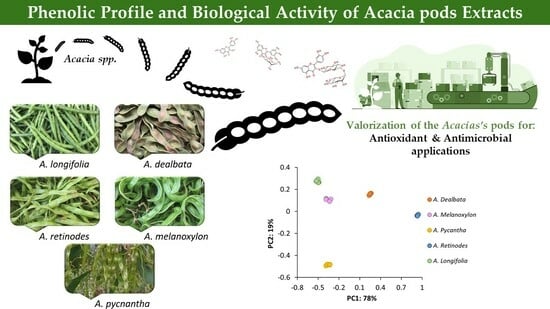
First Chemical Profile Analysis of Acacia Pods
Abstract
:1. Introduction
2. Results and Discussion
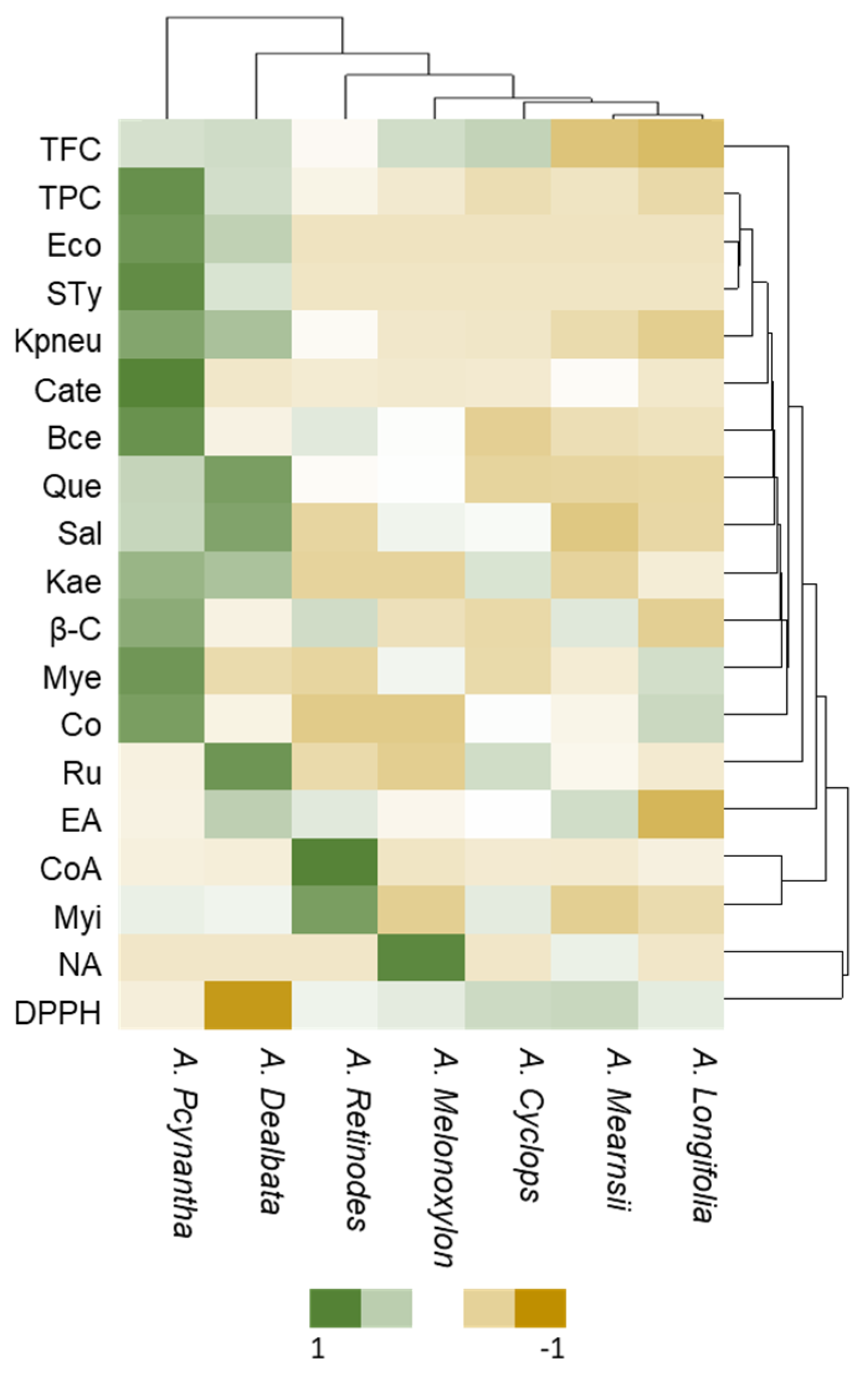
2.1. Determination of Total Phenolic and Flavonoid Contents
2.2. Determination of Antioxidant Properties
2.3. Determination of Antimicrobial Activity
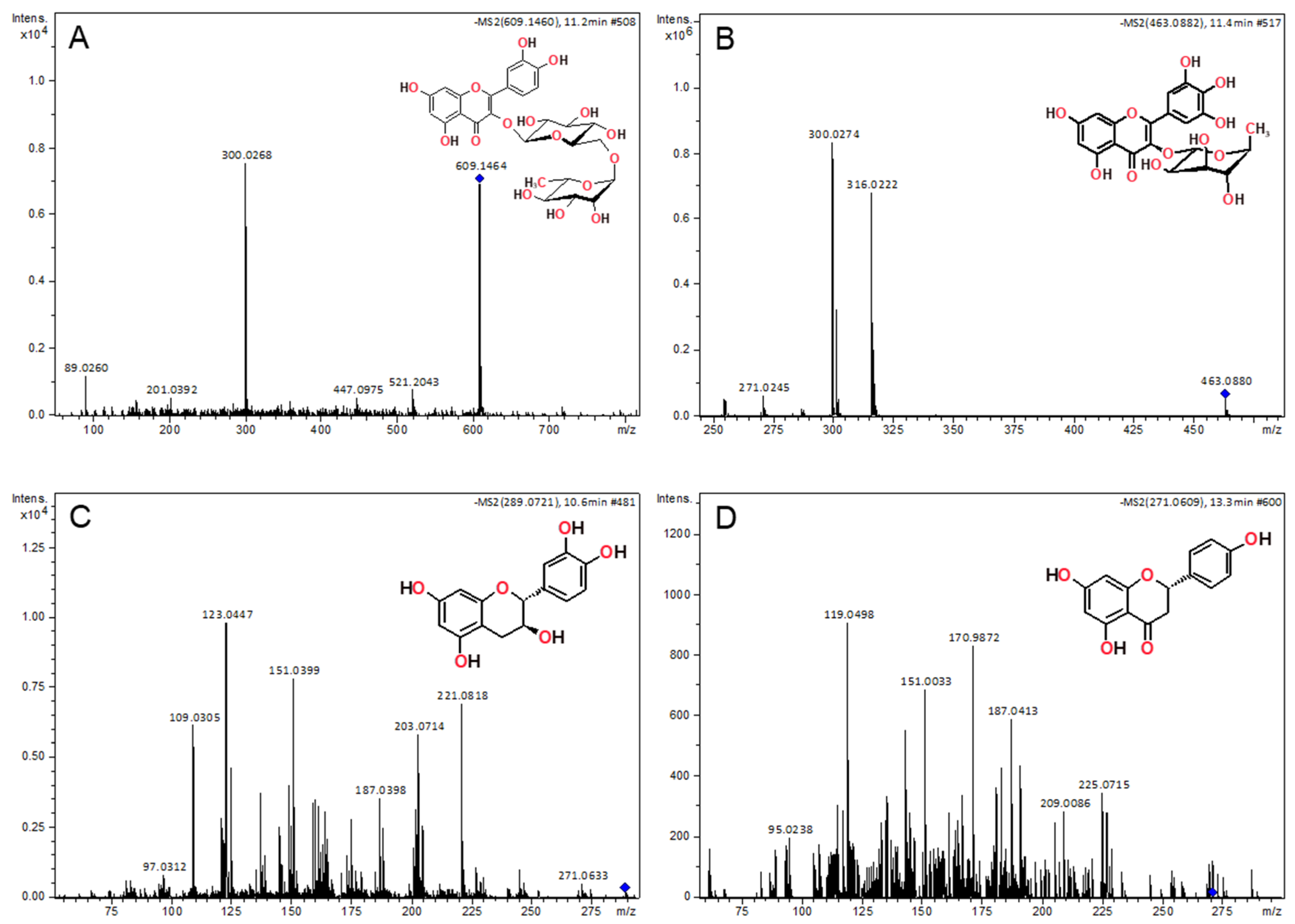
2.4. LC-ESI-HRMS/MS Analysis
2.5. HPLC-DAD Analysis
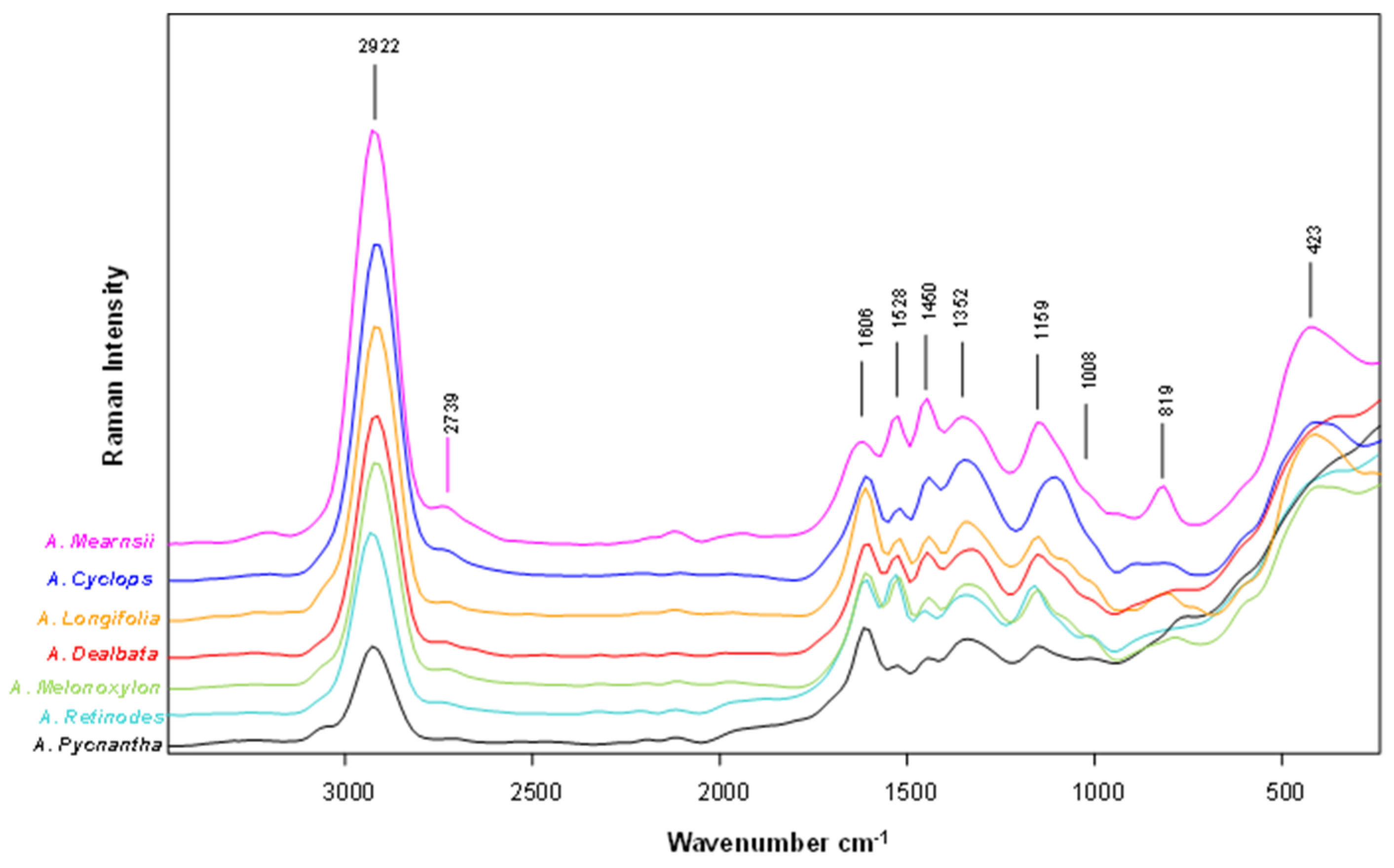
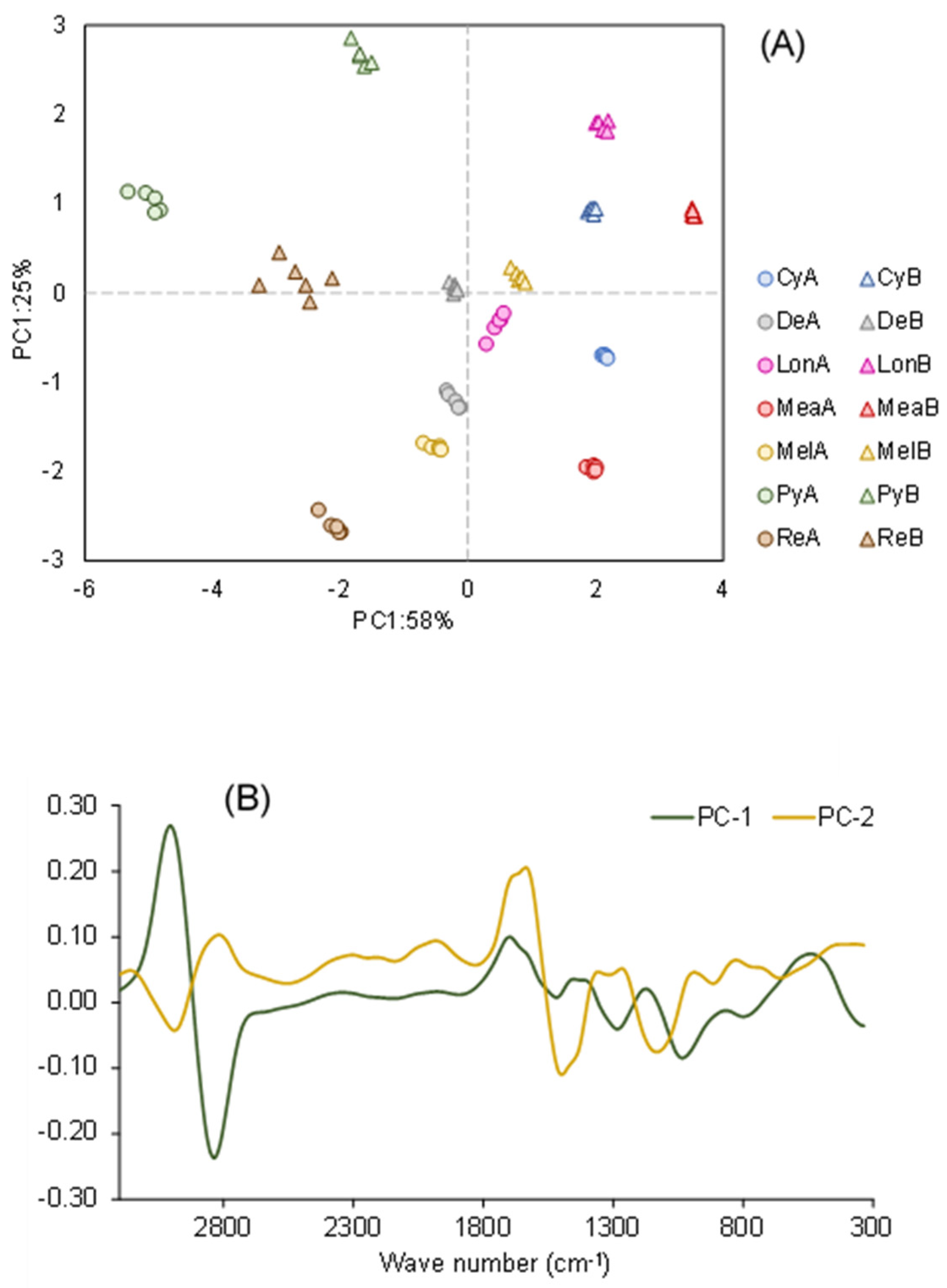
2.6. FT-Raman Spectral Analysis
3. Materials and Methods
3.1. Pods Material
3.2. Chemicals
3.3. Extraction Conditions
3.4. Total Phenolic Compounds Determination
3.5. Flavonoid Contents Determination
3.6. Evaluation of Antioxidant Properties
3.6.1. DPPH Scavenging Assay
3.6.2. β-Carotene Bleaching Test
3.7. Determination of Antimicrobial Activity
3.7.1. Test Microorganisms and Culture Media
3.7.2. Disc Diffusion Assay
3.7.3. Resazurin Microtiter Method
3.8. Chemical Analysis
3.8.1. LC-ESI-HRMS/MS Analysis
3.8.2. HPLC-DAD Analysis
3.8.3. Vibrational Spectroscopy
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Costa, R.M.F.; Bosch, M.; Simister, R.; Gomez, L.D.; Canhoto, J.M.; Batista de Carvalho, L.A.E. Valorisation Potential of Invasive Acacia dealbata, A. longifolia and A. melanoxylon from Land Clearings. Molecules 2022, 27, 7006. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Moreira, R.; Alves, P.; Gominho, J.; Fabião, A. Potential for Bioenergy Production of Eucalyptus and Invasive Acacias Under Mediterranean Climate. In Proceedings of the 8th Conference on Sustainable Development of Energy, Water and Environment Systems, Dubrovnik, Croatia, 22–27 September 2013; pp. 1–11. [Google Scholar]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Uushona, T.; Chikwanha, O.C.; Tayengwa, T.; Katiyatiya, C.L.F.; Strydom, P.E.; Mapiye, C. Nutraceutical and preservative potential of Acacia mearnsii and Acacia dealbata leaves for ruminant production and product quality enhancement. J. Agric. Sci. 2021, 159, 743–756. [Google Scholar] [CrossRef]
