Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Molecular Structures and Software
4.2. In Silico Molecular Docking Procedure
- (1)
- Monte Carlo (MC) conformational search of the ligand using the BOSS (Biochemical and Organic Simulation System) software, freely available to academic users. The structure of the ligand was optimized using a classical MC conformational search procedure, as described in BOSS [52]. A conformational analysis was performed to define the best starting geometries for each compound. An energy minimization was carried out to identify all minimum-energy conformers, leading to the identification of a unique conformer for the free ligand. Within BOSS, MC simulations were performed in the constant-temperature and constant-pressure ensemble (NPT).
- (2)
- Evaluation of the free energy of hydration for the chosen structure of the ligand. The molecular mechanics/generalized Born surface area (MM/GBSA) procedure was used to evaluate the free energies of hydration (ΔG) (Jorgensen and Tirado-Rives, 2005) [53]. MC search and computation of ΔG were performed within BOSS using the xMCGB script according to procedures given in references [53,54]. The best ligand structure was then used in the docking procedure.
- (3)
- Definition of the α-tubulin-ligand site of interaction. The pironetin binding site was defined as the binding site for all α-pyrone derivatives tested. With the 5FNV structure, based on shape complementarity criteria, the flexible amino acids are Phe135, Phe202, Leu248, Leu252, Phe255, Gln256, Leu259, Cys316, Lys352, and Leu378. Shape complementarity and geometry considerations favor a docking grid centered in the volume defined by the central amino acid. Within the binding site, the side chains of the specific amino acids were considered fully flexible during docking.
- (4)
- Docking procedure using GOLD. In our typical docking process, 100 energetically reasonable poses (according to the ChemPLP scoring function) are retained while searching for the correct binding mode of the ligand. The decision to maintain a trial pose is based on ranked poses, using the PLP fitness scoring function (which is the default in GOLD version 5.3 used here) [55]. Six poses are kept. The empirical potential energy of the interaction ΔE for the ranked complexes was evaluated using the simple expression