Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species
Abstract
1. Introduction
2. Results
2.1. Substrate Electrical Conductivity
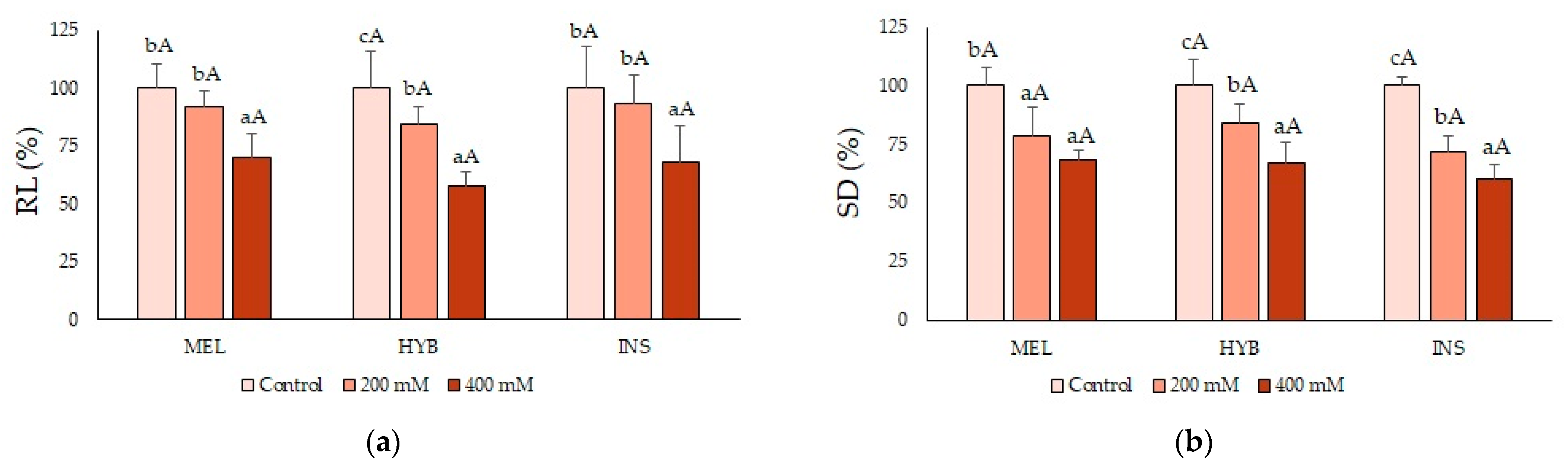
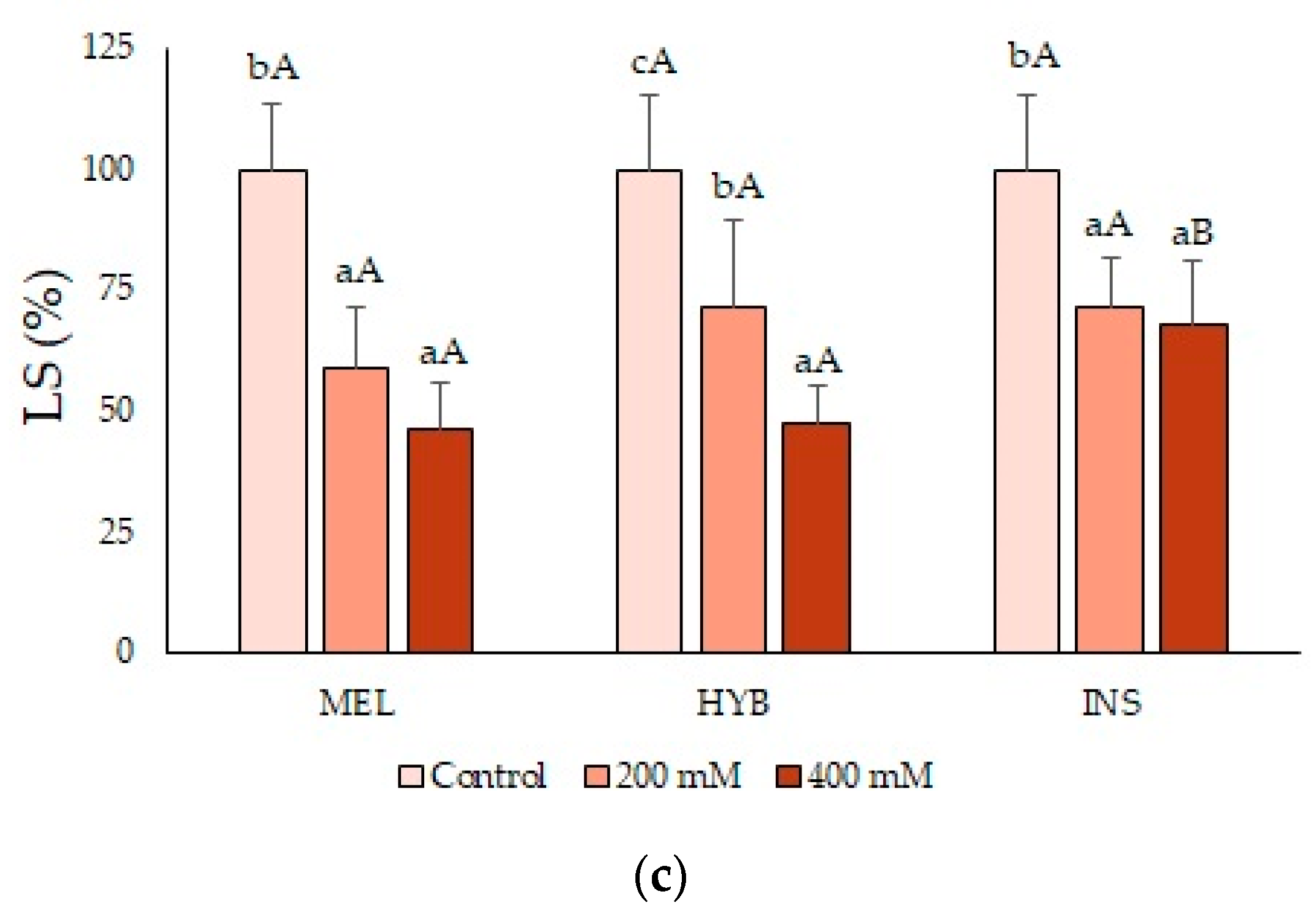
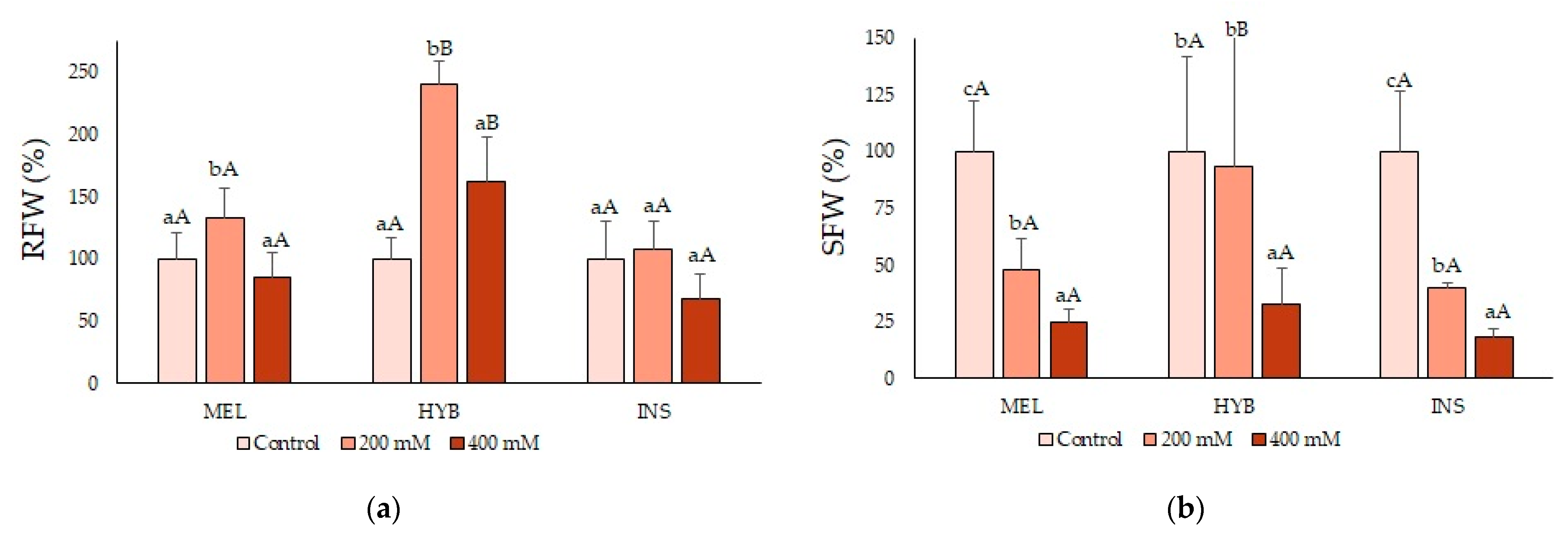
