | CTTGGACATCTGTTCCTCA |
| PP2C | PROTEIN PHOSPHATASE 2C | AGACAGCAGAGGTGGACATGAA | CGTCTTCTAGCCTCTGGAAACA |
| PS1 | PHOTOSYSTEM 1 | CCTGAAGGCCCCATCCAT | GGAGGGTCGTCTCCTTGTGA |
| LFY | Leafy | TCACCAGCTTCCTCGGTTTA | AACCCTCTTGCCAAATAGCC |
| FT-A | Flowering locus T (FT) homologue A | GACATGCGCACCTTCTACAC | CGAGCTGTTGGAAGAGCAG |
| Lg2 | Liguleless 2 | TTGCCAACTACACTGCTCTC | TCTGGTGCAACGTCTGCTG |
| PI | Pistillata | GCACAAGAGCCTTAGTGCAG | AGATCTTCACCTTTCAGGTGC |
| ScUBQ1 * | UBIQUITIN 1 | AGCCTCAGACCAGATTCCAA | AATCGCTGTCGAACTACTTGC |
| ScTUB * | TUBULIN | CTCCACATTCATCGGCAACTC | TCCTCCTCTTCTTCCTCCTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, L.; Huang, H.; Liao, L.; Duan, Z.; Zhang, X.; Wang, Z.; Lei, J.; Huang, W.; Chen, X.; Huang, D.; et al. Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum. Plants 2023, 12, 316. https://doi.org/10.3390/plants12020316
Shuai L, Huang H, Liao L, Duan Z, Zhang X, Wang Z, Lei J, Huang W, Chen X, Huang D, et al. Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum. Plants. 2023; 12(2):316. https://doi.org/10.3390/plants12020316
Chicago/Turabian StyleShuai, Liang, Hairong Huang, Lingyan Liao, Zhenhua Duan, Xiaoqiu Zhang, Zeping Wang, Jingchao Lei, Weihua Huang, Xiaohang Chen, Dongmei Huang, and et al. 2023. "Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum" Plants 12, no. 2: 316. https://doi.org/10.3390/plants12020316
APA StyleShuai, L., Huang, H., Liao, L., Duan, Z., Zhang, X., Wang, Z., Lei, J., Huang, W., Chen, X., Huang, D., Li, Q., Song, X., & Yan, M. (2023). Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum. Plants, 12(2), 316. https://doi.org/10.3390/plants12020316






