MsYSL6, A Metal Transporter Gene of Alfalfa, Increases Iron Accumulation and Benefits Cadmium Resistance
Abstract
:1. Introduction
2. Results
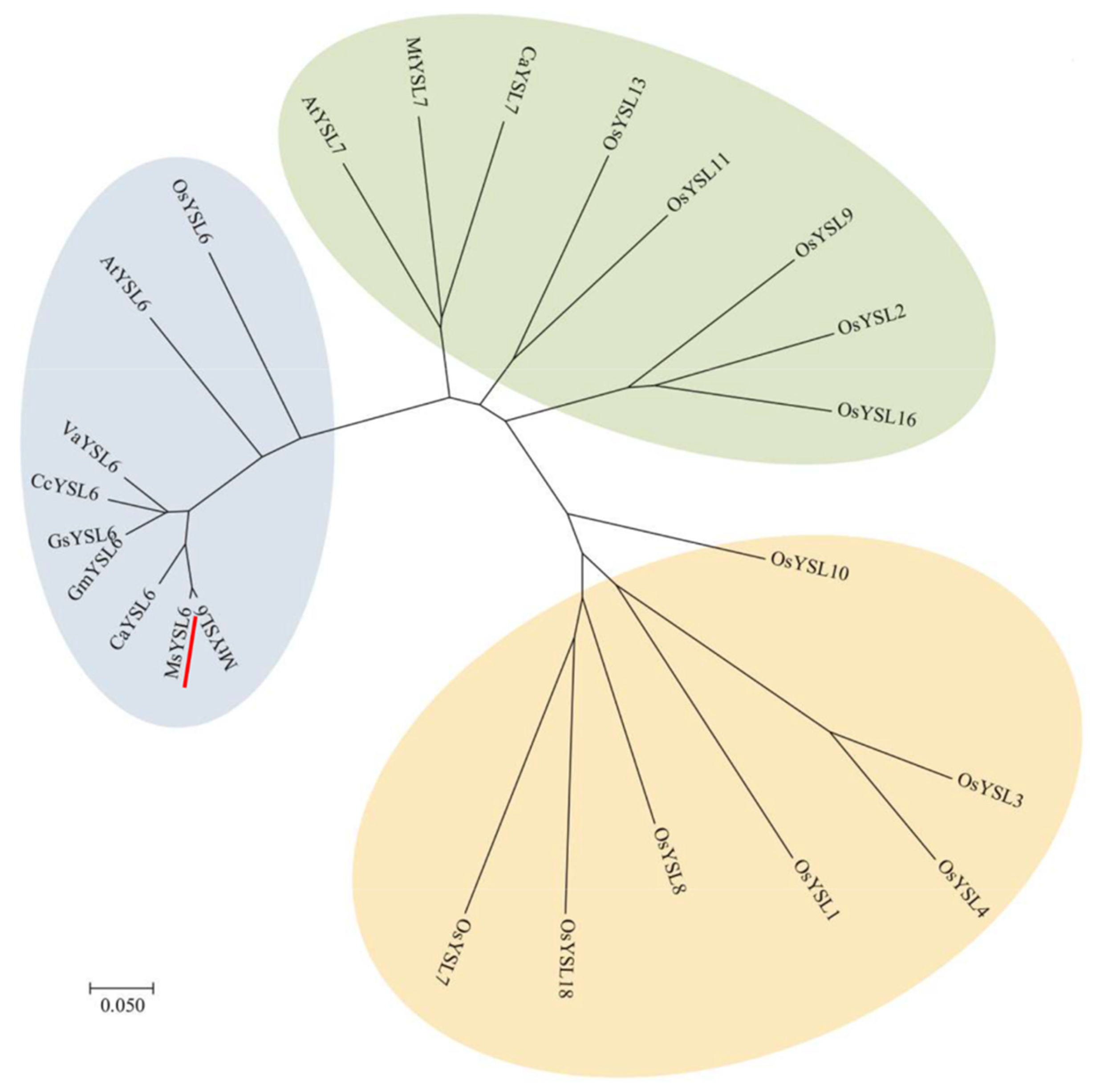
2.1. MsYSL6 Belongs to the YSL Transporter Family
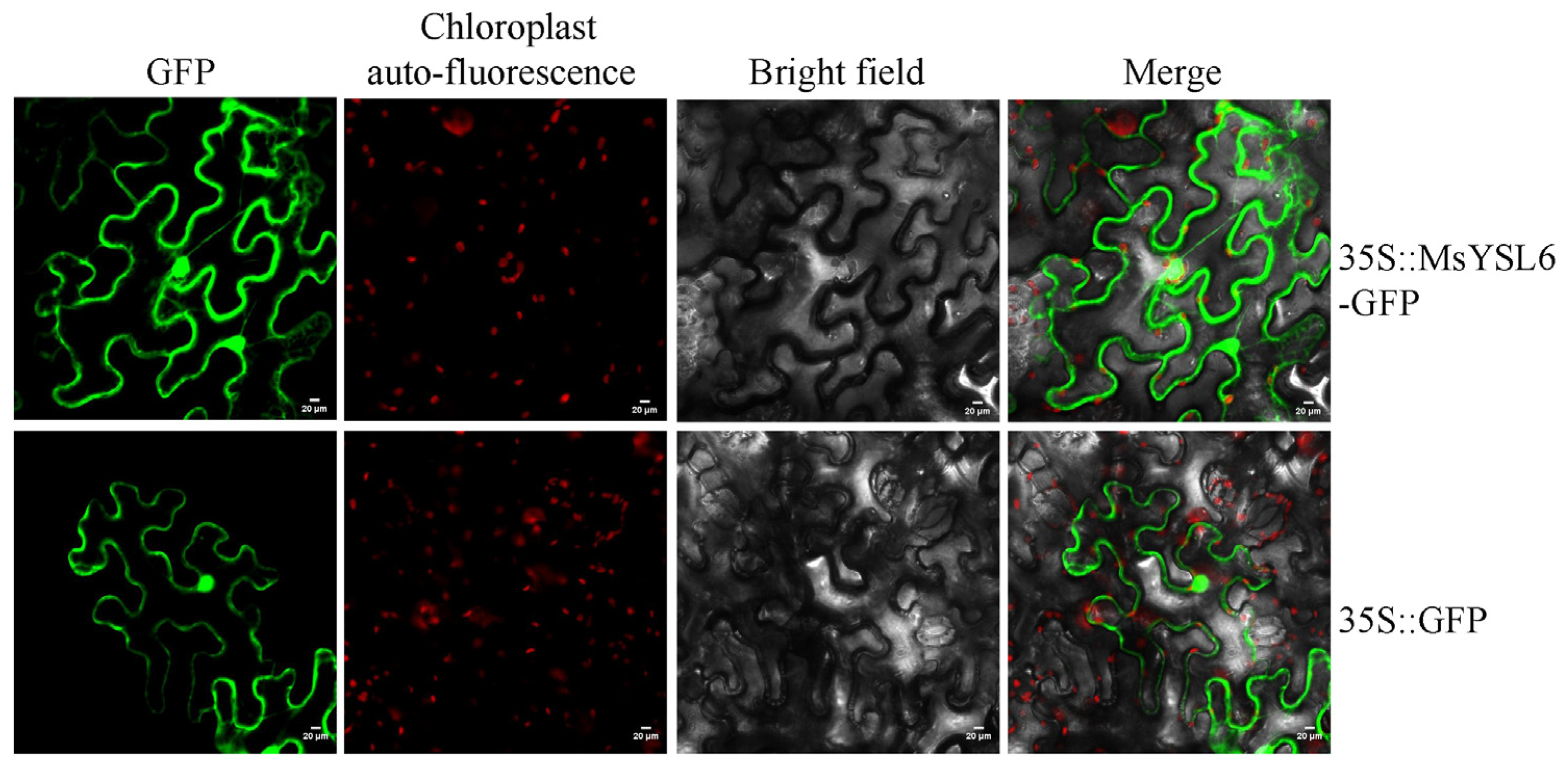
2.2. The Subcellular Localization of MsYSL6
2.3. The Expression of MsYSL6 in Alfalfa
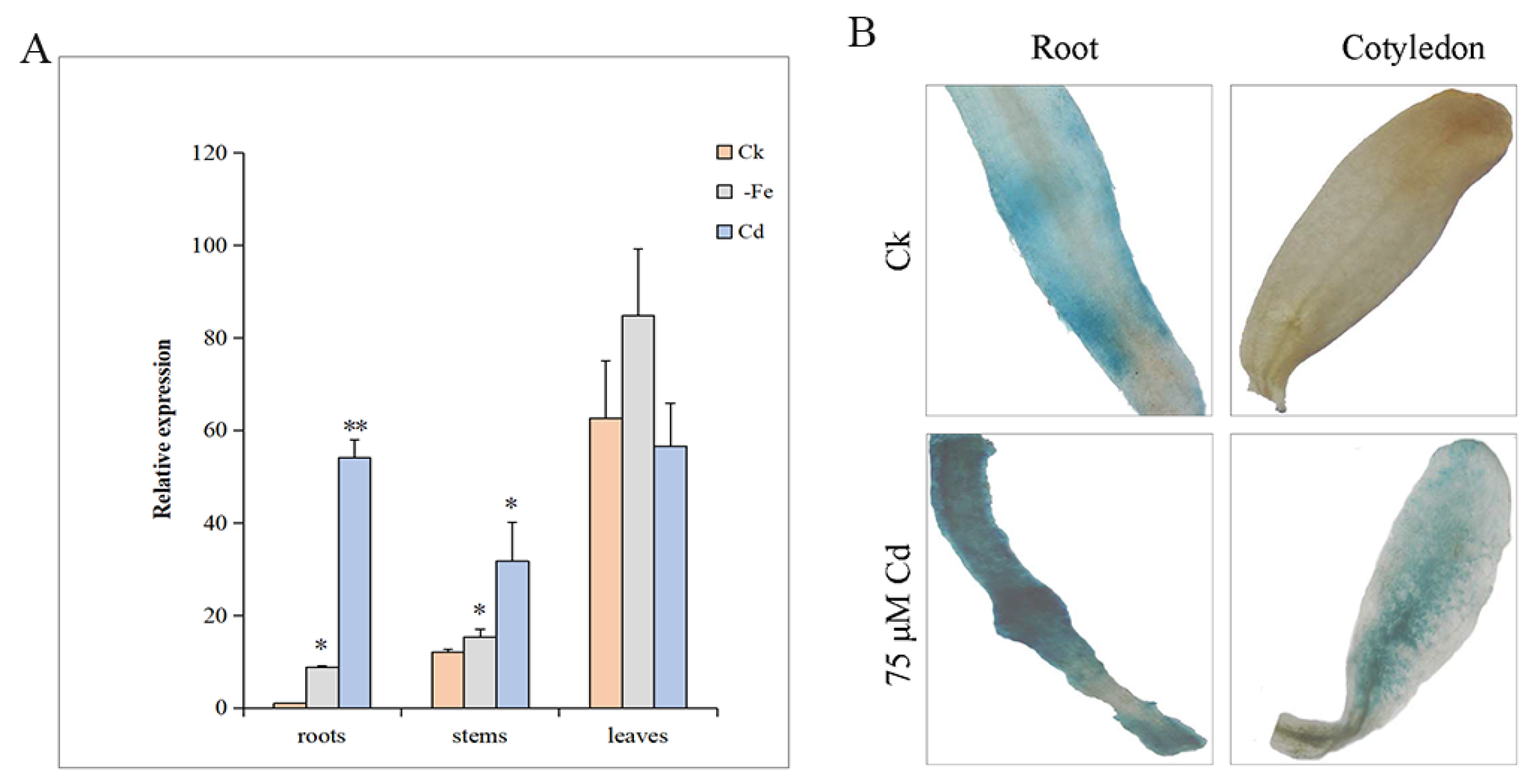
2.4. The GUS Activity
2.5. The Growth of MsYSL6 Transgenic Tobacco
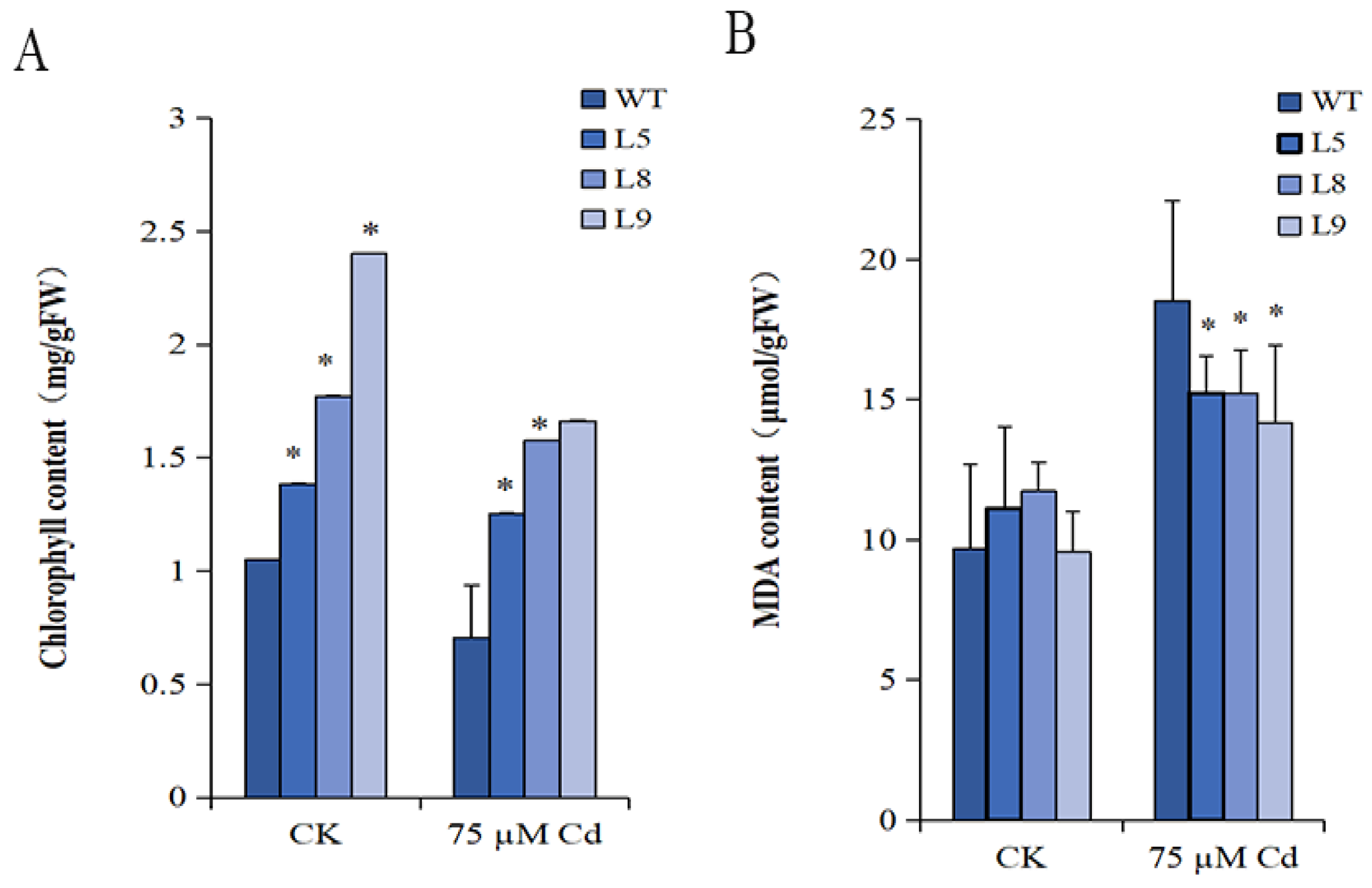
2.6. The Chlorophyll Content
2.7. The MDA Content
2.8. The DAB and NBT Staining
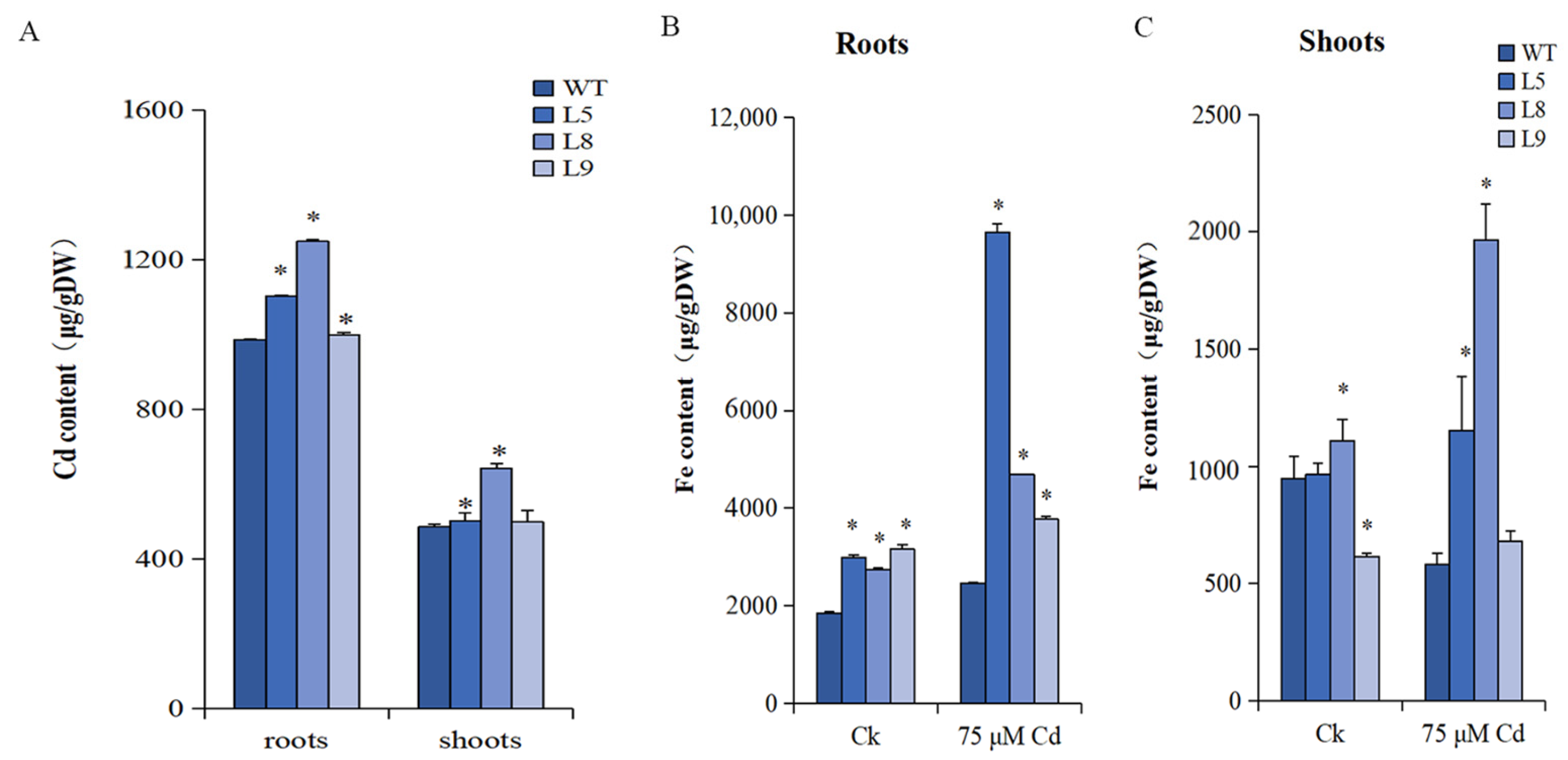
2.9. The Cd Content and Translocation Rate
2.10. The Fe Content and Translocation Rate
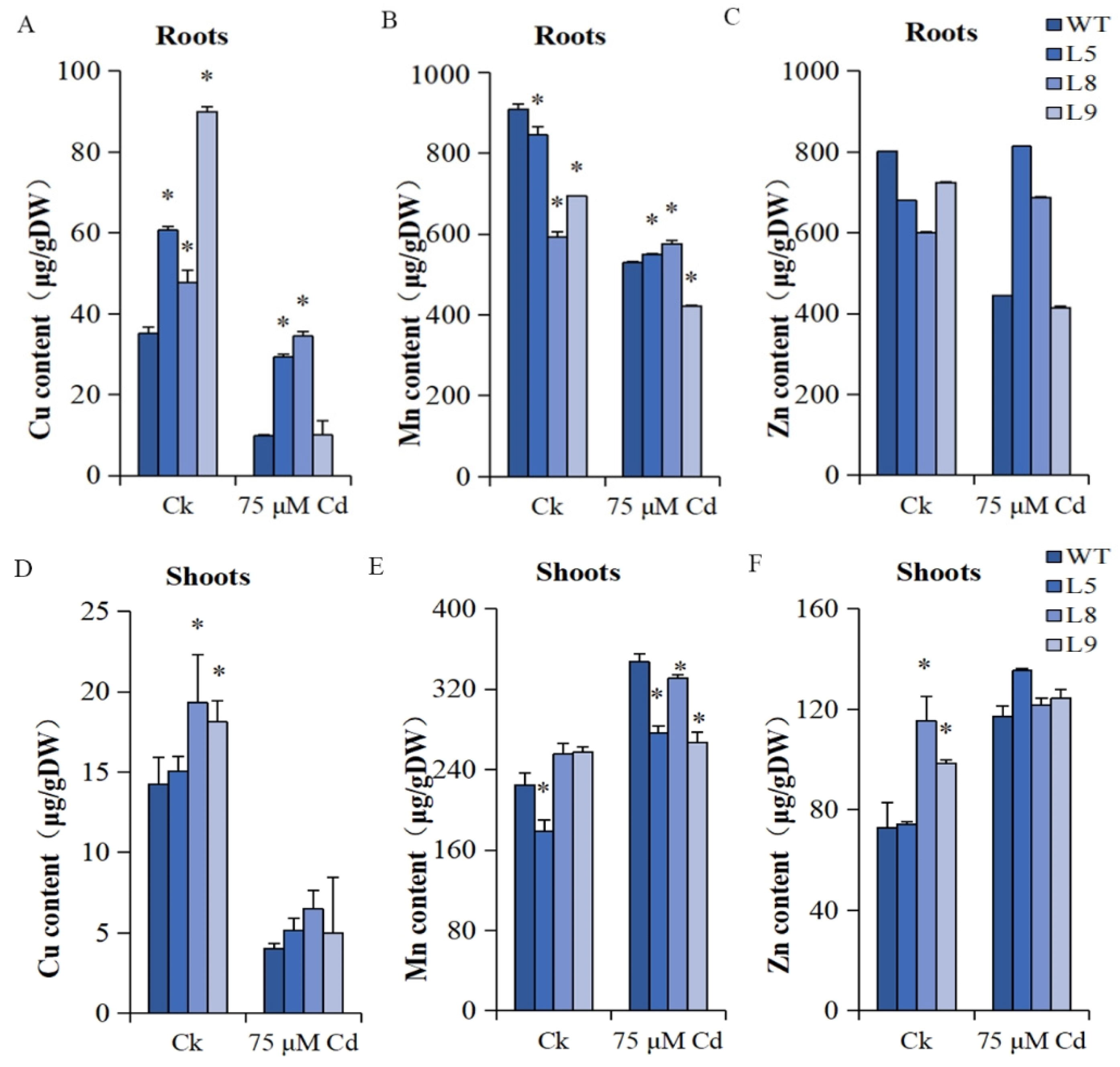
2.11. The Cu, Mn, and Zn Content
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. RNA and DNA Extraction
4.3. Cloning of MsYSL6 and MsYSL6pro
4.4. Subcellular Localization of MsYSL6
4.5. qRT-PCR
4.6. GUS Assay
4.7. Plant Transformation and Selection
4.8. Phenotypic and Physiological Analysis
4.9. Oxidative Analysis
4.10. Metal Content Determination
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merry, R.; Espina, M.J.; Lorenz, A.J.; Stupar, R.M. Development of a controlled-environment assay to induce iron deficiency chlorosis in soybean by adjusting calcium carbonates, pH, and nodulation. Plant Methods 2022, 18, 36. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M.L. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Fari, D.Q.; Ali, B.; Hayat, S.; Ahmad, A. Cd: Toxicity and tolerance in plants. J. Environ. Biol. 2009, 30, 165–174. [Google Scholar] [CrossRef]