- Zothantluanga, J.H.; Bhat, H.R.; Shakya, A. A systematic review on the nutraceutical potential of Acacia pennata (L.) Willd. Curr. Trends Pharm. Res. 2019, 6, 12–27. [Google Scholar]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-care products formulated with natural antioxidant extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef]
- Fajraoui, A.; Ben Nasr, J.; Lacoste, C.; Amar, M.B.; Dony, P.; Odof, S.; El Halouani, F. Coloration of the polylactic acid with the natural dye extracted from Acacia cyanophylla flowers. Polym. Test. 2019, 78, 105988. [Google Scholar] [CrossRef]
- Imperato, F. A new chalcone glucoside and cernuoside from the flowers of Acacia dealbata. Experientia 1982, 38, 67–68. [Google Scholar] [CrossRef]
- Imperato, F. A chalcone glycoside from Acacia dealbata. Phytochemistry 1982, 21, 480–481. [Google Scholar] [CrossRef]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the valorization of biomass from portuguese invasive Acacia spp. in a biorefinery perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Durgadevi, G.; Karthika, N. Screening of phytochemicals and pharmacological studies on Mimosa pudica L. Asian J. Innov. Res. 2018, 3, 19–28. [Google Scholar]
- Parvez, M.K.; Al-Dosari, M.S.; Arbab, A.H.; Al-Rehaily, A.J.; Abdelwahid, M.A.S. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm. J. 2020, 28, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Kim, A.; Choi, J.; Myo, K.; Chin, Y.; Kim, J.; Dong, K. Phytochemistry Flavonoid glycosides from the aerial parts of Acacia pennata in Myanmar. Phytochemistry 2015, 118, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef]
- Pedro, S.I.; Rosado, T.; Barroca, C.; Neiva, D.; Alonso-herranz, V.; Gradillas, A.; Garc, A.; Gominho, J.; Gallardo, E. Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts. Plants 2022, 11, 1442. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Tharaphan, P.; Chotivanich, K.; Tarning, J.; Anal, A.K. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Jelassi, A.; El Ayeb-Zakhama, A.; Nejma, A.B.; Chaari, A.; Harzallah-Skhiri, F.; Jannet, H. Ben Phytochemical composition and allelopathic potential of three Tunisian Acacia species. Ind. Crops Prod. 2016, 83, 339–345. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- March, R.E.; Miao, X.S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Sarma, B.K.; Singh, H.B. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem. Toxicol. 2009, 47, 778–786. [Google Scholar] [CrossRef]
