Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators
Abstract
1. Introduction
2. Results
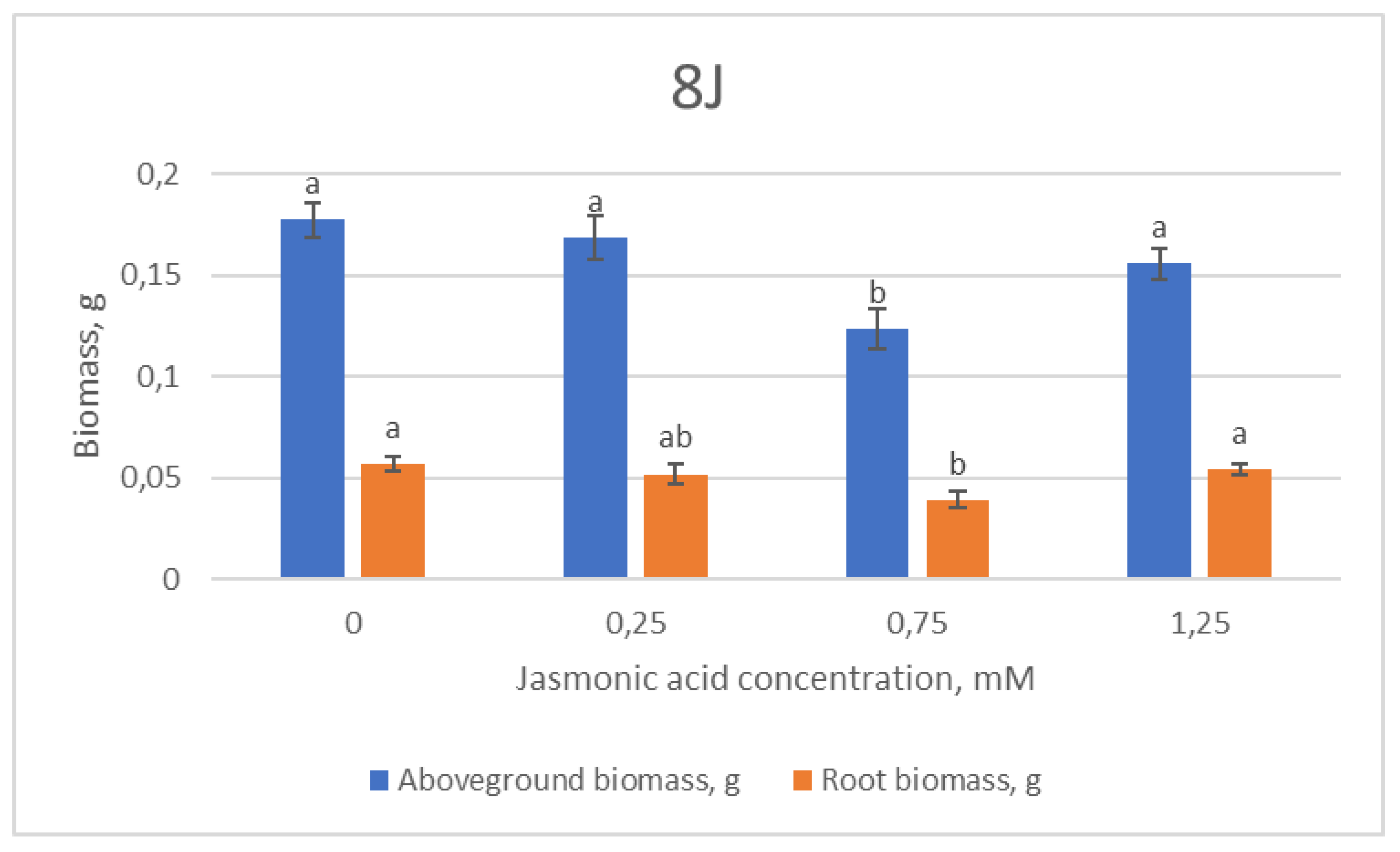
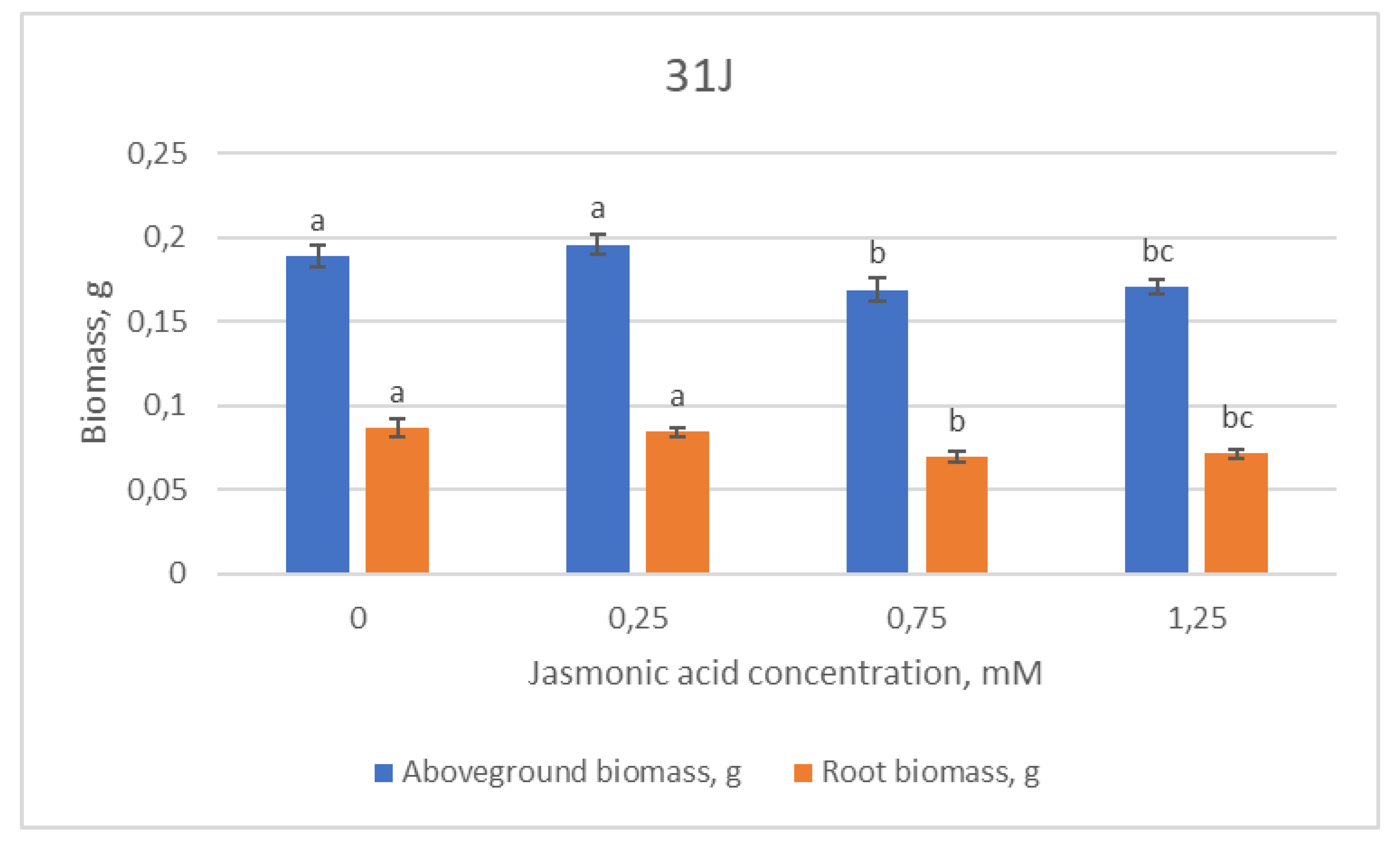
2.1. Growth Parameters
2.2. Secondary Metabolites
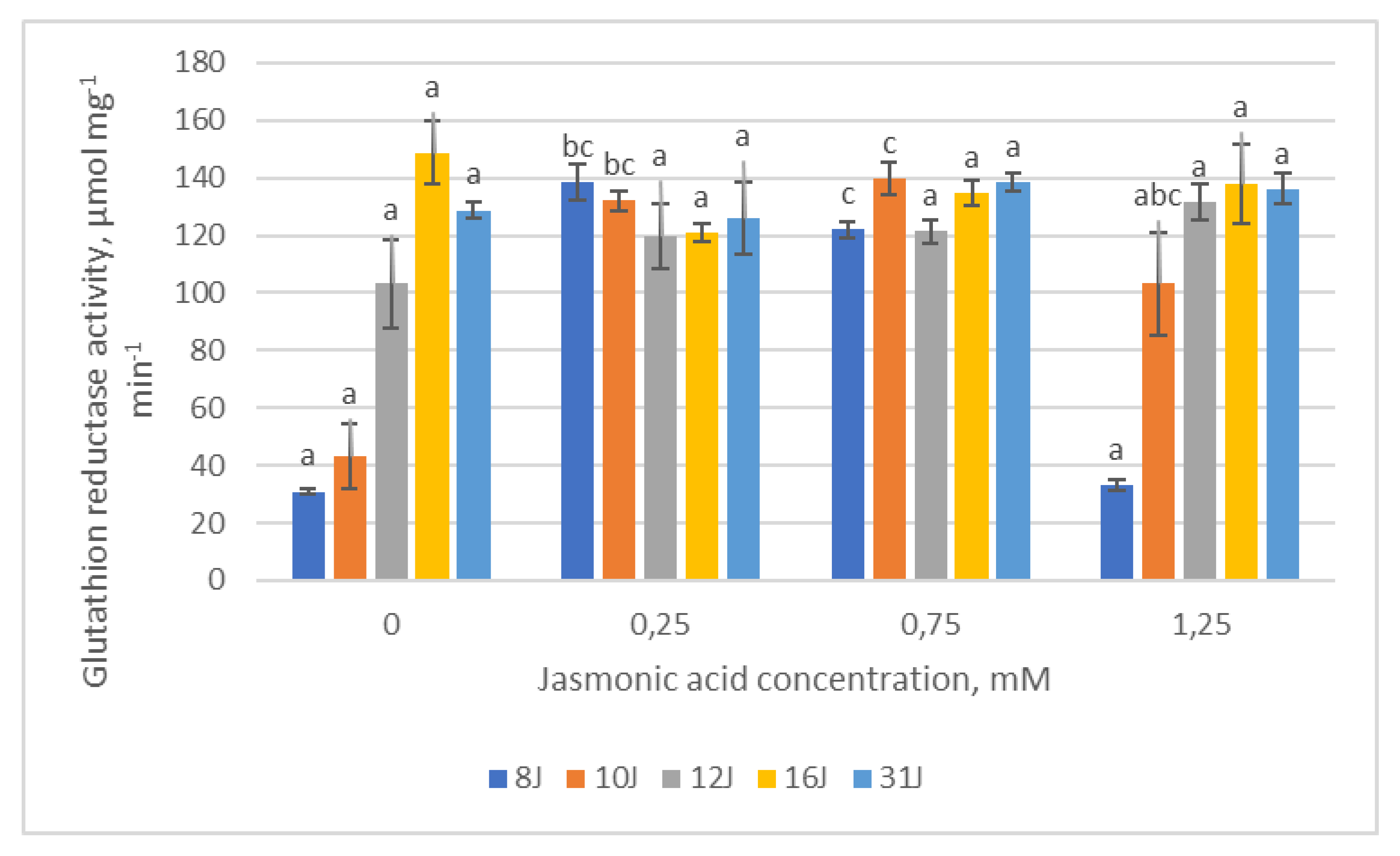
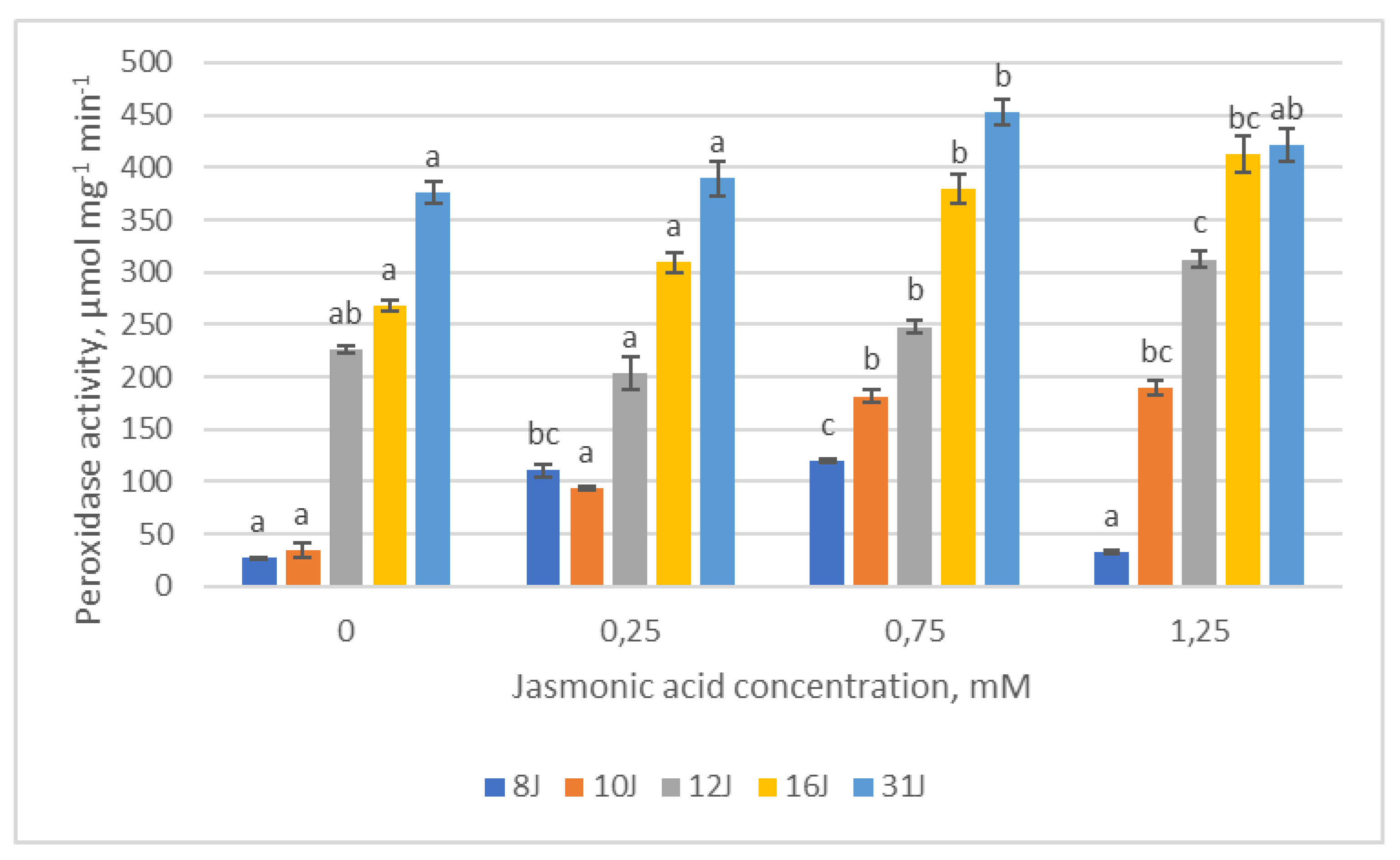
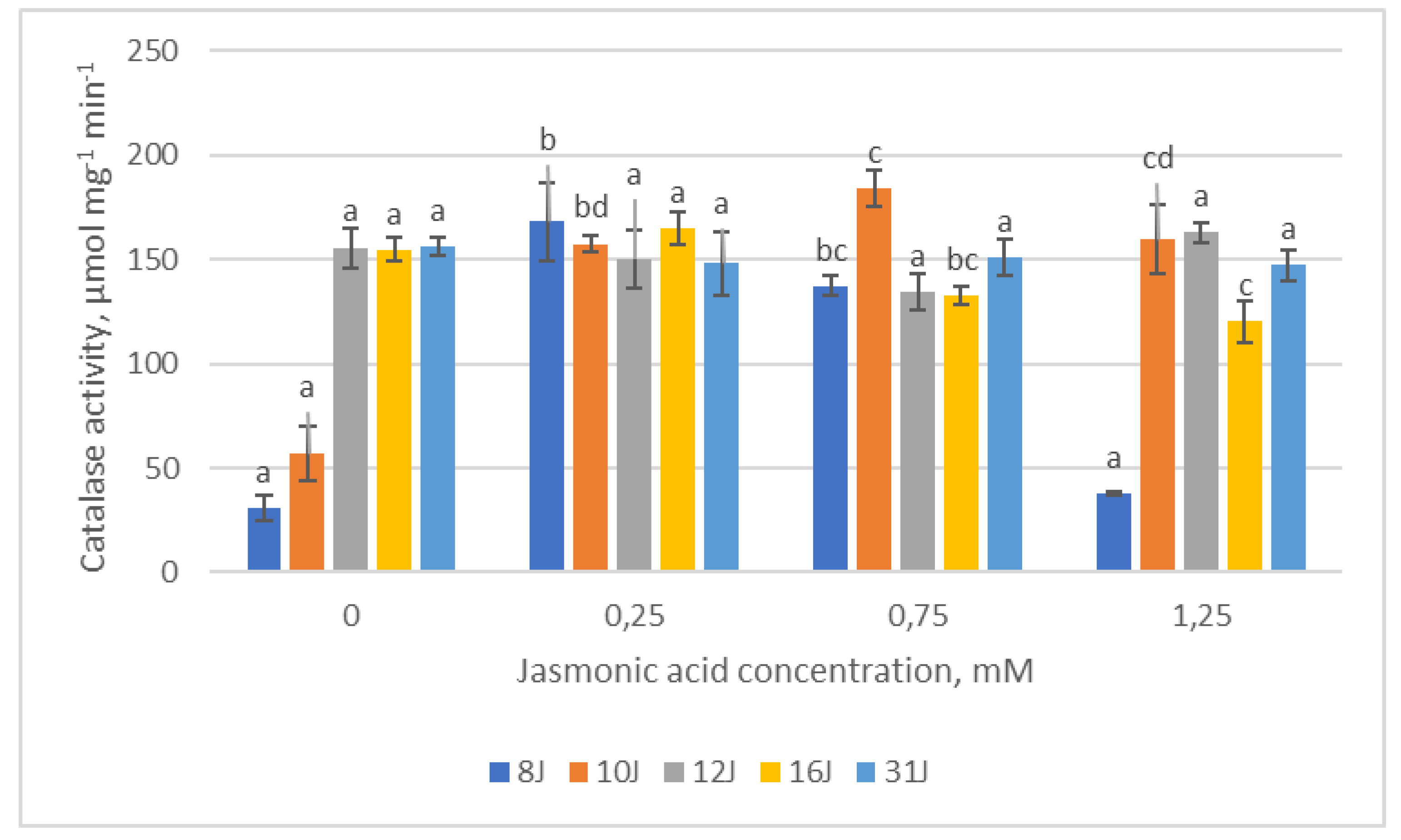
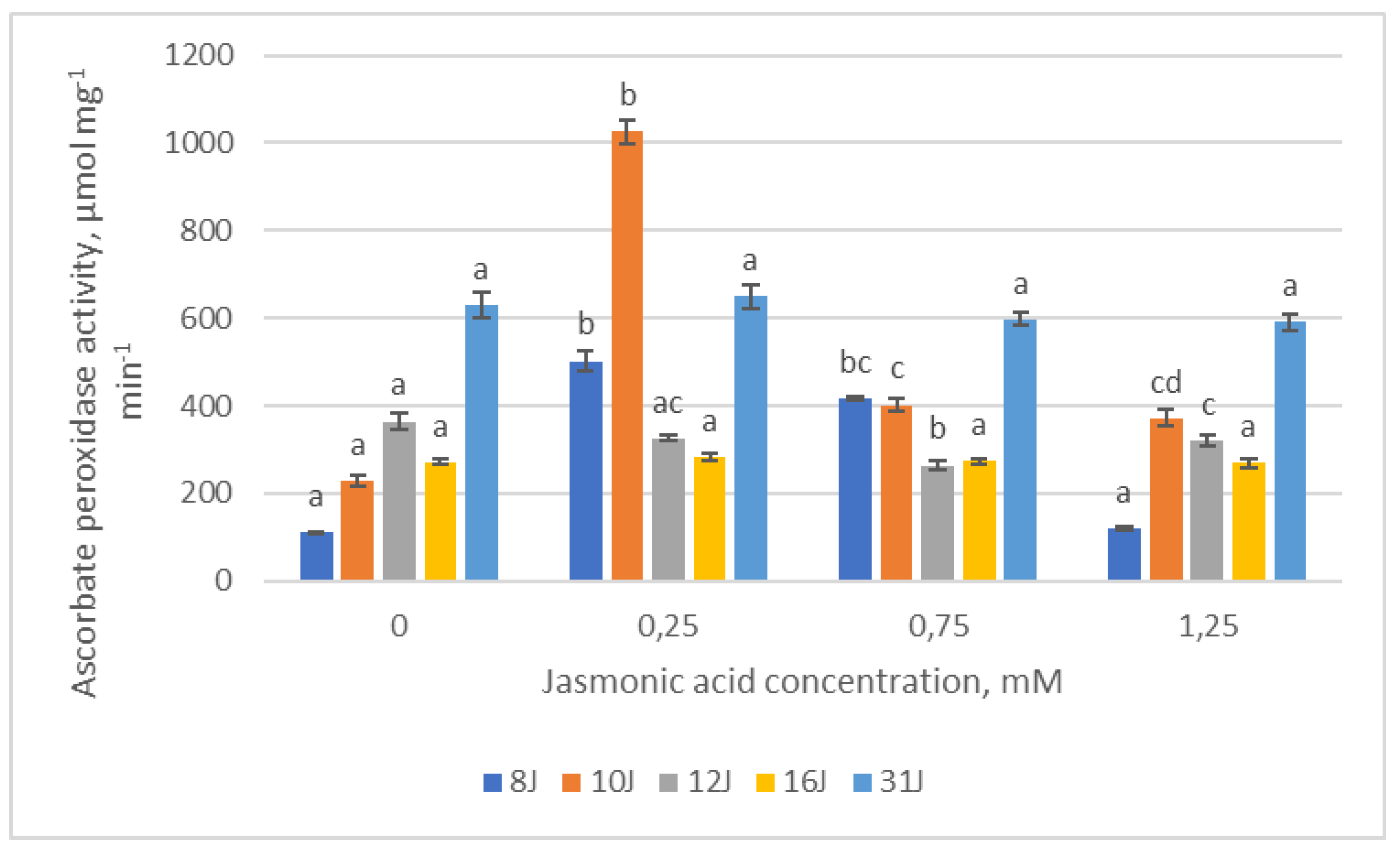
2.3. Antioxidative Enzymes
3. Discussion
4. Materials and Methods
4.1. Seeds
4.2. Sowing
4.3. Treatment with Jasmonic Acid
4.4. Collection of Samples
4.5. Preparation of Extracts for Secondary Metabolite and Photosynthetic Pigment Analysis
4.6. Quantification of Chlorophyll a, b and Total Carotenoids
4.7. Determination of TPC
4.8. Determination of TFC
4.9. Sample Preparation for Antioxidant Enzyme Tests
4.9.1. Total Protein (PROT)
4.9.2. Catalase (CAT)
4.9.3. Ascorbate Peroxidase (APX)
4.9.4. Guaiacol Peroxidase (POX)
4.9.5. Glutathione Reductase (GR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallad, G.E.; Goodman, R.M. Systemic Acquired Resistance and Induced Systemic Resistance in Conventional Agriculture. Crop. Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced Resistance to Pests and Pathogens in Trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Bostock, R.M. Induced Systemic Resistance (ISR) against Pathogens in the Context of Induced Plant Defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zas, R.; Björklund, N.; Nordlander, G.; Cendán, C.; Hellqvist, C.; Sampedro, L. Exploiting Jasmonate-Induced Responses for Field Protection of Conifer Seedlings against a Major Forest Pest, Hylobius Abietis. For. Ecol. Manage. 2014, 313, 212–223. [Google Scholar] [CrossRef]