2.2. Young Plant Growth Parameters
2.3. Photosynthetic Pigments
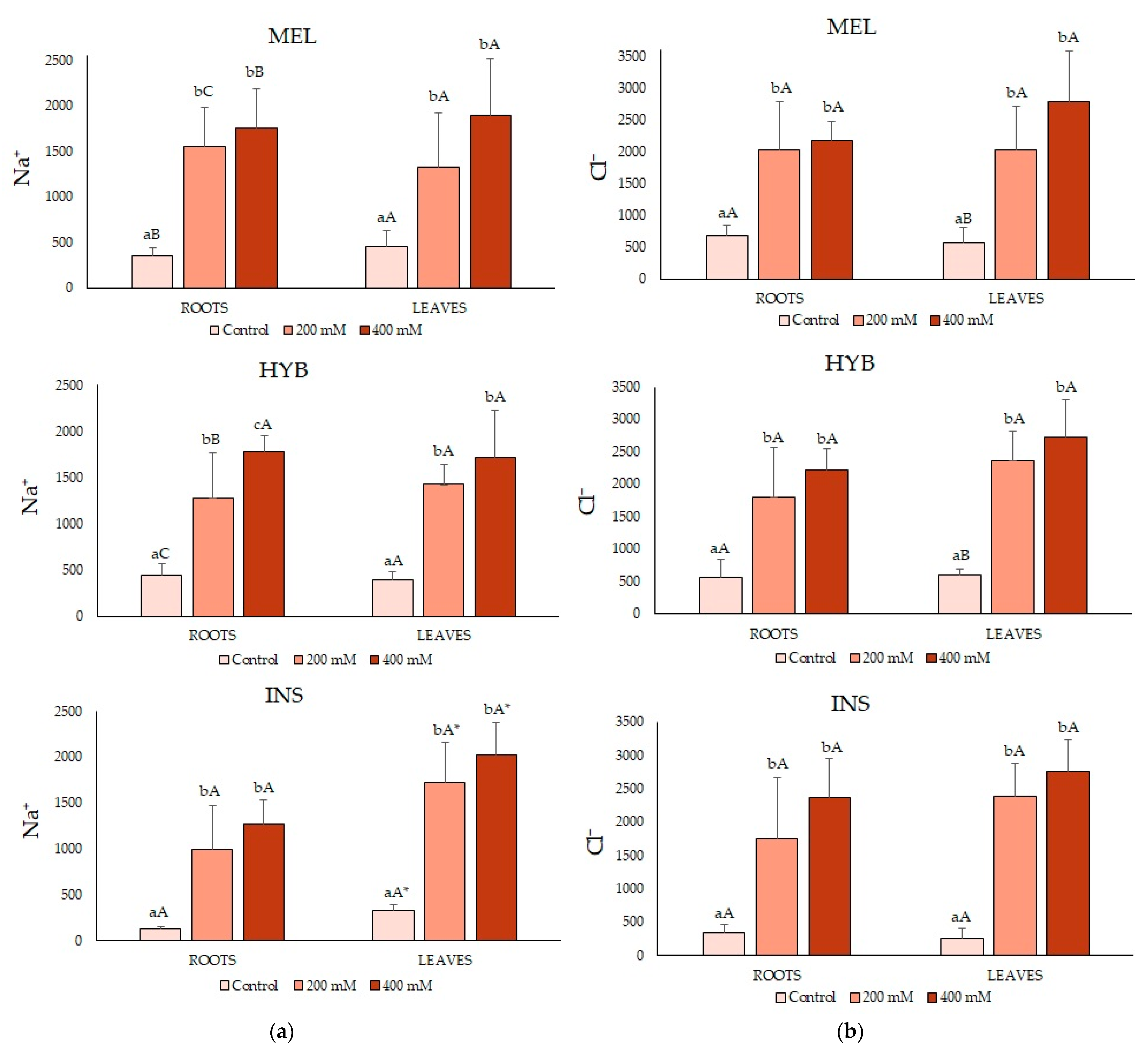
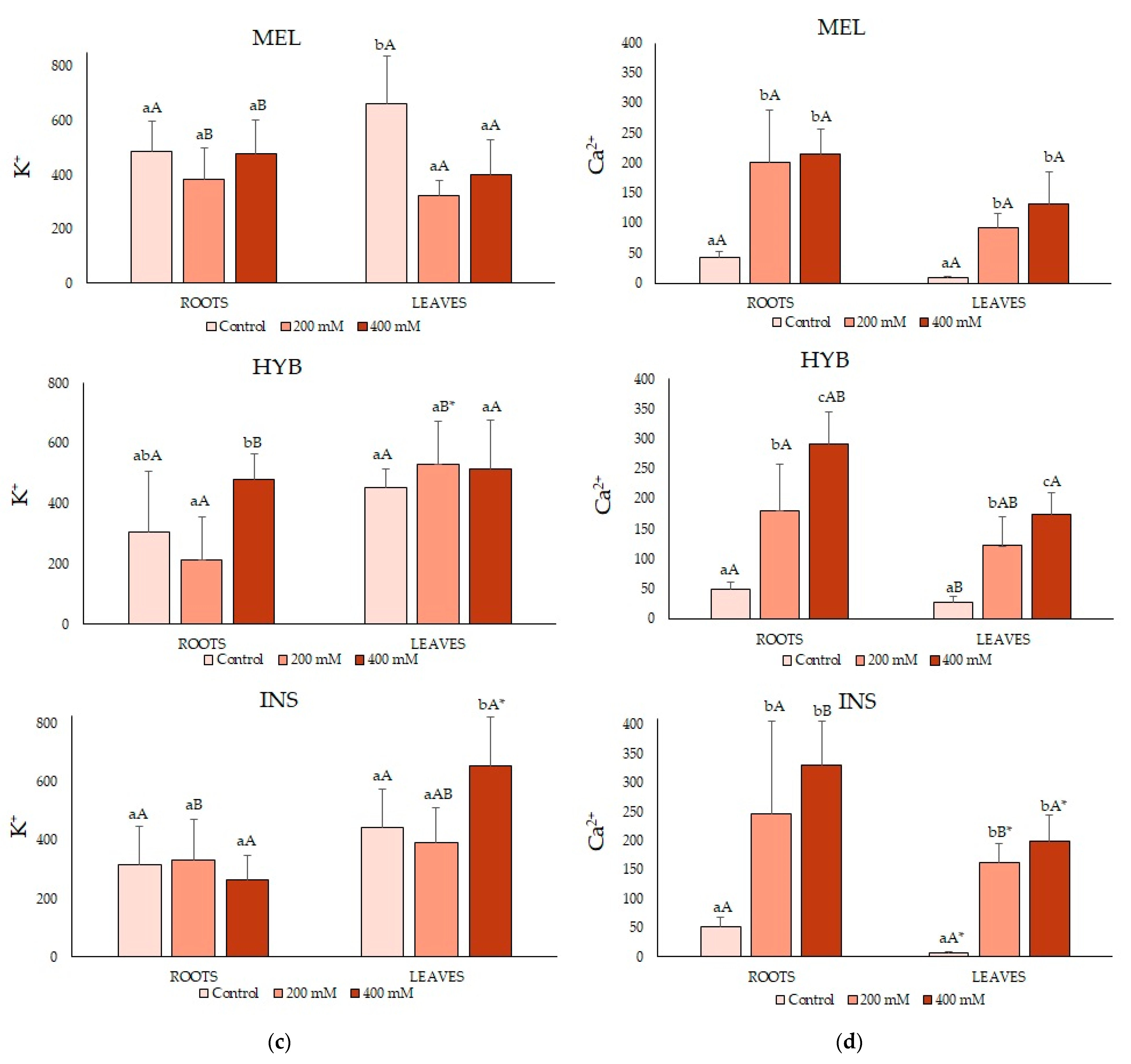
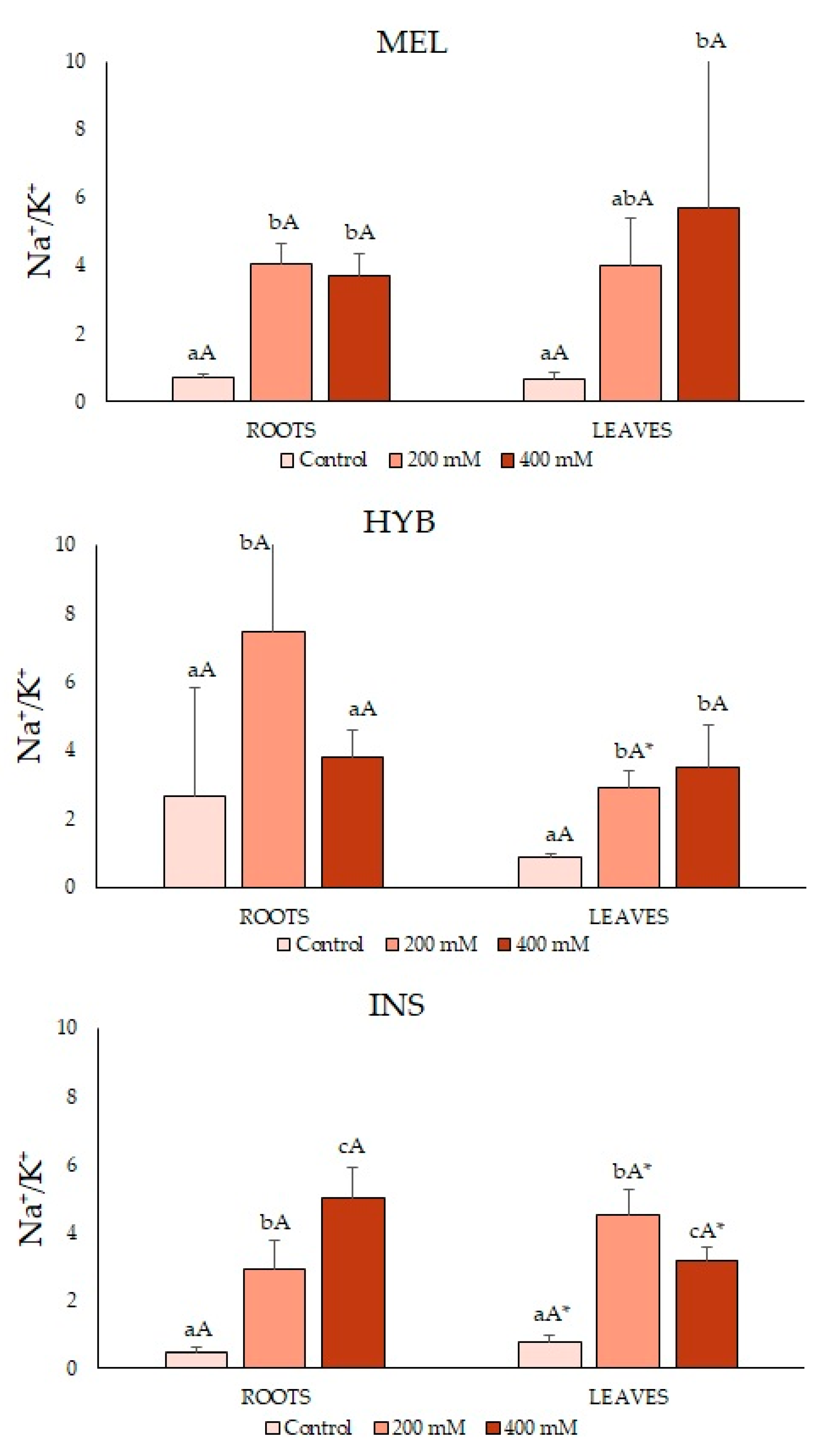
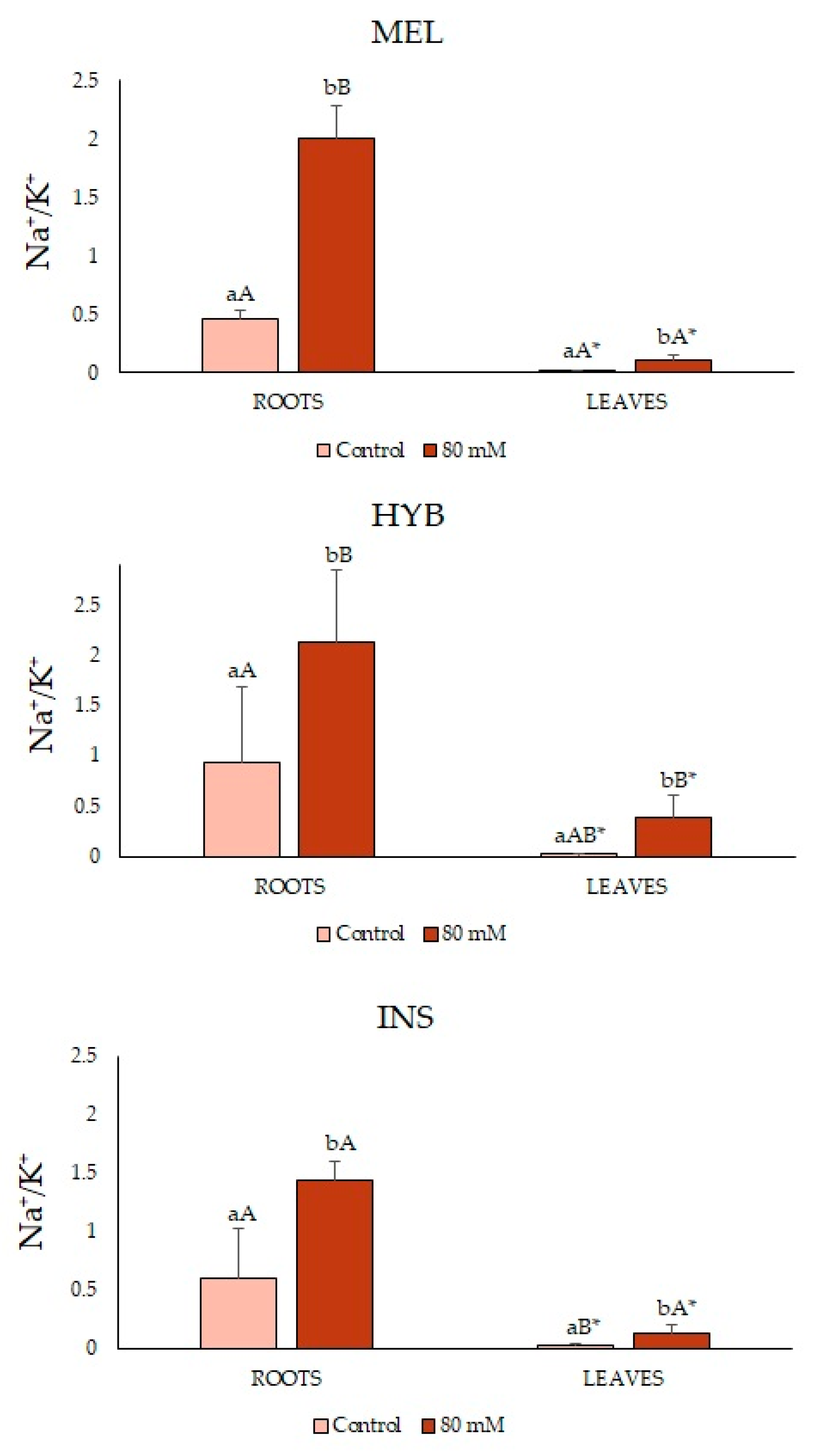