- Faria, A.F.; Marques, M.C.; Mercadante, A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011, 126, 1571–1578. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-L.; Wan, D.; Fang, L.-Z.; Zou, D.-Z.; Zhang, S.-H. α-Glucosidase Inhibitory Activity-guided Identification of Compounds from Clerodendrum bungei Steud by HPLC-ESI- QTOF-MS/MS. Digit. Chin. Med. 2019, 2, 41–49. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Li, Q.M.; Van Den Heuvel, H.; Claeys, M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Constant, H.L.; Slowing, K.; Graham, J.G.; Pezzuto, J.M.; Cordell, G.A.; Beecher, C.W.W. A general method for the dereplication of flavonoid glycosides utilizing high performance liquid chromatography/mass spectrometric analysis. Phytochem. Anal. 1997, 8, 176–180. [Google Scholar] [CrossRef]
- Costa, A.R.M.; Freitas, L.A.P.; Mendiola, J.; Ibáñez, E. Copaifera langsdorffii supercritical fluid extraction: Chemical and functional characterization by LC/MS and in vitro assays. J. Supercrit. Fluids 2015, 100, 86–96. [Google Scholar] [CrossRef]
- Edimecheva, I.P.; Kisel, R.M.; Shadyro, O.I.; Kazem, K.; Murase, H.; Kagiya, T. Homolytic cleavage of the O-glycoside bond in carbohydrates: A steady-state radiolysis study. J. Radiat. Res. 2005, 46, 319–324. [Google Scholar] [CrossRef]
- Islam, S.; Alam, M.B.; Ann, H.J.; Park, J.H.; Lee, S.H.; Kim, S. Metabolite profiling of Manilkara zapota L. Leaves by high-resolution mass spectrometry coupled with esi and apci and in vitro antioxidant activity, α-glucosidase, and elastase inhibition assays. Int. J. Mol. Sci. 2021, 22, 132. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Nowacka, N. Plant Polyphenols as Chemopreventive Agents. Polyphenols Hum. Heal. Dis. 2013, 2, 1289–1307. [Google Scholar] [CrossRef]