- Barka, E.A.; Nowak, J.; Clément, C. Enhancement of Chilling Resistance of Inoculated Grapevine Plantlets with a Plant Growth-Promoting Rhizobacterium, Burkholderia Phytofirmans Strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Fedderwitz, F.; Nordlander, G.; Ninkovic, V.; Björklund, N. Effects of Jasmonate-Induced Resistance in Conifer Plants on the Feeding Behaviour of a Bark-Chewing Insect, Hylobius Abietis. J. Pest Sci. 2016, 89, 97–105. [Google Scholar] [CrossRef]
- Sabbagh, E.; Sabbagh, S.K.; Panjehkeh, N.; Bolok-Yazdi, H.R. Jasmonic Acid Induced Systemic Resistance in Infected Cucumber by Pythium Aphanidermatum. Tarim Bilim. Derg. 2018, 24, 143–152. [Google Scholar] [CrossRef]
- Moreira, X.; Sampedro, L.; Zas, R. Defensive Responses of Pinus Pinaster Seedlings to Exogenous Application of Methyl Jasmonate: Concentration Effect and Systemic Response. Environ. Exp. Bot. 2009, 67, 94–100. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Christiansen, E.; Långström, B.; Borg-Karlson, A.; Solheim, H.; Björklund, N.; Zhao, T.; Schmidt, A.; Fossdal, C.G.; Krokene, P. Priming of Inducible Defenses Protects Norway Spruce against Tree-killing Bark Beetles. Plant Cell Environ. 2020, 43, 420–430. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A. Phenolic Metabolites in the Resistance of Northern Forest Trees to Pathogens—Past Experiences and Future Prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Jasmonate-Mediated Induced Plant Resistance Affects a Community of Herbivores. Ecol. Entomol. 2001, 26, 312–324. [Google Scholar] [CrossRef]