2.4. Ion Contents
2.5. Osmolyte Quantification, MDA, H2O2 and Antioxidant Compounds
2.6. Antioxidant Enzymes
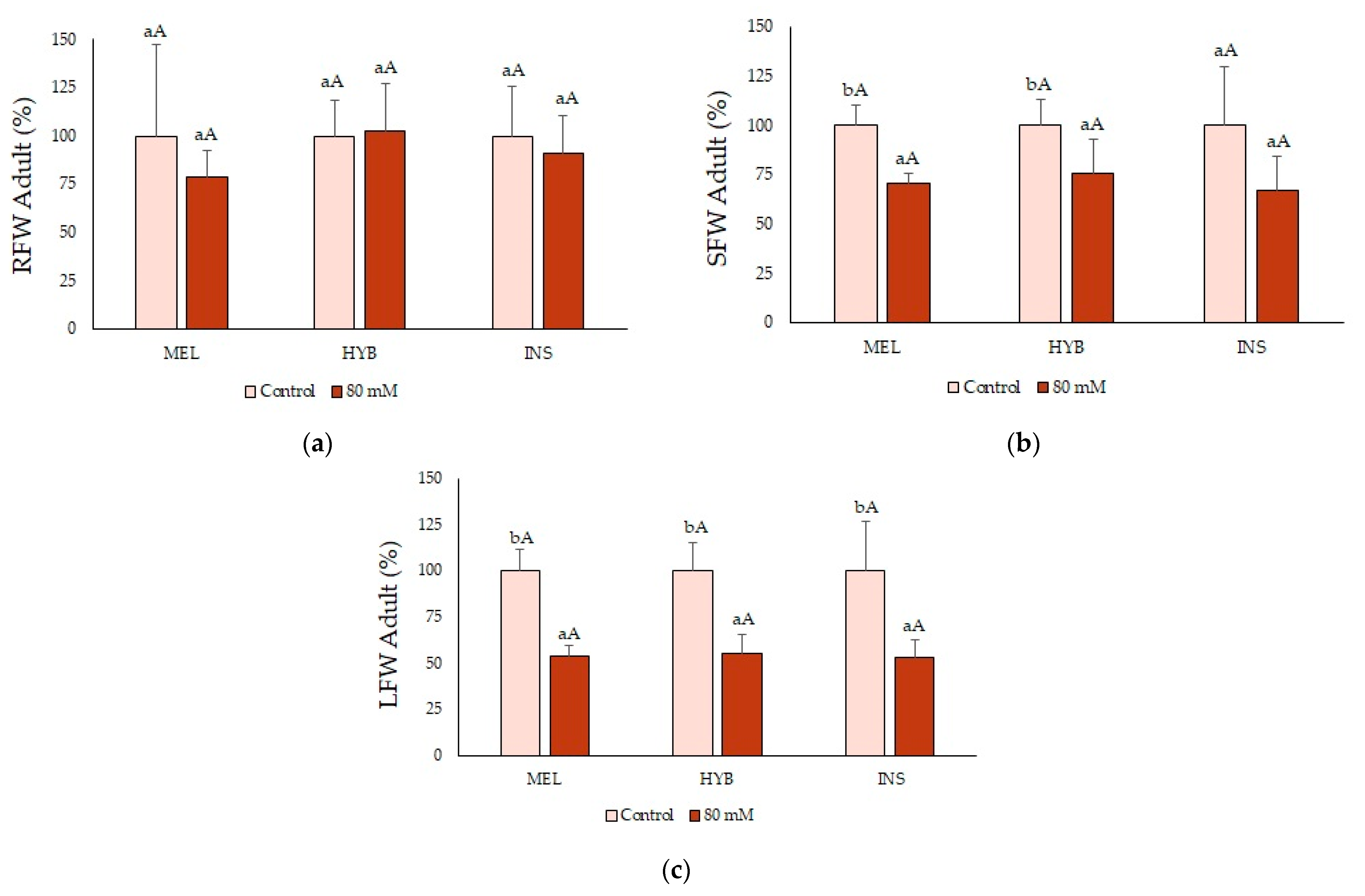
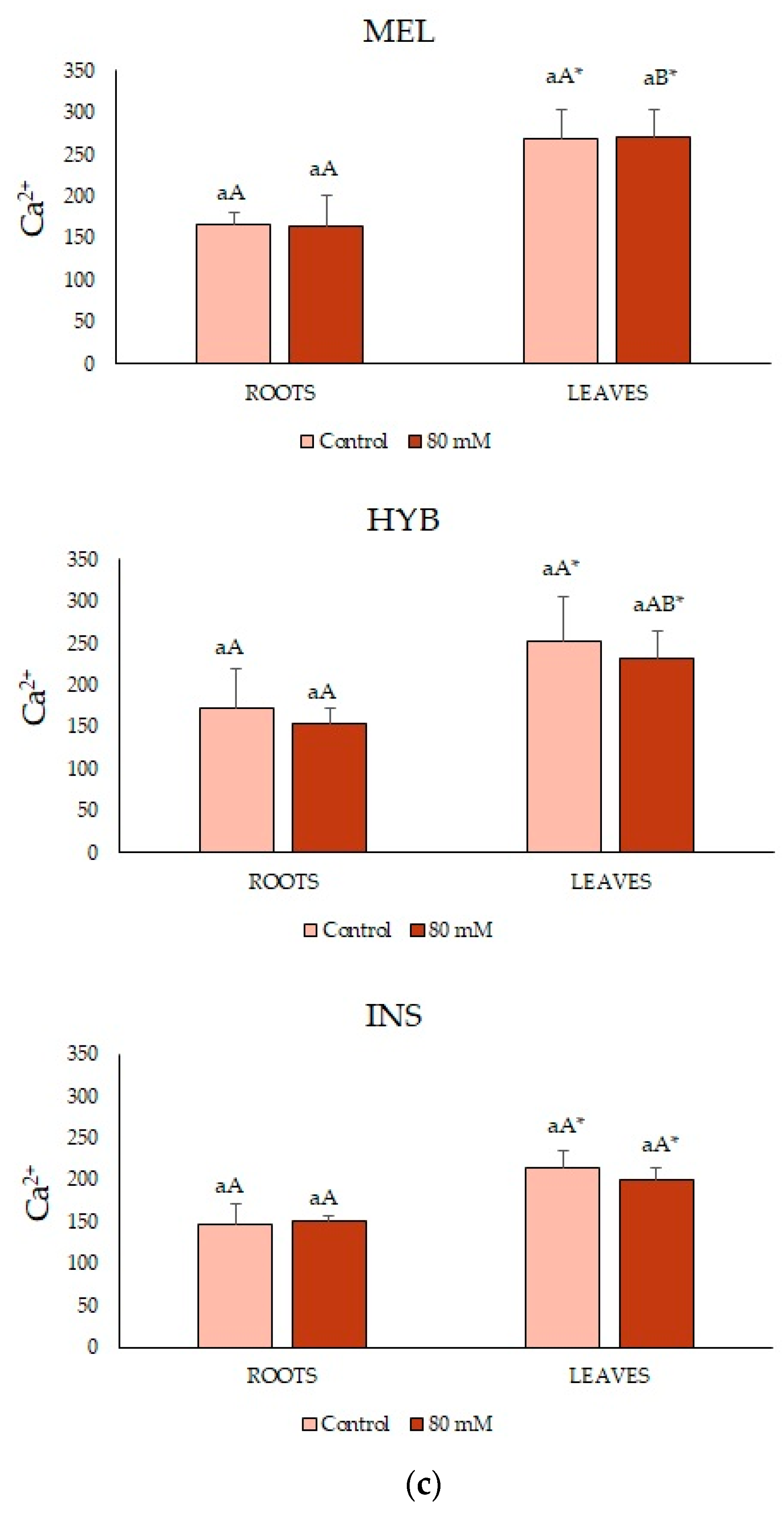
2.7. Adult Plant Performance
3. Discussion
4. Materials and Methods
4.1. Plant Material, Seed Germination and Plant Growth
4.2. Salt Treatments
4.3. Substrate Electrical Conductivity
4.4. Photosynthetic Pigments
4.5. Ion Content Measurement
4.6. Osmolyte Quantification
4.7. MDA, H2O2 and Antioxidant Compounds
4.8. Antioxidant Enzymes Activities
4.9. Adult Plant Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization Corporate Statistical Database. Available online: https://www.fao.org/statistics/es/ (accessed on 15 April 2022).