ΔE(interaction) = E(complex) − [E(protein) + E(ligand)]. Calculations of the final energy are performed on the basis of the SPASIBA spectroscopic force field. The corresponding parameters are derived from vibrational wavenumbers obtained in the infrared and Raman spectra of a large series of compounds including organic molecules, amino acids, saccharides, nucleic acids and lipids.
- (5)
- Validation using the SPASIBA force field. This last step is considered essential to define the best protein–ligand structure. The spectroscopic SPASIBA (Spectroscopic Potential Algorithm for Simulating Biomolecular conformational Adaptability) force field has been specifically developed to provide refined empirical molecular mechanics force field parameters [56]. SPASIBA empirical energies of interaction are calculated as described [57,58]. SPASIBA (integrated into CHARMM) [59] has been shown to be excellent in reproducing crystal phase infrared data. The same procedure was used to establish molecular models for the various drug–protein complexes.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Lu, L.; Song, X.; Qi, J.; Wang, J. Combination of microtubule targeting agents with other antineoplastics for cancer treatment. Biochim. Biophys. Acta Rev. Cancer. 2022, 1877, 188777. [Google Scholar] [CrossRef] [PubMed]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B. Drugs That Changed Society: Microtubule-Targeting Agents Belonging to Taxanoids, Macrolides and Non-Ribosomal Peptides. Molecules 2022, 27, 5648. [Google Scholar] [CrossRef]
- Eli, S.; Castagna, R.; Mapelli, M.; Parisini, E. Recent Approaches to the Identification of Novel Microtubule-Targeting Agents. Front. Mol. Biosci. 2022, 9, 841777. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization. Int. J. Mol. Sci. 2022, 23, 4001. [Google Scholar] [CrossRef]
- Coulup, S.K.; Georg, G.I. Revisiting microtubule targeting agents: α-Tubulin and the pironetin binding site as unexplored targets for cancer therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865–1873. [Google Scholar] [CrossRef]