- De Maria, S.; Pus, C.R.M.; Rivelli, A.R. Cd accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil. Environ. 2013, 59, 254–261. [Google Scholar] [CrossRef]
- Han, Z.; Wei, X.; Wan, D.; He, W.; Wang, X.; Xiong, Y. Effect of Molybdenum on Plant Physiology and Cd Uptake and Translocation in Rape (Brassica napus L.) under Different Levels of Cd Stress. Int. J. Environ. Res. Public Health 2020, 17, 2355. [Google Scholar] [CrossRef]
- Rodríguez, S.M.; Romero, P.M.C.; Paz, M.D.M.; Testi, L.P.S.; Risue, N.M.C.; Del, R.L.A.; Sanda, L.L.M. Cellular response of pea plants to Cd toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Th, M.S.; Wang, R.; Ward, J.M.; Crawford, N.M.; Schroeder, J.I. Cd and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Seno, U.T.; Shi, M.H.; Ishikawa, S.; Arao, T.; Naka, N.H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Ishi, M.Y.; Taki, H.R.; Bashir, K.; Shi, M.H.; Seno, U.T.; Sugi, M.K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T.; et al. Characterizing the role of rice NRAMP5 in Manganese, Iron and Cd Transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef]
- Chen, S.; Han, X.; Fang, J.; Lu, Z.; Qiu, W.; Liu, M.; Sang, J.; Jiang, J.; Zhuo, R. Sedum alfredii SaNramp6 Metal Transporter Contributes to Cd Accumulation in Transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 13318. [Google Scholar] [CrossRef]