- Dewir, Y.; Chakrabarty, D.; Ali, M.; Hahn, E.; Paek, K.Y. Lipid Peroxidation and Antioxidant Enzyme Activities of Euphorbia Millii Hyperhydric Shoots. Environ. Exp. Bot. 2005, 58, 93–99. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in Photosynthesis and Some Antioxidant Enzymatic Activities of Mungbean Subjected to Waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Li, B.; Wei, J.; Wei, X.; Tang, K.; Liang, Y.; Shu, K.; Wang, B. Effect of sound wave stress on antioxidant enzyme activities and lipid peroxidation of Dendrobium candidum. Coll. Surfaces B Biointerfaces 2008, 63, 269–275. [Google Scholar] [CrossRef]
- Striganavičiūtė, G.; Žiauka, J.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Impact of Plant-Associated Bacteria on the In Vitro Growth and Pathogenic Resistance against Phellinus Tremulae of Different Aspen (Populus) Genotypes. Microorganisms 2021, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Striganavičiūtė, G.; Žiauka, J.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Priming of Resistance-Related Phenolics: A Study of Plant-Associated Bacteria and Hymenoscyphus fraxineus. Microorganisms 2021, 9, 2504. [Google Scholar] [CrossRef] [PubMed]
- Bonello, P.; Gordon, T.R.; Herms, D.A.; Wood, D.L.; Erbilgin, N. Nature and ecological implications of pathogen-induced systemic resistance in conifers: A novel hypothesis. Physiol. Mol. Plant Pathol. 2006, 68, 95–104. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef]
- Grulke, N.E. The Nexus of Host and Pathogen Phenology: Understanding the Disease Triangle with Climate Change. New Phytol. 2011, 189, 8–11. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and Forest Functioning in Response to Global Warming. New Phytol. 2001, 149, 369–400. [Google Scholar] [CrossRef]