- Matthew, S.; Chen, Q.Y.; Ratnayake, R.; Fermaintt, C.S.; Lucena-Agell, D.; Bonato, F.; Prota, A.E.; Lim, S.T.; Wang, X.; Díaz, J.F.; et al. Gatorbulin-1, a distinct cyclodepsipeptide chemotype, targets a seventh tubulin pharmacological site. Proc. Natl. Acad. Sci. USA 2021, 118, e2021847118. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsuchiya, K.; Harada, T.; Nishide, M.; Kurokawa, T.; Nakagawa, T.; Shimada, N.; Kobayashi, K. Pironetin, a novel plant growth regulator produced by Streptomyces sp. NK10958. I. Taxonomy, production, isolation and preliminary characterization. J. Antibiot 1994, 47, 697–702. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsuchiya, K.; Kurokawa, T.; Nakagawa, T.; Shimada, N.; Iitaka, Y. Pironetin, a novel plant growth regulator produced by Streptomyces sp. NK10958. II. Structural elucidation. J. Antibiot. 1994, 47, 703–707. [Google Scholar] [CrossRef]
- Watanabe, H.; Watanabe, H.; Usui, T.; Kondoh, M.; Osada, H.; Kitahara, T. Synthesis of pironetin and related analogs: Studies on structure-activity relationships as tubulin assembly inhibitors. J. Antibiot. 2000, 53, 540–545. [Google Scholar] [CrossRef]
- Kondoh, M.; Usui, T.; Kobayashi, S.; Tsuchiya, K.; Nishikawa, K.; Nishikiori, T.; Mayumi, T.; Osada, H. Cell cycle arrest and antitumor activity of pironetin and its derivatives. Cancer Lett. 1998, 126, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Usui, T.; Nishikiori, T.; Mayumi, T.; Osada, H. Apoptosis induction via microtubule disassembly by an antitumour compound, pironetin. Biochem. J. 1999, 340, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Watanabe, H.; Nakayama, H.; Tada, Y.; Kanoh, N.; Kondoh, M.; Asao, T.; Takio, K.; Watanabe, H.; Nishikawa, K.; et al. The anticancer natural product pironetin selectively targets Lys352 of alpha-tubulin. Chem. Biol. 2004, 11, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Wang, T.; Jiang, J.; Botting, C.H.; Liu, H.; Chen, Q.; Yang, J.; Naismith, J.H.; Zhu, X.; et al. Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule. Nat. Commun. 2016, 7, 12103. [Google Scholar] [CrossRef]
- Prota, A.E.; Setter, J.; Waight, A.B.; Bargsten, K.; Murga, J.; Diaz, J.F.; Steinmetz, M.O. Pironetin Binds Covalently to αCys316 and Perturbs a Major Loop and Helix of α-Tubulin to Inhibit Microtubule Formation. J. Mol. Biol. 2016, 428, 2981–2988. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, A.E.; Mendoza-Espinoza, J.A.; Pereda-Miranda, R.; Cerda-García-Rojas, C.M. Studies of (-)-pironetin binding to α-tubulin: Conformation, docking, and molecular dynamics. J. Org. Chem. 2014, 79, 3752–3764. [Google Scholar] [CrossRef]