- Naka, N.H.; Oga, W.I.; Ishi, M.Y.; Mori, S.; Nishi, Z.N.K. Iron deficiency enhances Cd uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Mendoza, C.D.G.; Xie, Q.; Akma, J.G.Z.; Jobe, T.O.; Patel, A.; Stacey, M.G.; Song, L.; De, M.D.W.; Juri, S.S.S.; Stacey, G.; et al. OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of Cd in seeds. Mol. Plant 2014, 7, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, J.; Ahmadi, H.; Scheepers, M.; Weber, M.; Nitsche, S.; Carnol, M.; Bosman, B.; Kroy, M.J.; Motte, P.; Clemens, S.; et al. The two copies of the zinc and Cd ZIP6 transporter of Arabidopsis halleri have distinct effects on Cd tolerance. Plant Cell Environ. 2020, 43, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Wen, N.; Zhang, C.; Wang, J.; Liu, Z. Modeling uptake of Cd from solution outside of root to cell wall of shoot in rice seedling. Plant Growth Regul. 2017, 82, 11–20. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, H.; Upadhyay, S.K. Cation Transporters in Plants; Elsevier: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Morel, M.; Crouzet, J.; Gra, V.A.; Au, R.P.; Leon, H.N.; Vavasseur, A.; Rich, A.P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef]
- Gong, X.; Yin, L.; Chen, J.; Guo, C. Overexpression of the iron transporter NtPIC1 in tobacco mediates tolerance to Cd. Plant Cell Rep. 2015, 34, 1963–1973. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Guo, H.; Hu, Y.; He, Y.; Jiang, D. Overexpression of a Miscanthus sacchariflorus yellow stripe-like transporter MsYSL1 enhances resistance of Arabidopsis to Cd by mediating metal ion reallocation. Plant Growth Regul. 