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–53. ISBN 978-3-319-96189-7. [Google Scholar]
- Munns, R. Genes and Salt Tolerance: Bringing Them Together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, Salinity and Drought Stresses: An Overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The Global Technical and Economic Potential of Bioenergy from Salt-Affected Soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The Threat of Soil Salinity: A European Scale Review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Hesami, A.; Bazdar, L.; Shahriari, M.H. Effect of Soil Salinity on Growth, Proline, and Some Nutrient Accumulation in Two Genotypes Seedlings of Ziziphus spina-christi (L.) Willd. Commun. Soil Sci. Plant Anal. 2020, 51, 804–815. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceutical and Functional Value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global Plant-Responding Mechanisms to Salt Stress: Physiological and Molecular Levels and Implications in Biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-Tolerant Genes from Halophytes Are Potential Key Players of Salt Tolerance in Glycophytes; Elsevier: Amsterdam, The Netherlands, 2016; Volume 124, ISBN 0114802411244. [Google Scholar]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The Mechanism of Salt Tolerance in Halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, M.; Kim, S.R.; Ryu, H.; Cho, Y.G. Insights into Genomics of Salt Stress Response in Rice. Rice 2013, 6, 27. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium Transport and Plant Salt Tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genomics 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Murata, N. Revised Scheme for the Mechanism of Photoinhibition and Its Application to Enhance the Abiotic Stress Tolerance of the Photosynthetic Machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, Metabolomics, and Ionomics Perspectives of Salinity Tolerance in Halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of Five Mediterranean Halophytes to Seasonal Changes in Environmental Conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Salinity and Programmed Cell Death: Unravelling Mechanisms for Ion Specific Signalling. J. Exp. Bot. 2009, 60, 709–712. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J.M. Physiological Roles of Nonselective Cation Channels in Plants: From Salt Stress to Signalling and Development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signaling Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defence in Halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Ünlükara, A.; Kurunç, A.; Kesmez, G.D.; Yurtseven, E.; Suarez, D.L. Effects of Salinity on Eggplant (Solanum melongena L.) Growth and Evapotranspiration. Irrig. Drain. 2010, 59, 203–214. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodríguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific Hybridization between Eggplant and Wild Relatives from Different Genepools. J. Am. Soc. Hortic. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Á.; Prohens, J.; Plazas, M. Physiological and Biochemical Responses to Salt Stress in Cultivated Eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy 2020, 10, 651. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na + Compartmentalization Related to Salinity Stress Tolerance in Upland Cotton (Gossypium hirsutum) Seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in Plants: Perception, Signalling, and Regulation of Sodium Fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Kouassi, B.; Prohens, J.; Gramazio, P.; Kouassi, A.B.; Vilanova, S.; Galán-Ávila, A.; Herraiz, F.J.; Kouassi, A.; Seguí-Simarro, J.M.; Plazas, M. Development of Backcross Generations and New Interspecific Hybrid Combinations for Introgression Breeding in Eggplant (Solanum melongena). Sci. Hortic. 2016, 213, 199–207. [Google Scholar] [CrossRef]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Díez, M.J.; Fita, A.; Herraiz, F.J.; Rodríguez-Burruezo, A.; Soler, S.; et al. Introgressiomics: A New Approach for Using Crop Wild Relatives in Breeding for Adaptation to Climate Change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Mangino, G.; Plazas, M.; Vilanova, S.; Prohens, J.; Gramazio, P. Performance of a Set of Eggplant (Solanum melongena) Lines with Introgressions from Its Wild Relative S. Incanum under Open Field and Screenhouse Conditions and Detection of QTLs. Agronomy 2020, 10, 467. [Google Scholar] [CrossRef]
- Zayova, E.; Philipov, P.; Nedev, T.; Stoeva, D. Response of in vitro cultivated eggplant (Solanum melongena L.) to salt and drought stress. AgroLife Sci. J. 2017, 6, 276–282. [Google Scholar]
- Hannachi, S.; Van Labeke, M.C. Salt Stress Affects Germination, Seedling Growth and Physiological Responses Differentially in Eggplant Cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Mustafa, Z.; Ayyub, C.M.; Amjad, M.; Ahmad, R. Assesment of Biochemical and Ionic Attributes against Salt Stress in Eggplant (Solanum melongena L.) Genotypes. J. Anim. Plant Sci. 2017, 27, 503–509. [Google Scholar]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative Analysis of the Responses to Water Stress in Eggplant (Solanum melongena) Cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef]
- Brenes, M.; Pérez, J.; González-Orenga, S.; Solana, A.; Boscaiu, M.; Prohens, J.; Plazas, M.; Fita, A.; Vicente, O. Comparative Studies on the Physiological and Biochemical Responses to Salt Stress of Eggplant (Solanum melongena) and Its Rootstock s. Torvum. Agriculture 2020, 10, 328. [Google Scholar] [CrossRef]
- Ünlükara, A.; Kurunç, A.; Kesmez, G.D.; Yurtseven, E. Growth and Evapotranspiration of Okra (Abelmoschus esculentus L.) as Influenced by Salinity of Irrigation Water. J. Irrig. Drain. Eng. 2008, 134, 160–166. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Li, X. Auxin Redistribution Modulates Plastic Development of Root System Architecture under Salt Stress in Arabidopsis Thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef]