- Keck, G.E.; Knutson, C.E.; Wiles, S.A. Total synthesis of the immunosupressant (-)-pironetin (PA48153C). Org. Lett. 2001, 3, 707–710. [Google Scholar] [CrossRef]
- Dias, L.C.; De Oliveira, L.G.; De Sousa, M.A. Total synthesis of (-)-pironetin. Org. Lett. 2003, 5, 265–268. [Google Scholar] [CrossRef]
- Shen, X.; Wasmuth, A.S.; Zhao, J.; Zhu, C.; Nelson, S.G. Catalytic asymmetric assembly of stereodefined propionate units: An enantioselective total synthesis of (-)-pironetin. J. Am. Chem. Soc. 2006, 128, 7438–7439. [Google Scholar] [CrossRef]
- Bressy, C.; Vors, J.P.; Hillebrand, S.; Arseniyadis, S.; Cossy, J. Asymmetric total synthesis of the immunosuppressant (-)-pironetin. Angew. Chem. Int. Ed. Engl. 2008, 47, 10137–10140. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T.; Dechert, A.M. Enantioselective total synthesis of (-)-pironetin: Iterative aldol reactions of thiazolidinethiones. Org. Lett. 2009, 11, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; García-Pla, J.; Carda, M.; Murga, J.; Falomir, E.; Trigili, C.; Notararigo, S.; Díaz, J.F.; Barasoain, I. Design and synthesis of pironetin analogues with simplified structure and study of their interactions with microtubules. Eur. J. Med. Chem. 2011, 46, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Paños, J.; Díaz-Oltra, S.; Sánchez-Peris, M.; García-Pla, J.; Murga, J.; Falomir, E.; Carda, M.; Redondo-Horcajo, M.; Díaz, J.F.; Barasoain, I.; et al. Synthesis and biological evaluation of truncated α-tubulin-binding pironetin analogues lacking alkyl pendants in the side chain or the dihydropyrone ring. Org. Biomol. Chem. 2013, 11, 5809–5826. [Google Scholar] [CrossRef] [PubMed]
- Roldán, S.; Cardona, A.; Conesa, L.; Murga, J.; Falomir, E.; Carda, M.; Marco, J.A. Synthesis and biological evaluation of simplified pironetin analogues with modifications in the side chain and the lactone ring. Org. Biomol. Chem. 2016, 15, 220–232. [Google Scholar] [CrossRef]
- Huang, D.S.; Wong, H.L.; Georg, G.I. Synthesis and Cytotoxicity Evaluation of C4- and C5-Modified Analogues of the α,β-Unsaturated Lactone of Pironetin. ChemMedChem 2017, 12, 520–528. [Google Scholar] [CrossRef]
- Huang, D.S.; Wong, H.L.; Georg, G.I. Synthesis and evaluation of C2 functionalized analogs of the α-tubulin-binding natural product pironetin. Bioorg. Med. Chem. Lett. 2018, 28, 2789–2793. [Google Scholar] [CrossRef]