2018, 85, 101–111. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Guo, H.; Dean, J. MsYSL1 of Miscanthus sacchariflorus enhances resistance of Arabidopsis to cadmium by mediating metal ion reallocation. In Proceedings of the 7th Yangtze River Delta Plant Science Seminar and Youth Academic Report, Shanghai, China, 12 October 2018. [Google Scholar]
- Shu, M.A.; Tyagi, S.; Sharma, Y.; Ma, D.H.; Sharma, A.; Pandey, A.; Sin, G.K.; Upadhyay, S.K. Expression of TaNCL2-A ameliorates cadmium toxicity by increasing calcium and enzymatic antioxidants activities in Arabidopsis. Chemosphere 2023, 329, 138636. [Google Scholar] [CrossRef]
- Ban, S.R.; Priya, S.; Dikshit, H.K.; Jacob, S.R.; Rao, M.; Bana, R.S.; Ku, M.J.; Tripathi, K.; Ku, M.A.; Kumar, S.H.M.; et al. Growth and Antioxidant Responses in Iron-Biofortified Lentil under Cd Stress. Toxics 2021, 9, 182. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Du, J.; Liu, H.; Cui, Y.; Zhang, Y.; He, Y.; Wang, Y.; Chu, C.; Feng, Z.; et al. Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced Cd tolerance via increased Cd sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012, 158, 790–800. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; He, X.; Xu, L.; Huang, Y.; Shao, H.; Zhang, D.; Tang, B.; Ma, H. The Soybean Basic Helix-Loop-Helix Transcription Factor ORG3-Like Enhances Cd Tolerance via Increased Iron and Reduced Cd Uptake and Transport from Roots to Shoots. Front. Plant Sci. 2017, 8, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Siedlecka, A.; Kru, P.Z. Cd/Fe interaction in higher plants-its consequences for the photosynthetic apparatus. Photosynthetica 1999, 36, 321–331. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Xiao, D.S.; Qiu, C.P.; Han, D.G.; Zhang, X.Z.; Wu, T.; Han, Z.H. Comparison of Cd-induced iron-deficiency responses and genuine iron-deficiency responses in Malus xiaojinensis. Plant Sci. 2011, 181, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.S.; Hakeem, K.R.; Moha, M.R.; Lee, J.H. Cd toxicity induced alterations in the root proteome of green gram in contrasting response towards iron supplement. Int. J. Mol. Sci. 2014, 15, 6343–6355. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Moul, I.D.; Choudhury, S. Iron (Fe3+)-mediated redox responses and amelioration of oxidative stress in Cd (Cd2+) stressed mung bean seedlings: A biochemical and computational analysis. J. Plant Biochem. Biotechnol. 2022, 31, 49–60. [Google Scholar] [CrossRef]
- Mu, N.S.; Kim, T.H.; Qureshi, M.I. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata. Plant Growth Regul. 2012, 68, 421–433. [Google Scholar] [CrossRef]
- Elazab, D.S.; Abdel, W.D.A.; El, M.M.T. Iron and zinc supplies mitigate Cd toxicity in micropropagated banana (Musa spp.). Plant Cell Tissue Organ. Cult. 2021, 145, 367–377. [Google Scholar] [CrossRef]
- Kabir, A.H.; Hossain, M.M.; Khatun, M.A.; Man, D.A.; Haider, S.A. Role of Silicon Counteracting Cd Toxicity in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2016, 7, 11–17. [Google Scholar] [CrossRef]