- Zolla, G.; Heimer, Y.M.; Barak, S. Mild Salinity Stimulates a Stress-Induced Morphogenic Response in Arabidopsis Thaliana Roots. J. Exp. Bot. 2010, 61, 211–224. [Google Scholar] [CrossRef]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root Development in Horticultural Plants Grown under Abiotic Stress Conditions—A Review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; De Boer, G.J.; et al. Genetic Components of Root Architecture Remodeling in Response to Salt Stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef] [PubMed]
- Botet, R.; Keurentjes, J.J.B. The Role of Transcriptional Regulation in Hybrid Vigor. Front. Plant Sci. 2020, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, J.P.; Stelkens, R.B.; Greig, D. Heterosis in Hybrids within and between Yeast Species. J. Evol. Biol. 2017, 30, 538–548. [Google Scholar] [CrossRef]
- Kutka, F. Open-Pollinated vs. Hybrid Maize Cultivars. Sustainability 2011, 3, 1531–1554. [Google Scholar] [CrossRef]
- Cheng, S.H.; Cao, L.Y.; Zhuang, J.Y.; Chen, S.G.; Zhan, X.D.; Fan, Y.Y.; Zhu, D.F.; Min, S.K. Super Hybrid Rice Breeding in China: Achievements and Prospects. J. Integr. Plant Biol. 2007, 49, 805–810. [Google Scholar] [CrossRef]
- Krieger, U.; Lippman, Z.B.; Zamir, D. The Flowering Gene single flower truss Drives Heterosis for Yield in Tomato. Nat. Genet. 2010, 42, 459–463. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Jain, B.T.; Kaushik, P. Heterosis Breeding in Eggplant (Solanum melongena L). Plants 2020, 9, 403. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of Salt Stress on Basic Processes of Photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of Leaf Gas Exchange Attributes, Photosynthetic Pigments and Antioxidant Enzymes in NaCl-Stressed Cotton (Gossypium hirsutum L.) Seedlings to Exogenous Glycine Betaine and Salicylic Acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef]
- Shaheen, S.; Naseer, S.; Ashraf, M.; Akram, N.A. Salt Stress Affects Water Relations, Photosynthesis, and Oxidative Defense Mechanisms in Solanum melongena L. J. Plant Interact. 2013, 8, 85–96. [Google Scholar] [CrossRef]
- Shahbaz, M.; Mushtaq, Z.; Andaz, F.; Masood, A. Does Proline Application Ameliorate Adverse Effects of Salt Stress on Growth, Ions and Photosynthetic Ability of Eggplant (Solanum melongena L.)? Sci. Hortic. 2013, 164, 507–511. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of Ion Homeostasis under Salt Stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Akram, N.A. Improving Salinity Tolerance of Plants through Conventional Breeding and Genetic Engineering: An Analytical Comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.; Zhou, L.; Li, L.; Zhang, J.; Liu, Y.; Chen, H. Comparative Transcriptome Analysis Reveals K + Transporter Gene Contributing to Salt Tolerance in Eggplant. BMC Plant Biol. 2019, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and Salt-Stress Signaling in Plants: Shedding Light on SOS Pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Ábrahám, E.; Rigó, G.; Székely, G.; Nagy, R.; Koncz, C.; Szabados, L. Light-Dependent Induction of Proline Biosynthesis by Abscisic Acid and Salt Stress Is Inhibited by Brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Al Hassan, M.; López-Gresa, M.D.P.; Boscaiu, M.; Vicente, O. Stress Tolerance Mechanisms in Juncus: Responses to Salinity and Drought in Three Juncus Species Adapted to Different Natural Environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Rahat, N., Khan, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. ISBN 978-81-322-2615-4. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Pipatsitee, P.; Theerawitaya, C.; Tiasarum, R.; Samphumphuang, T.; Singh, H.P.; Datta, A.; Cha-um, S. Physio-Morphological Traits and Osmoregulation Strategies of Hybrid Maize (Zea Mays) at the Seedling Stage in Response to Water-Deficit Stress. Protoplasma 2022, 259, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Gouiaa, S.; Khoudi, H.; Leidi, E.O.; Pardo, J.M.; Masmoudi, K. Expression of Wheat Na +/H + Antiporter TNHXS1 and H +- Pyrophosphatase TVP1 Genes in Tobacco from a Bicistronic Transcriptional Unit Improves Salt Tolerance. Plant Mol. Biol. 2012, 79, 137–155. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.; Di Marco, G.; Bruno, L.; Gismondi, A.; Canini, A. Investigating the Drought and Salinity Effect on the Redox Components of Sulla coronaria (L.) Medik. Antioxidants 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.N.; Shakeel, A.; Lei, S.; et al. Characterization of SOD and GPX Gene Families in the Soybeans in Response to Drought and Salinity Stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Birhanie, Z.M.; Yang, D.; Luan, M.; Xiao, A.; Liu, L.; Zhang, C.; Biswas, A.; Dey, S.; Deng, Y.; Li, D. Salt Stress Induces Changes in Physiological Characteristics, Bioactive Constituents, and Antioxidants in Kenaf (Hibiscus cannabinus L.). Antioxidants 2022, 11, 2005. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Naranjo, M.Á.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to Salt Stress in the Halophyte Plantago Crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Ragab, K.; Taha, N. Evaluation of Nine Egyptian Bread Wheat Cultivars for Salt Tolerance At Seedling and Adult-Plant Stages. J. Plant Prod. 2016, 7, 147–159. [Google Scholar] [CrossRef]
- Johnson, D.W.; Smith, S.E.; Dobrenz, A.K. Genetic and Phenotypic Relationships in Response to NaCl at Different Developmental Stages in Alfalfa. Theor. Appl. Genet. 1992, 83, 833–838. [Google Scholar] [CrossRef]
- Ranil, R.H.G.; Niran, H.M.L.; Plazas, M.; Fonseka, R.M.; Fonseka, H.H.; Vilanova, S.; Andújar, I.; Gramazio, P.; Fita, A.; Prohens, J. Improving Seed Germination of the Eggplant Rootstock Solanum Torvum by Testing Multiple Factors Using an Orthogonal Array Design. Sci. Hortic. 2015, 193, 174–181. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Yangen, F.; Xiuxiu, Z.; Hanyue, W.; Yueyue, T.; Qinzeng, X.; Lixia, Z. Effects of Light Intensity on Metabolism of Light-Harvesting Pigment and Photosynthetic System in Camellia Sinensis L. Cultivar ‘Huangjinya’. Environ. Exp. Bot. 2019, 166, 103796. [Google Scholar] [CrossRef]