- Wang, J.; Ting, Y.; Chena, Y.; Wang, Y. Rapid And Sensitive High-performance Liquid Chromatography/tandem Mass Spectrometry Method For Determination of Danshensu In Rat Plasma. J. Proteomics Genomics Res. 2013, 1, 43–49. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Antunes, A.M.M.; Caldeira, I.; Anjos, O.; de Freitas, V.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Identification of gallotannins and ellagitannins in aged wine spirits: A new perspective using alternative ageing technology and high-resolution mass spectrometry. Food Chem. 2022, 382, 132322. [Google Scholar] [CrossRef]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Sohn, M.; Himmelsbach, D.S.; Kays, S.E.; Archibald, D.D.; Barton, F.E. NIR-FT/Raman Spectroscopy for Nutritional Classification of Cereal Foods. Cereal Chem. J. 2005, 82, 660–665. [Google Scholar] [CrossRef]
- White, J.L. Interpretation of infrared spectra of soil minerals. Soil Sci. 1971, 112, 22–31. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Payne, W.Z.; Kurouski, D. Raman-Based Diagnostics of Biotic anAbiotic Stresses in Plants. A Reviewd. Front. Plant Sci. 2021, 11, 616672. [Google Scholar] [CrossRef]
- Krimmer, M.; Farber, C.; Kurouski, D. Rapid and Noninvasive Typing and Assessment of Nutrient Content of Maize Kernels Using a Handheld Raman Spectrometer. ACS Omega 2019, 4, 16330–16335. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, Y.; Yin, Y.; Pu, Y.; Jackson, L.A.; Engle, N.L.; Martin, M.Z.; Tschaplinski, T.J.; Ding, S.; Ragauskas, A.J.; et al. Phytochemistry Pinoresinol reductase 1 impacts lignin distribution during secondary cell wall biosynthesis in Arabidopsis. Phytochemistry 2015, 112, 170–178. [Google Scholar] [CrossRef]
- Abdalla, M.S.A.; Babiker, I.A.; Idris, A.M.; Elkalifa, K.F. Potential Nutrient Composition of Acacia seyal Fruits as Fodder for Livestock in the Dry Lands in Sudan. Dev. Anal. Chem. 2014, 1, 25–30. [Google Scholar]