- Pautasso, M.; Dehnen-Schmutz, K.; Holdenrieder, O.; Pietravalle, S.; Salama, N.; Jeger, M.J.; Lange, E.; Hehl-Lange, S. Plant Health and Global Change—Some Implications for Landscape Management. Biol. Rev. 2010, 85, 729–755. [Google Scholar] [CrossRef]
- EEA. Climate Change, Impacts and Vulnerability in Europe 2012, An Indicator-Based Report; European Environment Agency (EEA), Office for Official Publications of the European Union: Luxembourg, 2012. [Google Scholar] [CrossRef]
- Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. Jasmonate Biosynthesis in Arabidopsis Thaliana—Enzymes, Products, Regulation. Plant Biol. 2006, 8, 297–306. [Google Scholar] [CrossRef]
- Šņepste, I.; Krivmane, B.; Šķipars, V.; Zaluma, A.; Ruņģis, D.E. Induction of Defense Responses in Pinus Sylvestris Seedlings by Methyl Jasmonate and Response to Heterobasidion Annosum and Lophodermium Seditiosum Inoculation. Forests 2021, 12, 628. [Google Scholar] [CrossRef]
- Wilkinson, S.W.; Dalen, L.S.; Skrautvol, T.O.; Ton, J.; Krokene, P.; Mageroy, M.H. Transcriptomic Changes during the Establishment of Long-term Methyl Jasmonate-induced Resistance in Norway Spruce. Plant, Cell Environ. 2021, 45, 1891–1913. [Google Scholar] [CrossRef]
- Moreira, X.; Zas, R.; Sampedro, L. Methyl Jasmonate as Chemical Elicitor of Induced Responses and Anti-Herbivory Resistance in Young Conifer Trees. Plant Def. Biol. Control 2012, 12, 345–362. [Google Scholar] [CrossRef]