- Coulup, S.K.; Huang, D.S.; Wong, H.L.; Georg, G.I. Identification of the Metabolic Profile of the α-Tubulin-Binding Natural Product (-)-Pironetin. J. Med. Chem. 2019, 62, 1684–1689. [Google Scholar] [CrossRef]
- Noman, M.A.A.; Huang, D.S.; Coulup, S.K.; Syeda, S.S.; Henry; Wong, L.; Georg, G.I. Cytotoxicity of phenylpironetin analogs and the metabolic fate of pironetin and phenylpironetin. Bioorg. Chem. 2022, 125, 105915. [Google Scholar] [CrossRef]
- Vilanova, C.; Torijano-Gutiérrez, S.; Díaz-Oltra, S.; Murga, J.; Falomir, E.; Carda, M.; Alberto Marco, J. Design and synthesis of pironetin analogue/combretastatin A-4 hybrids containing a 1,2,3-triazole ring and evaluation of their cytotoxic activity. Eur. J. Med. Chem. 2014, 87, 125–130. [Google Scholar] [CrossRef]
- Vilanova, C.; Díaz-Oltra, S.; Murga, J.; Falomir, E.; Carda, M.; Redondo-Horcajo, M.; Díaz, J.F.; Barasoain, I.; Marco, J.A. Design and synthesis of pironetin analogue/colchicine hybrids and study of their cytotoxic activity and mechanisms of interaction with tubulin. J. Med. Chem. 2014, 57, 10391–10403. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, C.; Díaz-Oltra, S.; Murga, J.; Falomir, E.; Carda, M.; Marco, J.A. Inhibitory effect of pironetin analogue/colchicine hybrids on the expression of the VEGF, hTERT and c-Myc genes. Bioorg. Med. Chem. Lett. 2015, 25, 3194–3198. [Google Scholar] [CrossRef] [PubMed]
- Bonandi, E.; Foschi, F.; Marucci, C.; Dapiaggi, F.; Sironi, M.; Pieraccini, S.; Christodoulou, M.S.; de Asís Balaguer, F.; Díaz, J.F.; Zidar, N.; et al. Synthesis of Thicolchicine-Based Conjugates: Investigation towards Bivalent Tubulin/Microtubules Binders. ChemPlusChem 2019, 84, 98–102. [Google Scholar] [CrossRef]
- Yang, B.Y.; Kong, L.Y.; Wang, X.B.; Zhang, Y.M.; Li, R.J.; Yang, M.H.; Luo, J.G. Nitric Oxide Inhibitory Activity and Absolute Configurations of Arylalkenyl α,β-Unsaturated δ/γ-Lactones from Cryptocarya concinna. J. Nat. Prod. 2016, 79, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Shi, Y.M.; Luo, J.G.; Kong, L.Y. Two new arylalkenyl α,β-unsaturated δ-lactones with cytotoxic activity from the leaves and twigs of Cryptocarya concinna. Nat. Prod. Res. 2017, 31, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, A.J.; Yoshida, M. 6-[omega-arylalkenyl]-5,6-dihydro-alpha-pyrones from Cryptocarya moschata (Lauraceae). Phytochemistry 2000, 53, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, V.; Van Hung, N.; Adeline, M.T.; Riche, C.; Chiaroni, A.; Sévenet, T.; Guéritte, F. Cytotoxic flavonoids and alpha-pyrones from Cryptocarya obovata. J. Nat. Prod. 2004, 67, 858–862. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Cinel, B.; Díaz-Marrero, A.R.; McHardy, L.M.; Ngo, M.; Andersen, R.J.; Roberge, M. Abrogation of ionizing radiation-induced G2 checkpoint and inhibition of nuclear export by Cryptocarya pyrones. Cancer Chemother. Pharmacol. 2008, 61, 407–413. [Google Scholar] [CrossRef]
- Meragelman, T.L.; Scudiero, D.A.; Davis, R.E.; Staudt, L.M.; McCloud, T.G.; Cardellina, J.H., 2nd; Shoemaker, R.H. Inhibitors of the NF-kappaB activation pathway from Cryptocarya rugulosa. J. Nat. Prod. 2009, 72, 336–339. [Google Scholar] [CrossRef]
- Falomir, E.; Murga, J.; Ruiz, P.; Carda, M.; Marco, J.A.; Pereda-Miranda, R.; Fragoso-Serrano, M.; Cerda-García-Rojas, C.M. Stereoselective synthesis and determination of the cytotoxic properties of spicigerolide and three of its stereoisomers. J. Org. Chem. 2003, 68, 5672–5676. [Google Scholar] [CrossRef]
- de Kok, R.P.J. A revision of Cryptocarya (Lauraceae) from Thailand and Indochina. Gard. Bull. Singap. 2015, 67, 309–350. [Google Scholar] [CrossRef]
- Zhang, M.; Yahara, T.; Tagane, S.; Rueangruea, S.; Suddee, S.; Moritsuka, E.; Suyama, Y. Cryptocarya kaengkrachanensis, a new species of Lauraceae from Kaeng Krachan National Park, southwest Thailand. PhytoKeys 2020, 140, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Zheng, J.; Yu, Z.C.; Huang, X.D.; Zhang, Q.L.; Tian, X.S.; Peng, C.L. Functional characteristics of phenolic compounds accumulated in young leaves of two subtropical forest tree species of different successional stages. Tree Physiol. 2018, 38, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.T.M.; Chung, N.T.; Huong, L.T.; Hung, N.H.; Ogunwande, I.A.; Dai, D.N.; Setzer, W.N. Chemical Compositions, Mosquito Larvicidal and Antimicrobial Activities of Leaf Essential Oils of Eleven Species of Lauraceae from Vietnam. Plants 2020, 9, 606. [Google Scholar] [CrossRef]
- Della-Felice, F.; Sarotti, A.M.; Pilli, R.A. Catalytic Asymmetric Synthesis and Stereochemical Revision of (+)-Cryptoconcatone H. J. Org. Chem. 2017, 82, 9191–9197. [Google Scholar] [CrossRef]