- Pedro, S.I.; Antunes, C.A.L.; Horta, C.; Pitacas, I.; Gonçalves, J.; Gominho, J.; Gallardo, E.; Anjos, O. Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods. Plants 2023, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Anjos, O.; Santos, A.J.A.; Paixão, V.; Estevinho, L.M. Physicochemical characterization of Lavandula spp. honey with FT-Raman spectroscopy. Talanta 2018, 178, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.L.R.; Stephani, R.; Poppi, R.J.; De Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a source of antioxidant and antimicrobial polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef]
- Anjos, O.; Caldeira, I.; Pedro, S.I.; Canas, S. FT-Raman methodology applied to identify different ageing stages of wine spirits. LWT 2020, 134, 1–9. [Google Scholar] [CrossRef]

| Acacia species | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| A. melanoxylon | 0.32 ± 0.02 c | 6.38 ± 0.42 b |
| A. longifolia | 0.12 ± 0.00 a | 4.48 ± 0.13 a |
| A. cyclops | 0.17 ± 0.00 ab | 6.55 ± 0.15 b |
| A. retinodes | 0.44 ± 0.02 d | 5.66 ± 0.17 ab |
| A. pycnantha | 1.75 ± 0.06 f | 6.32 ± 0.58 b |
| A. mearnsii | 0.25 ± 0.06 bc | 4.62 ± 0.86 a |
| A. dealbata | 0.92 ± 0.01 e | 6.39 ± 0.22 b |
| Acacia Species | DPPH Free Radical Scavenging Assay | β-Carotene Bleaching Test | |
|---|---|---|---|
| IC50 (mg/L) | AAI | IC50 (mg/L) | |
| A. melanoxylon | 7165 ± 730 bc | 0.00 ± 0.00 a | 3526 ± 1997 abc |
| A. longifolia | 7158 ± 741 bc | 0.00 ± 0.00 a | 6582± 547 cd |
| A. cyclops | 7969 ± 474 c | 0.01 ± 0.00 a | 6048 ± 961 bcd |
| A. retinodes | 6815 ± 516 bc | 0.01 ± 0.00 a | 2890 ± 395 a |
| A. pycnantha | 5399 ± 2519 b | 0.04 ± 0.05 a | 4397 ± 398 abc |
| A. mearnsii | 8111 ± 570 c | 0.01 ± 0.00 a | 8786 ± 2817 d |
| A. dealbata | 1033 ± 7 a | 0.04 ± 0.00 a | 3148 ± 208 ab |
| Positive control—BHT | - | - | 78.0 ± 6.2 |
| Acacia Species (4 mg/Disk) | Gram-Negative Bacteria | Gram-Positive Bacteria | Candida albicans ATCC 90028 | ||||
|---|---|---|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 | Salmonella Typhimurium ATCC 13311 | Bacillus cereus ATCC 11778 | Staphylococcus aureus ATCC 29213 | Listeria monocytogenes LMG 16779 | ||