- Liu, J.-J.; Williams, H.; Li, X.R.; Schoettle, A.W.; Sniezko, R.A.; Murray, M.; Zamany, A.; Roke, G.; Chen, H. Profiling Methyl Jasmonate-Responsive Transcriptome for Understanding Induced Systemic Resistance in Whitebark Pine (Pinus Albicaulis). Plant Mol. Biol. 2017, 95, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How Can Salicylic Acid and Jasmonic Acid Mitigate Salt Toxicity in Soybean Plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Jasmonic Acid Signalling and the Plant Holobiont. Curr. Opin. Microbiol. 2017, 37, 42–47. [Google Scholar] [CrossRef]
- Halim, V.A.; Vess, A.; Scheel, D.; Rosahl, S. The Role of Salicylic Acid and Jasmonic Acid in Pathogen Defence. Plant Biol. 2006, 8, 307–313. [Google Scholar] [CrossRef]
- Kozlowski, G.; Buchala, A.; Metraux, J.-P. Methyl Jasmonate Protects Norway Spruce [Picea abies (L.) Karst.] Seedlings against Pythium Ultimum Trow. Physiol. Mol. Plant Pathol. 1999, 55, 53–58. [Google Scholar] [CrossRef]
- Kānberga-siliņa, K.; Rauda, E.; Šķipars, V.; Vivian-smith, A.; Yakovlev, I.; Krivmane, B.; Šņepste, I.; Ruņģis, D. Transcriptomic Response to Methyl Jasmonate Treatment of Scots Pine (Pinus sylvestris) Seedlings. Environ. Exp. Biol. 2017, 257–274. [Google Scholar] [CrossRef]
- Lone, W.A.; Majeed, N.; Yaqoob, U.; John, R. Exogenous Brassinosteroid and Jasmonic Acid Improve Drought Tolerance in Brassica rapa L. Genotypes by Modulating Osmolytes, Antioxidants and Photosynthetic System. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, N.; Shamsi, I.H.; Jabeen, Z.; Siddiqui, M.H.; Jiang, L.-X. Role of Jasmonic Acid in Improving Tolerance of Rapeseed (Brassica napus L.) to Cd Toxicity. J. Zhejiang Univ. Sci. B 2018, 19, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P.; Nagy, N.E.; Solheim, H. Methyl Jasmonate and Oxalic Acid Treatment of Norway Spruce: Anatomically Based Defense Responses and Increased Resistance against Fungal Infection. Tree Physiol. 2008, 28, 29–35. [Google Scholar] [CrossRef]