- Acharyya, R.K.; Pal, P.; Chatterjee, S.; Nanda, S. Asymmetric total synthesis of cryptoconcatone I. Org. Biomol. Chem. 2019, 17, 3552–3566. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, F.; Celik, I.E.; Kirsch, S.F. Total Synthesis of Cryptoconcatone D via Construction of 1,3-Diol Units Using Chiral Horner-Wittig Reagents. J. Org. Chem. 2022, 87, 14899–14908. [Google Scholar] [CrossRef]
- Martínez-Fructuoso, L.; Pereda-Miranda, R.; Rosas-Ramírez, D.; Fragoso-Serrano, M.; Cerda-García-Rojas, C.M.; da Silva, A.S.; Leitão, G.G.; Leitão, S.G. Structure Elucidation, Conformation, and Configuration of Cytotoxic 6-Heptyl-5,6-dihydro-2 H-pyran-2-ones from Hyptis Species and Their Molecular Docking to α-Tubulin. J. Nat. Prod. 2019, 82, 520–531. [Google Scholar] [CrossRef]
- da Silva, A.S.; Martínez-Fructuoso, L.; Simas, R.C.; Leitão, G.G.; Fragoso-Serrano, M.; Barros, Y.S.; de Souza, D.R.; Pereda-Miranda, R.; Leitão, S.G. Distribution of 5,6-dihydro-α-pyrones by electrospray ionization ion trap mass spectrometry in different aerial parts of Hyptis monticola. Phytochemistry 2021, 185, 112706. [Google Scholar] [CrossRef]
- Juliawaty, L.D.; Ra’idah, P.N.; Abdurrahman, S.; Hermawati, E.; Alni, A.; Tan, M.I.; Ishikawa, H.; Syah, Y.M. 5,6-Dihydro-α-pyrones from the leaves of Cryptocarya pulchinervia (Lauraceae). J. Nat. Med. 2020, 74, 584–590. [Google Scholar] [CrossRef]
- Liu, Y.; Rakotondraibe, L.H.; Brodie, P.J.; Wiley, J.D.; Cassera, M.B.; Miller, J.S.; Ratovoson, F.; Rakotobe, E.; Rasamison, V.E.; Kingston, D.G. Antimalarial 5,6-Dihydro-α-pyrones from Cryptocarya rigidifolia: Related Bicyclic Tetrahydro-α-Pyrones Are Artifacts1. J. Nat. Prod. 2015, 78, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Tirado-Rives, J. Monte Carlo versus Molecular Dynamics for conformational sampling. J. Phys. Chem. 1996, 100, 14508–14513. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689–1700. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Ulmschneider, J.P.; Tirado-Rives, J. Free energies of hydration from a generalized Born model and an ALL-atom force field. J. Phys. Chem. B 2004, 108, 16264–16270. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Meziane-Tani, M.; Lagant, P.; Semmoud, A.; Vergoten, G. The SPASIBA force field for chondroitin sulfate: Vibrational analysis of D-glucuronic and N-acetyl-D-galactosamine 4-sulfate sodium salts. J. Phys. Chem. A 2006, 110, 11359–11370. [Google Scholar] [CrossRef]