| A. melanoxylon | 6.00 ± 0.00 a | 9.26 ± 0.41 c | 6.00 ± 0.00 a | 9.19 ± 0.26 c | 10.59 ± 0.78 b | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. longifolia | 6.00 ± 0.00 a | 7.98 ± 0.36 a | 6.00 ± 0.00 a | 8.07 ± 0.43 b | 8.12 ± 0.12 a | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. cyclops | 6.00 ± 0.00 a | 9.25 ± 0.35 c | 6.00 ± 0.00 a | 7.17 ± 0.19 a | 10.27 ± 0.61 b | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. retinodes | 6.00 ± 0.00 a | 10.55 ± 0.20 d | 6.00 ± 0.00 a | 9.94 ± 0.41 c | 8.14 ± 0.74 a | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. pycnantha | 8.26 ± 0.29 c | 14.47 ± 0.47 f | 9.36 ± 0.06 c | 12.76 ± 0.23 d | 12.07 ± 0.19 c | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. mearnsii | 6.00 ± 0.00 a | 8.52 ± 0.52 b | 6.00 ± 0.00 a | 8.27 ± 0.91 b | 7.35 ± 0.18 a | 6.00 ± 0.00 | 6.00 ± 0.00 |
| A. dealbata | 7.56 ± 0.51 b | 14.39 ± 0.61 e | 7.30 ± 0.06 b | 8.41 ± 0.36 b | 14.20 ± 0.44 d | 6.00 ± 0.00 | 6.00 ± 0.00 |
| DMSO (20 µL/disk) | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| Tetracycline (30 µg/disk) | 23.25 ± 0.50 | 22.25 ± 0.50 | 11.50 ± 0.58 | 30.00 ± 0.82 | 30.25 ± 0.50 | 18.25 ± 0.60 | - |
| Amphotericin B (25 µg/disk) | - | - | - | - | - | - | 20.33 ± 0.58 |
| Acacia Species | Gram-Negative Bacteria | Gram-Positive Bacteria | Candida albicans ATCC 90028 | ||||
|---|---|---|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 | Salmonella Typhimurium ATCC 13311 | Bacillus cereus ATCC 11778 | Staphylococcus aureus ATCC 29213 | Listeria monocytogenes LMG 16779 | ||
| A. melanoxylon | >10 | 10 | >10 | 1.25 | 10 | >10 | >10 |
| A. longifolia | >10 | >10 | >10 | 5 | 10 | >10 | >10 |
| A. cyclops | >10 | 10 | >10 | 2.5 | >10 | >10 | >10 |
| A. retinodes | >10 | 10 | >10 | 1.25 | >10 | >10 | >10 |
| A. pycnantha | 5 | 2.5 | 10 | 0.313 | 2.5 | >10 | >10 |
| A. mearnsii | >10 | >10 | >10 | 1.25 | 10 | >10 | >10 |
| A. dealbata | 5 | 2.5 | 10 | 0.625 | 5 | >10 | >10 |
| Tetracycline (µg/mL) | 0.06 | 0.06 | 0.24 | 0.06 | 0.06 | 0.06 | - |
| Amphotericin B (µg/mL) | - | - | - | - | - | - | 0.25 |
| Identified Compound | RTa (min) | Molecular Formula | [M+H]+ (m/z Exp) | Error (ppm) | Fragment Ions m/z (Error ppm, Molecular Formula) |
|---|---|---|---|---|---|
| kaempferol | 13.2 | C15H10O6 | 287.0567 | 5.9 | 269.0464 (7.2, C15H9O5+) 153.0184 (1.1, C7H5O4+) 135.0445 (3.3, C8H7O2+) |