- Berglund, T.; Lindström, A.; Aghelpasand, H.; Stattin, E.; Ohlsson, A.B. Protection of Spruce Seedlings against Pine Weevil Attacks by Treatment of Seeds or Seedlings with Nicotinamide, Nicotinic Acid and Jasmonic Acid. Forestry 2016, 89, 127–135. [Google Scholar] [CrossRef]
- Heijari, J.; Nerg, A.; Kainulainen, P.; Viiri, H.; Vuorinen, M.; Holopainen, J.K. Application of Methyl Jasmonate Reduces Growth but Increases Chemical Defence and Resistance against Hylobius Abietis in Scots Pine Seedlings. Entomol. Exp. Appl. 2005, 115, 117–124. [Google Scholar] [CrossRef]
- Martin, M.T.; Pedranzani, H.; García-Molinero, P.; Pando, V.; Sierra-De-Grado, R. Inhibitory Effect of Jasmonic Acid and Ethylene on Epicotyl Growth and Bud Induction in the Maritime Pine, Pinus Pinaster Soland. Ait. Biocell 2009, 33, 141–148. [Google Scholar] [CrossRef]
- Regvar, M.; Gogala, N. Changes in Root Growth Patterns of (Picea Abies) Spruce Roots by Inoculation with an Ectomycorrhizal Fungus Pisolithus Tinctorlus and Jasmonic Acid Treatment. Trees Struct. Funct. 1996, 10, 410–414. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Strzałka, K.; Kostecka-Gugała, A.; Latowski, D. Carotenoids and Environmental Stress in Plants: Significance of Carotenoid-Mediated Modulation of Membrane Physical Properties. Russ. J. Plant Physiol. 2003, 50, 168–173. [Google Scholar] [CrossRef]