- Vergoten, G.; Mazur, I.; Lagant, P.; Michalski, J.C.; Zanetta, J.P. The SPASIBA force field as an essential tool for studying the structure and dynamics of saccharides. Biochimie 2003, 85, 65–73. [Google Scholar] [CrossRef]
- Lagant, P.; Nolde, D.; Stote, R.; Vergoten, G.; Karplus, M. Increasing Normal Modes Analysis Accuracy: The SPASIBA Spectroscopic Force Field Introduced into the CHARMM Program. J. Phys. Chem. A 2004, 108, 4019–4029. [Google Scholar] [CrossRef]
- Homans, S.W. A molecular mechanical force field for the conformational analysis of oligosaccharides: Comparison of theoretical and crystal structures of Man alpha 1-3Man beta 1-4GlcNAc. Biochemistry 1990, 29, 9110–9118. [Google Scholar] [CrossRef] [PubMed]
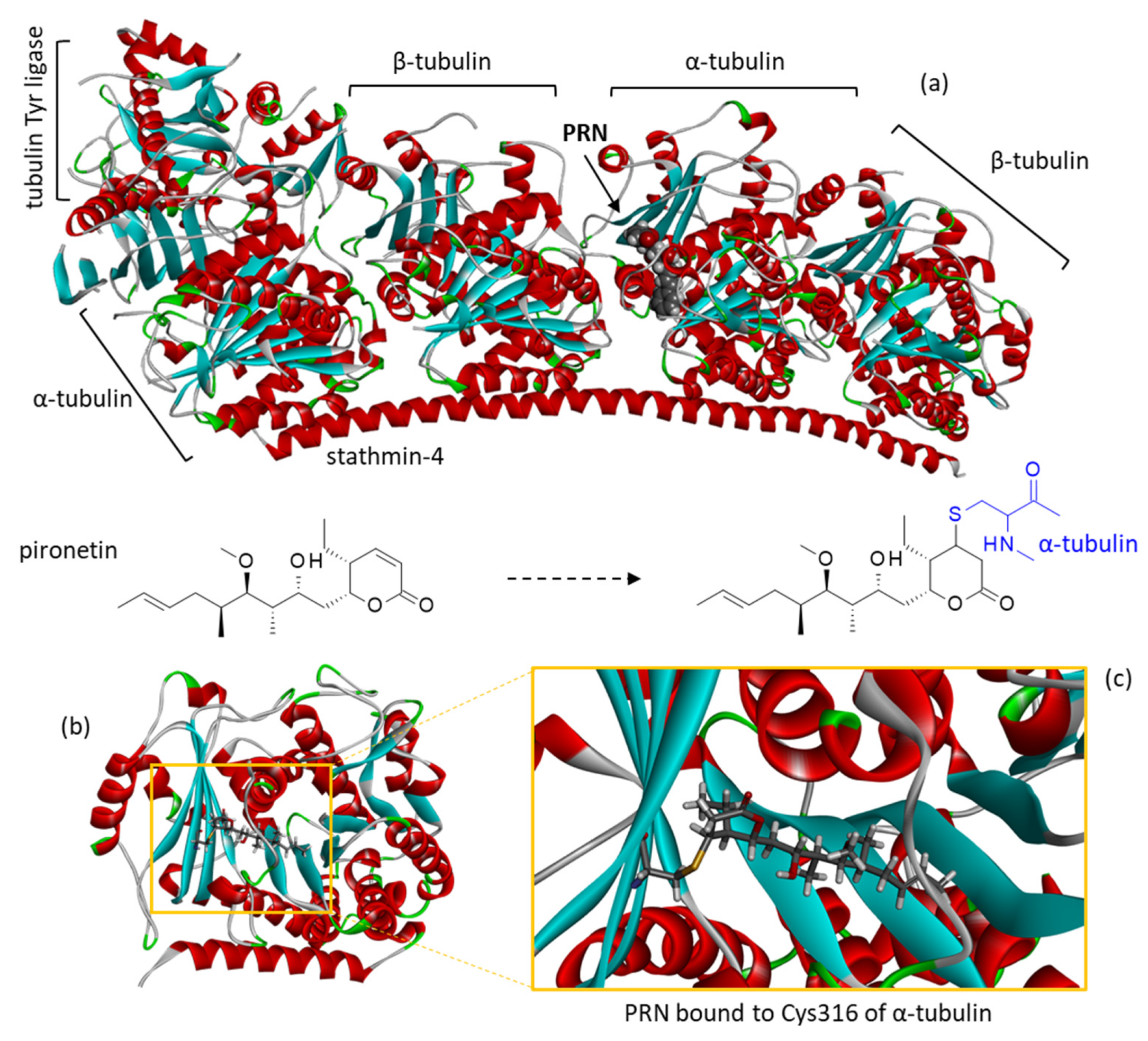

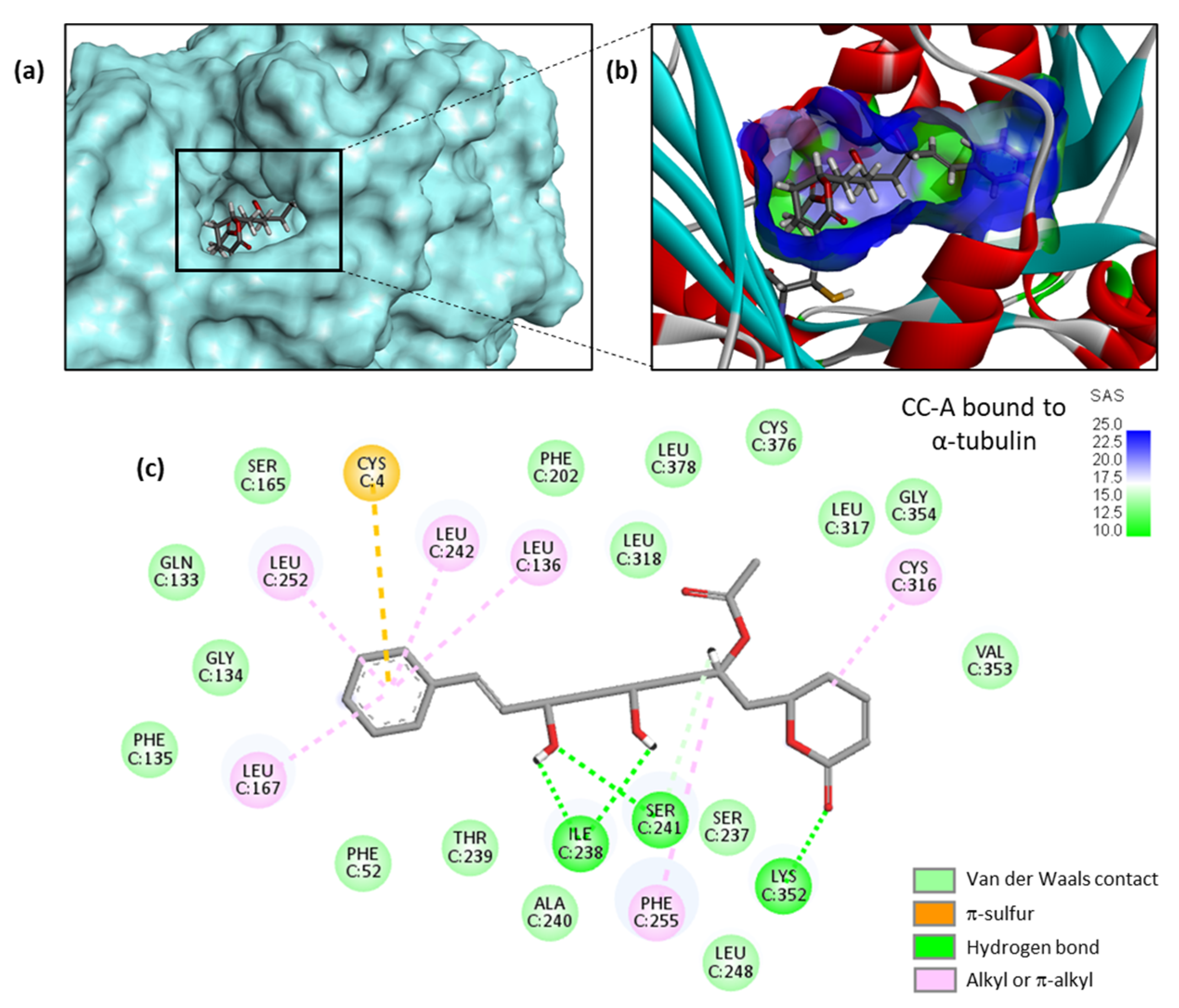
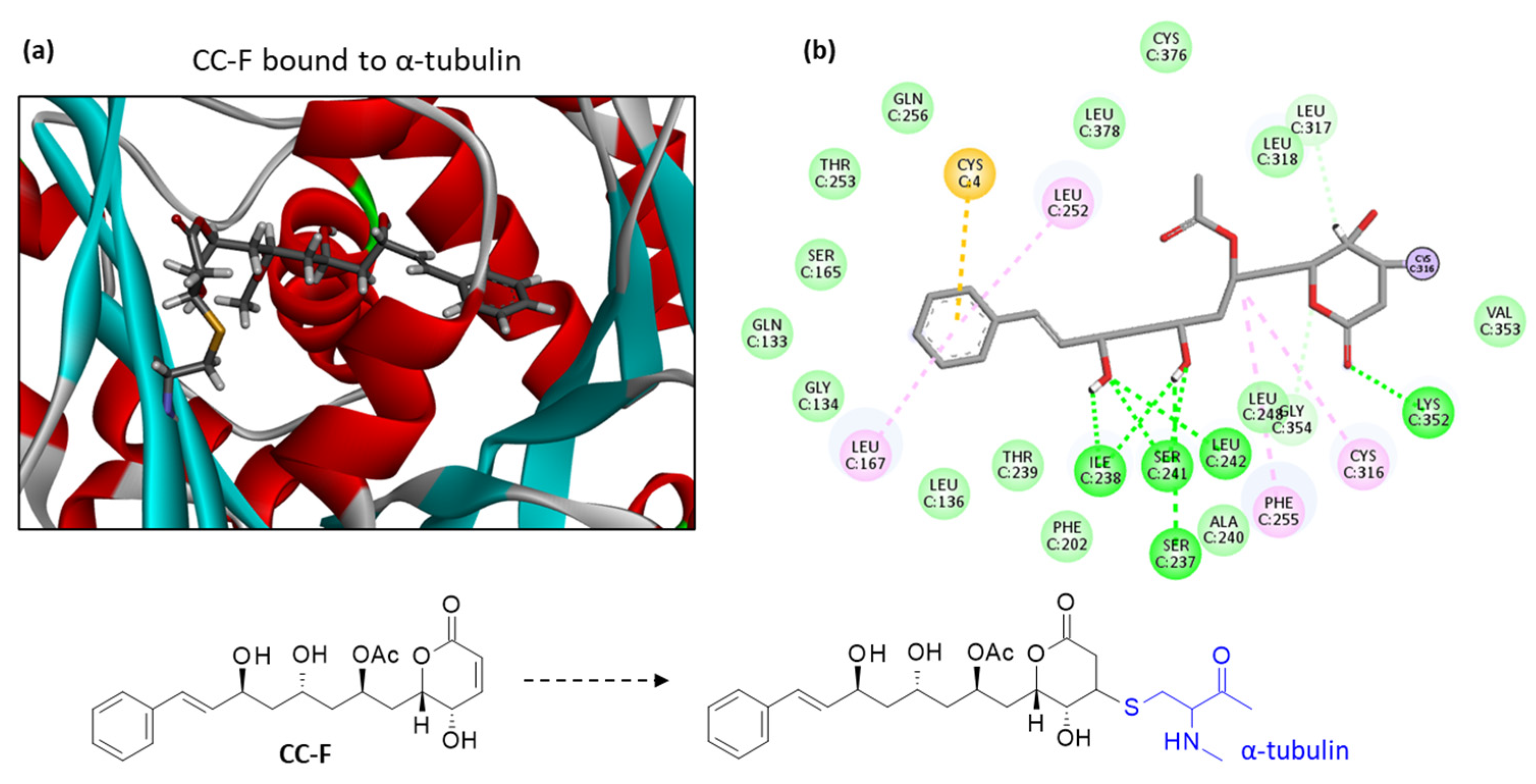


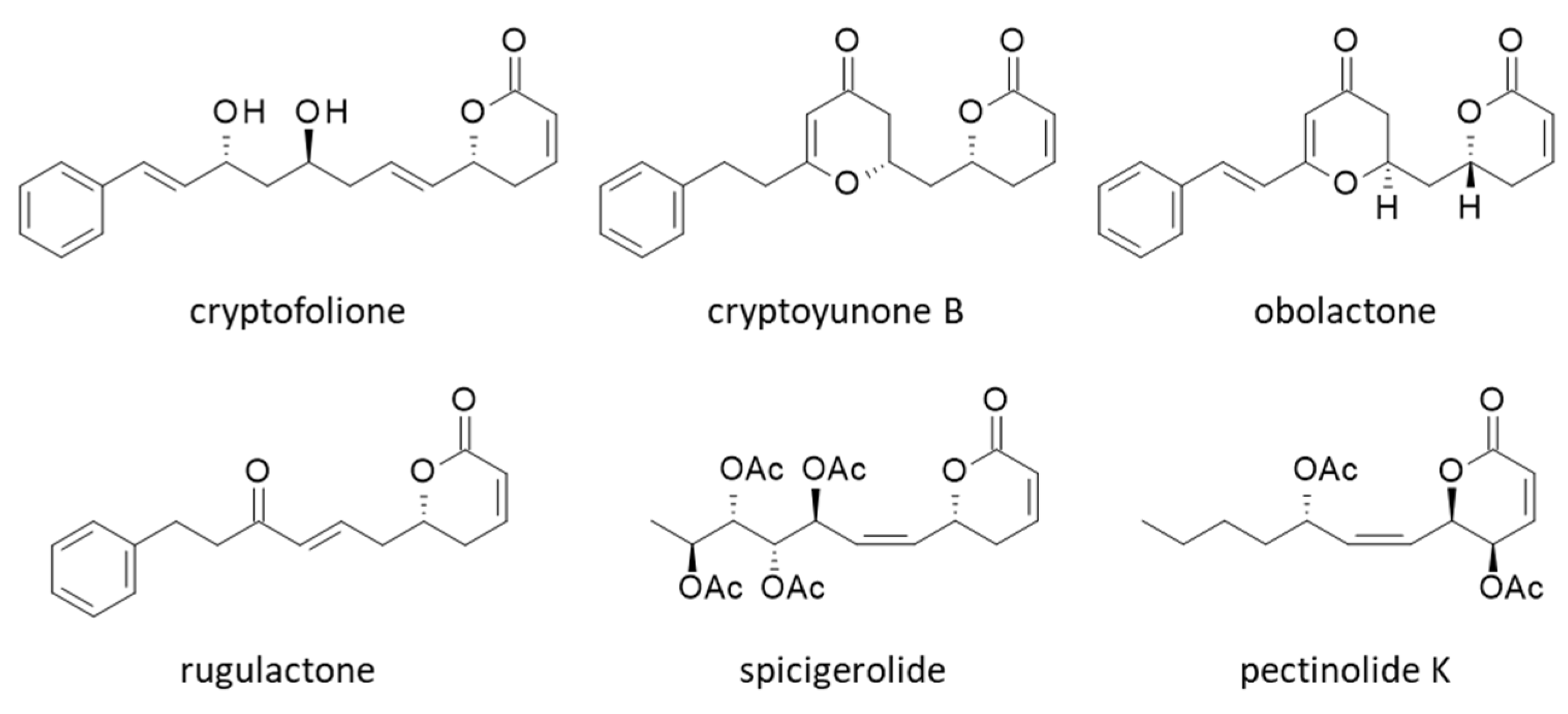
| Compounds | ΔE (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|
| Pironetin | −57.32 | −24.20 |
| Cryptocontanone A | −67.80 | −25.00 |
| Cryptocontanone B | −63.10 | −24.25 |
| Cryptocontanone C | −70.30 | −26.25 |
| Cryptocontanone D | −60.70 | −27.50 |
| Cryptocontanone E | −65.40 | −22.00 |
| Cryptocontanone F | −74.15 | −27.60 |
| Cryptocontanone G | −66.00 | −24.40 |
| Cryptocontanone H | −57.70 | −23.30 |
| Cryptocontanone I | −66.65 | −29.10 |
| Cryptocontanone J | −66.75 | −30.75 |
| Cryptocontanone K | −52.30 | −22.85 |
| Cryptocontanone L | −73.40 | −26.50 |
| Cryptofolione | −56.20 | −21.75 |
| Cryptoyunone B | −60.20 | −19.80 |
| Obolactone | −52.50 | −18.50 |
| Rugulactone | −59.50 | −16.80 |
| Spicigerolide | −67.70 | −21.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. https://doi.org/10.3390/plants12020296
Vergoten G, Bailly C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants. 2023; 12(2):296. https://doi.org/10.3390/plants12020296
Chicago/Turabian StyleVergoten, Gérard, and Christian Bailly. 2023. "Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues" Plants 12, no. 2: 296. https://doi.org/10.3390/plants12020296
APA StyleVergoten, G., & Bailly, C. (2023). Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants, 12(2), 296. https://doi.org/10.3390/plants12020296