- Yao, X.; Cai, Y.; Yu, D.; Liang, G. bHLH104 confers tolerance to Cd stress in Arabidopsis thaliana. J. Integr. Plant Biol. 2018, 60, 691–702. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Du, W.X.; Fang, X.Z.; Zhang, L.L.; Jin, C.W. Knockdown of BTS may provide a new strategy to improve Cd-phytoremediation efficiency by improving iron status in plants. J. Hazard. Mater. 2020, 384, 121–163. [Google Scholar] [CrossRef]
- Inoue, H.; Kob, A.T.; No, Z.T.; Taka, H.M.; Ka, K.Y.; Suzu, K.K.; Naka, Z.M.; Nakani, S.H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef]
- Zheng, L.; Fu, J.M.; Ya, M.N.; Sa, S.A.; Ya, M.M.; Saku, R.I.; Sato, K.; Ma, J.F. Isolation and characterization of a barley yellow stripe-like gene, HvYSL5. Plant Cell Physiol. 2011, 52, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ka, K.Y.; Ishi, M.Y.; Koba, Y.T.; Yama, K.T.; Naka, N.H.; Nishizawa, N.K. OsYSL16 plays a role in the allocation of iron. Plant Mol. Biol. 2012, 79, 583–594. [Google Scholar] [CrossRef]
- Ao, Y.T.; Koba, Y.T.; Taka, H.M.; Naga, S.S.; Usuda, K.; Ka, K.Y.; Ishi, M.Y.; Naka, N.H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron (III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef]
- Zang, J.; Huo, Y.; Liu, J.; Zhang, H.; Liu, J.; Chen, H. Maize YSL2 is required for iron distribution and development in kernels. J. Exp. Bot. 2020, 20, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Di, V.F.; Couch, D.; Con, E.G.; Rosch, Z.H.; Ma, R.S.; Cu, R.C. The Arabidopsis YELLOW STRIPE LIKE4 and 6 transporters control iron release from the chloroplast. Plant Cell 2013, 25, 1040–1055. [Google Scholar] [CrossRef]