- Šėžienė, V.; Lučinskaitė, I.; Mildažienė, V.; Ivankov, A.; Koga, K.; Shiratani, M.; Laužikė, K.; Baliuckas, V. Changes in Content of Bioactive Compounds and Antioxidant Activity Induced Antioxidants Changes in Content of Bioactive Compounds and Antioxidant Activity Induced in Needles of Different Half-Sib Families of Norway Spruce (Picea abies (L.) H. Karst). Antioxidants 2022, 11, 1558. [Google Scholar] [CrossRef]
- Gould, N.; Reglinski, T.; Northcott, G.L.; Spiers, M.; Taylor, J.T. Physiological and Biochemical Responses in Pinus Radiata Seedlings Associated with Methyl Jasmonate-Induced Resistance to Diplodia Pinea. Physiol. Mol. Plant Pathol. 2009, 74, 121–128. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Properties and Functions of Glutathione Reductase in Plants. Physiol. Plant. 1989, 77, 449–456. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and Glutathione Reductase: A Boon in Disguise for Plant Abiotic Stress Defense Operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and Ascorbate Peroxidase—Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.M.; Scandalios, J.G. Hydrogen Peroxide-Mediated Catalase Gene Expression in Response to Wounding. Free Radic. Biol. Med. 2000, 28, 1182–1190. [Google Scholar] [CrossRef]
- Bandurska, H.Z.; Stroiński, A.; Kubiś, J. The Effect of Jasmonic Acid on the Accumulation of ABA, Proline and Spermidine and Its Influence on Membrane Injury under Water Deficit in Two Barley Genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthhofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1974, 299, 152–178. [Google Scholar] [CrossRef]
- DeAngelis, K.M. Phosphate Buffer Preparation. Available online: https://www.unl.edu/cahoonlab/phosphate buffer.pdf (accessed on 7 December 2022).