| quercetin | 13.0 | C15H10O7 | 303.0522 | 7.5 | 257.0461 (6.4, C14H9O5+) 229.0508 (5.5, C13H9O4+) 201.0549 (1.4, C12H9O3..+) 153.0176 (−4.1, C7H5O4+) 137.0225 (−6.0, C7H5O3+) |
| myricetin | 11.5 | C15H10O8 | 319.0465 | 5.2 | 273.0426 (11.8, C14H9O6+) 245.0456 (0.4, C13H9O5+) 217.0516 (9.5, C12H9O4+) 153.0174 (−5.5, C7H5O4+) 137.0592 (−3.7, C8H9O2+) |
| Identified Compound | RTa (min) | Molecular Formula | [M−H]− (m/z Exp) | Error (ppm) | Fragment Ions, m/z (Error ppm, Molecular Formula) |
|---|---|---|---|---|---|
| rutin | 11.2 | C27H30O16 | 609.1464 | 0.5 | 300.0263 (−4.2, C15H8O7●−) |
| myricitrin | 11.4 | C21H20O12 | 463.0880 | −0.4 | 316.0222 (−0.8, C15H8O7●−) 300.0263 (−4.2, C15H8O7●−) |
| (+)-catechin | 10.6 | C15H14O6 | 289.0721 | 1.2 | 245.0828 (3.5, C14H13O4−) 221.0818 (−0.6, C12H13O4−) 203.0714 (0.2, C12H11O3−) 187.0398 (−1.4, C11H7O3−) 151.0399 (2.0, C7H3O4−) 123.0447 (−3.6, C7H7O2−) 109.0305 (9.1, C6H5O2−) |
| naringenin | 13.4 | C15H12O5 | 271.0609 | −1.1 | 187.0413 (6.5, C11H7O3−) 119.0498 (−3.7, C8H7O−) 151.0033 (−2.5, C7H3O4−) |
| Identified Compound | RTa (min) | Molecular Formula | [M−H]− (m/z Exp) | Error (ppm) | Fragment Ions m/z (Error ppm, Molecular Formula) |
|---|---|---|---|---|---|
| 4-hydroxybenzoic acid | 13.4 | C7H6O3 | 137.0236 | −6.0 | 93.0344 (−2.0, C6H5O−) |
| ellagic acid | 11.7 | C14H6O8 | 300.9989 602.9995 [2M−H]− | −0.3 | 283.9976 (4.7, C14H4O7−) 229.0144 (0.7, C12H5O5−) 185.0254 (5.3, C11H5O3−) |
| p-coumaric acid | 12.0 | C9H8O3 | 163.0394 | −4.1 | 191.0491 (−5.0, C8H7O−) 93.0351 (5.5, C6H5O−) |
| Compound | tR (min) | λmax (nm) | De | Lon | Re | Py | Mel | Cy | Mea |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||||
| 4-hydroxybenzoic acid | 9.8 | 255 | <LOQ ▪ | <LOQ ▪ | <LOQ ▪ | <LOQ ▪ | 0.21 ± 0.03 b | <LOQ ▪ | 0.06 ± 0.00 a |
| ellagic acid | 25.66 | 255 | 0.57 ± 0.53 a | <LOQ ▪▪ | 0.45 ± 0.00 a | 0.30 ± 0.00 a | 0.32 ± 0.00 a | 0.35 ± 0.06 a | <LOQ ▪▪ |
| Hydroxycinnamic acids | |||||||||
| p-coumaric acid | 19.8 | 291 | 0.40 ± 0.04 a | 0.47 ± 0.03 a | 4.12 ± 0.35 b | 0.45 ± 0.01 a | 0.13 ± 0.01 | 0.29 ± 0.11 a | 0.28 ± 0.01 a |
| Hydroxycinnamic aldehydes | |||||||||