- Weimberg, R. Solute Adjustments in Leaves of Two Species of Wheat at Two Different Stages of Growth in Response to Salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Osmotic Stress-Induced Changes in Germination, Growth and Soluble Sugar Content of Sorghum bicolor (L.) Moench Seeds. Bulg. J. Plant Physiol. 2002, 40, 157–162. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.K.; Taulavuori, K.; Laine, K. Comparison of Two Methods Used to Analyse Lipid Peroxidation from Vaccinium myrtillus (L.) during Snow Removal, Reacclimation and Cold Acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Portein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pedrol, N.; Ramos, P. Protein Content Quantification By Bradford Method. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 283–295. [Google Scholar]
- Beyer, W.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.S.; Lee, C.B. The Inductive Responses of the Antioxidant Enzymes by Salt Stress in the Rice (Oryza sativa L.). J. Plant Physiol. 2001, 158, 737–745. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinache Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Neto, A.D.D.A.; Prisco, J.T.; Enéas-Filho, J.; Rolim Medeiros, J.V.; Gomes-Filho, E. Hydrogen Peroxide Pre-Treatment Induces Salt-Stress Acclimation in Maize Plants. J. Plant Physiol. 2005, 162, 1114–1122. [Google Scholar] [CrossRef]
- Connell, J.P.; Mullet, J.E. Pea Chloroplast Glutathione Reductase: Purification and Characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef]
- Madhu; Kaur, A.; Tyagi, S.; Shumayla; Singh, K.; Upadhyay, S.K. Exploration of Glutathione Reductase for Abiotic Stress Response in Bread Wheat (Triticum aestivum L.). Plant Cell Rep. 2022, 41, 639–654. [Google Scholar] [CrossRef]





| Treatment (NaCl Concentrations) | ||||
|---|---|---|---|---|
| Control | 200 mM | 400 mM | ||
| EC1:5 | MEL | 5.2 ± 1.6 aA | 21.8 ± 3.2 bA | 32.2 ± 4.1 cA |
| HYB | 5.2 ± 2.001 aA | 26.4 ± 3.2 bA | 31 ± 5.9 bA | |
| INS | 4.4 ± 1.9 aA | 22.1 ± 2.9 bA | 31.8 ± 3.9 cA | |
| Treatment (NaCl Concentrations) | ||||
|---|---|---|---|---|
| Control | 200 mM | 400 mM | ||
| RWC | MEL | 77.6 ± 1.4 aA | 84.2 ± 1.2 bB | 83.5 ± 0.9 bB |
| HYB | 75.4 ± 1.6 aA | 83.1 ± 0.5 bB | 83.6 ± 0.4 bB | |
| INS | 77.5 ± 1.8 aA | 78.3 ± 0.4 aA | 78.2 ± 0.3 aA | |
| SWC | MEL | 90.1 ± 4.5 bA | 84.3 ± 1.7 aA | 83.5 ± 0.9 aA |
| HYB | 79.9 ± 11.3 aA | 77.3 ± 26.2 aA | 75.1 ± 18.9 aA | |
| INS | 78.7 ± 3.02 bA | 76.2 ± 1.8 bA | 71.1 ± 0.9 aA | |
| LWC | MEL | 81.8 ± 5.1 aA | 86.1 ± 2.4 aA | 85.6 ± 1.4 aB |
| HYB | 87.9 ± 6.3 aA | 85.9 ± 0.9 aA | 83.6 ± 0.4 aB | |
| INS | 82.6 ± 3.2 aA | 83.8 ± 1.3 aA | 81.2 ± 1.5 aA | |
| Treatment (NaCl Concentrations) | ||||
|---|---|---|---|---|
| Control | 200 mM | 400 mM | ||
| ChlA | MEL | 2.8 ± 0.8 aA | 3.1 ± 1.7 aA | 2.2 ± 0.9 aA |
| HYB | 2.7 ± 1.3 aA | 2.7 ± 1.4 aA | 4.4 ± 1.9 aB | |
| INS | 1.5 ± 0.9 aA | 2.9 ± 1.9 aA | 1.8 ± 1.1 aA | |
| ChlB | MEL | 0.9 ± 1.4 aA | 1.6 ± 1.3 aA | 0.8 ± 0.9 aA |
| HYB | 0.5 ± 0.4 aA | 0.3 ± 0.1 aA | 0.7 ± 0.5 aA | |
| INS | 1.0 ± 1.1 aA | 0.9 ± 0.3 aA | 0.4 ± 0.2 aA | |
| Caro | MEL | 0.8 ± 0.4 aA | 0.9 ± 0.4 aA | 0.6 ± 0.4 aA |
| HYB | 1.0 ± 0.3 aA | 0.6 ± 0.2 aA | 0.8 ± 0.3 aA | |
| INS | 0.9 ± 0.3 bA | 1.0 ± 0.2 bA | 0.5 ± 0.2 aA | |
| Treatment (NaCl Concentrations) | ||||
|---|---|---|---|---|
| Control | 200 mM | 400 mM | ||
| Pro | MEL | 11.9 ± 1.4 aA | 85.7 ± 13.9 bA | 134.1 ± 22.6 cA |
| HYB | 107.8 ± 42.4 aB | 2155.9 ± 258.9 bB | 3217.5 ± 697.9 bB | |
| INS | 27.3 ± 35.5 aA | 2233.5 ± 265.5 bB | 3717.1 ± 318.1 cB | |
| TSS | MEL | 160.2 ± 39.7 aA | 133.0 ± 63.7 aA | 224.1 ± 60.6 aA |
| HYB | 205.8 ± 69.9 aA | 180.9 ± 50.1 aA | 209.9 ± 32.8 aA | |
| INS | 227.4 ± 120.0 aA | 144.6 ± 78.9 aA | 187.9 ± 31.4 aA | |
| MDA | MEL | 623.7 ± 277.5 aA | 544.4 ± 119.8 aA | 493.9 ± 77.3 aA |
| HYB | 658.3 ± 111.6 aA | 437.2 ± 147.5 aA | 443.3 ± 162.0 aA | |
| INS | 569.1 ± 323.2 aA | 597.3 ± 131.9 aA | 477.9 ± 171.3 aA | |
| H2O2 | MEL | 6.6 ± 0.9 aA | 5.6 ± 1.8 aA | 11.4 ± 7.33 aAB |
| HYB | 5.5 ± 1.6 aA | 5.1 ± 2.8 aA | 9.4 ± 4.7 aA | |
| INS | 28.5 ± 6.7 aB | 21.3 ± 5.5 aB | 50.8 ± 11.8 bB | |
| TPC | MEL | 23.3 ± 8.6 aA | 26.7 ± 9.6 aA | 36.2 ± 11.4 aA |
| HYB | 37.8 ± 11.6 aB | 25.2 ± 3.3 aA | 32.5 ± 14.2 aA | |
| INS | 31.2 ± 6.5 aAB | 34.5 ±4.6 aA | 41.3 ± 11.3 aA | |
| MEL | 4.6 ± 3.5 aA | 7.8 ± 4.4 aA | 11.8 ± 6.6 aA | |
| TF | HYB | 11.9 ± 4.2 aB | 11.3 ± 2.8 aAB | 9.7 ± 5.5 aA |
| INS | 12.3 ± 1.5 aB | 15.2 ± 2.7 aB | 16.6 ± 5.1 aA | |
| Treatment (NaCl Concentrations) | ||||
|---|---|---|---|---|
| Control | 200 mM | 400 mM | ||
| APx | MEL | 48.9 ± 29.2 aA | 89.9 ± 58.2 aB | 87.4 ± 28.2 aA |
| HYB | 98.5 ± 65.3 aA | 124.5 ± 20.7 aB | 267.2 ± 161.8 aA | |
| INS | 62.7 ± 64.0 aA | 16.7 ± 11.2 aA | 83.0 ± 28.6 aA | |
| SOD | MEL | 90.2 ± 47.3 aA | 84.3 ± 34.9 aA | 89.2 ± 35.3 aAB |
| HYB | 144.2 ± 55.0 aB | 92.3 ± 24.8 aA | 128.3 ± 34.4 aB | |
| INS | 61.9 ± 20.1 aA | 56.3 ± 13.1 aA | 55.2 ± 21.7 aA | |
| GR | MEL | 948.1 ± 203.5 aA | 703.7 ± 196.8 aAB | 638.4 ± 68.6 aA |
| HYB | 1018.9 ± 363.5 aA | 863.9 ± 369.0 aB | 1175.6 ± 438.7 aA | |
| INS | 349.1 ± 172.7 aA | 313.6 ± 157.0 aA | 359.7 ± 232.6 aA | |
| Treatment (NaCl Concentrations) | |||
|---|---|---|---|
| Control | 80 mM | ||
| Pro | MEL | 13.6 ± 3.7 aA | 125.1 ± 54.1 bB |
| HYB | 9.8 ± 6.1 aA | 46.5 ± 22.8 bA | |
| INS | 6.7 ± 4.8 aA | 18.6 ± 18.8 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Albero, N.; González-Orenga, S.; Vicente, O.; Rodríguez-Burruezo, A.; Fita, A. Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants 2023, 12, 295. https://doi.org/10.3390/plants12020295
Ortega-Albero N, González-Orenga S, Vicente O, Rodríguez-Burruezo A, Fita A. Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants. 2023; 12(2):295. https://doi.org/10.3390/plants12020295
Chicago/Turabian StyleOrtega-Albero, Neus, Sara González-Orenga, Oscar Vicente, Adrián Rodríguez-Burruezo, and Ana Fita. 2023. "Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species" Plants 12, no. 2: 295. https://doi.org/10.3390/plants12020295
APA StyleOrtega-Albero, N., González-Orenga, S., Vicente, O., Rodríguez-Burruezo, A., & Fita, A. (2023). Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants, 12(2), 295. https://doi.org/10.3390/plants12020295