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Bio 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Wu, Y.X.; von Tiedemann, A. Impact of Fungicides on Active Oxygen Species and Antioxidant Enzymes in Spring Barley (Hordeum vulgare L.) Exposed to Ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavanged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of Glutathione Reductase in Crude Tissue Homogenates Using 5,5′-Dithiobis(2-Nitrobenzoic Acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]




| Analysis | Reaction Solutions |
|---|---|
| Extraction | Solution:
|
| Total protein (PROT) | Biuret reagent:
|
| H2O2 | 200 μL 30% H2O2 added to 10 mL of dH2O. |
| Catalase (CAT) | 40 mL of 50 mM K-phosphate buffer (pH 7.0) [54]. |
| Ascorbate peroxidase (APX) | Solution:
|
| Guaiacol peroxidase (POX) | Solution:
|
| Glutathione reductase (GR) | Solution:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beniušytė, E.; Čėsnienė, I.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators. Plants 2023, 12, 255. https://doi.org/10.3390/plants12020255
Beniušytė E, Čėsnienė I, Sirgedaitė-Šėžienė V, Vaitiekūnaitė D. Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators. Plants. 2023; 12(2):255. https://doi.org/10.3390/plants12020255
Chicago/Turabian StyleBeniušytė, Emilija, Ieva Čėsnienė, Vaida Sirgedaitė-Šėžienė, and Dorotėja Vaitiekūnaitė. 2023. "Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators" Plants 12, no. 2: 255. https://doi.org/10.3390/plants12020255
APA StyleBeniušytė, E., Čėsnienė, I., Sirgedaitė-Šėžienė, V., & Vaitiekūnaitė, D. (2023). Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators. Plants, 12(2), 255. https://doi.org/10.3390/plants12020255