| coniferaldehyde | 29.20 | 322 | 0.05 ± 0.00 a | 0.12 ± 0.00 b | <LOQ ▪▪ | 0.19 ± 0.01 c | <LOQ ▪▪ | 0.07 ± 0.01 ab | 0.06 ± 0.00 d |
| Flavan-3-ol | |||||||||
| (+)-catechin | 9.0 | 280 | <LOQ ▪▪▪ | 0.01 ± 0.00 ab | 0.04 ± 0.01 b | 1.19 ± 0.01 d | 0.02 ± 0.00 ab | 0.03 ± 0.00 ab | 0.18 ± 0.01 c |
| Flavonols | |||||||||
| rutin | 31.9 | 255 | 25.91 ± 1.31 d | 5.07 ± 2.31 a | 2.11 ± 0.30 a | 6.35 ± 0.74 a | <LOQ ▪▪ | 14.35 ± 2.19 c | 8.57 ± 6.03 b |
| myricitrin | 33.4 | 263 | 2.44 ± 0.71 ab | 0.52 ± 0.07 a | 5.71 ± 1.21 c | 2.61 ± 0.13 ab | <LOQ ▪▪ | 2.80 ± 0.13 b | <LOQ ▪▪ |
| myricetin | 34.5 | 360 | 0.05 ± 0.01 a | 0.56 ± 0.03 d | <LOQ ▪▪ | 1.06 ± 0.01 e | 0.39 ± 0.07 c | 0.04 ± 0.00 a | 0.18 ± 0.01 b |
| quercetin | 41.0 | 360 | 1.20 ± 0.13 d | 0.24 ± 0.02 a | 0.51 ± 0.06 b | 0.83 ± 0.08 c | 0.55 ± 0.04 b | 0.21 ± 0.08 a | 0.22 ± 0.02 a |
| kaempferol | 45.3 | 360 | 0.10 ± 0.01 b | 0.03 ± 0.00 a | <LOQ ▪▪ | 0.11 ± 0.00 b | <LOQ ▪▪ | 0.07 ± 0.03 | <LOQ ▪▪ |
| Flavanone | |||||||||
| naringenin | 43.5 | 280 | 0.17 ± 0.01 ab | 0.45 ± 0.07 bc | <LOQ ▪▪ | 0.74 ± 0.14 c | 0.07 ± 0.02 a | 0.07 ± 0.03 | 0.02 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, S.I.; Fernandes, T.A.; Luís, Â.; Antunes, A.M.M.; Gonçalves, J.C.; Gominho, J.; Gallardo, E.; Anjos, O. First Chemical Profile Analysis of Acacia Pods. Plants 2023, 12, 3486. https://doi.org/10.3390/plants12193486
Pedro SI, Fernandes TA, Luís Â, Antunes AMM, Gonçalves JC, Gominho J, Gallardo E, Anjos O. First Chemical Profile Analysis of Acacia Pods. Plants. 2023; 12(19):3486. https://doi.org/10.3390/plants12193486
Chicago/Turabian StylePedro, Soraia I., Tiago A. Fernandes, Ângelo Luís, Alexandra M. M. Antunes, José C. Gonçalves, Jorge Gominho, Eugenia Gallardo, and Ofélia Anjos. 2023. "First Chemical Profile Analysis of Acacia Pods" Plants 12, no. 19: 3486. https://doi.org/10.3390/plants12193486
APA StylePedro, S. I., Fernandes, T. A., Luís, Â., Antunes, A. M. M., Gonçalves, J. C., Gominho, J., Gallardo, E., & Anjos, O. (2023). First Chemical Profile Analysis of Acacia Pods. Plants, 12(19), 3486. https://doi.org/10.3390/plants12193486