- DiDonato, R.J.J.r.; Roberts, L.A.; Sander, S.T.; Eisley, R.B.; Walker, E.L. Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 2004, 39, 403–414. [Google Scholar] [CrossRef]
- Waters, B.M.; Chu, H.H.; Didona, T.R.J.; Roberts, L.A.; Eisley, R.B.; Lahner, B.; Salt, D.E.; Walker, E.L. Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 2006, 141, 1446–1458. [Google Scholar] [CrossRef]
- Gendre, D.; Czernic, P.; Con, E.G.; Pianelli, K.; Briat, J.F.; Lebrun, M.; Mari, S. TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J. 2007, 49, 1–15. [Google Scholar] [CrossRef]
- Sasa, K.A.; Ya, M.N.; Xia, J.; Ma, J.F. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef]
- Sheng, H.; Jiang, Y.; Rahmati, M.; Chia, J.C.; Doku, C.T.; Ka, V.Y.; Za, V.T.O.; Mendoza, P.N.; Huang, R.; Smieshka, L.M.; et al. YSL3-mediated copper distribution is required for fertility, seed size and protein accumulation in Brachypodium. Plant Physiol. 2021, 186, 655–676. [Google Scholar] [CrossRef]
- Feng, S.; Tan, J.; Zhang, Y.; Liang, S.; Xiang, S.; Wang, H.; Chai, T. Isolation and characterization of a novel Cd-regulated Yellow Stripe-Like transporter (SnYSL3) in Solanum nigrum. Plant Cell Rep. 2017, 36, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Li, Y.; Zhang, Y.X.; Chai, T.Y. Molecular cloning and characterization of a Brassica juncea yellow stripe-like gene, BjYSL7, whose overexpression increases heavy metal to OPT3lerance of tobacco. Plant Cell Rep. 2013, 32, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cao, Q.; Jiang, Q.; Li, J.; Yu, R.; Shi, G. Comparative transcriptome analysis reveals gene network regulating Cd uptake and translocation in peanut roots under iron deficiency. BMC Plant Biol. 2019, 19, 35. [Google Scholar] [CrossRef]
- Meda, A.R.; Scheuermann, E.B.; Prechsl, U.E.; Ere, N.B.; Schaaf, G.; Ha, Y.H.; Weber, G.; von Wi, R.N. Iron acquisition by phytosiderophores contributes to cadmium tolerance. Plant Physiol. 2007, 143, 1761–1773. [Google Scholar] [CrossRef]
- Fang, L.; Ju, W.; Yang, C.; Duan, C.; Cui, Y.; Han, F.; Shen, G.; Zhang, C. Application of signaling molecules in reducing metal accumulation in alfalfa and alleviating metal-induced phytotoxicity in Pb/Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 182, 109–129. [Google Scholar] [CrossRef]
- Gut, S.A.; Hend, R.S.; Guer, R.G.; Ren, A.J.; Lutts, S.; Alse, E.S.; Fernie, A.R.; Hausman, J.F.; Vang, R.J.; Cuy, P.A.; et al. Long-Term Cd Exposure Alters the Metabolite Profile in Stem Tissue of Medicago sativa. Cells 2020, 9, 2707. [Google Scholar] [CrossRef]
- Gut, S.A.; Ser, G.K.; Keu, N.E.; Prin, S.E.; Guer, R.G.; Rena, U.J.; Hausman, J.F.; Cuy, P.A. Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? BMC Plant Biol. 2019, 19, 271. [Google Scholar] [CrossRef]
- Chang, J.D.; Huang, S.; Koni, S.N.; Wang, P.; Chen, J.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Overexpression of the manganese/Cd transporter OsNRAMP5 reduces Cd accumulation in rice grain. J. Exp. Bot. 2020, 71, 5705–5715. [Google Scholar] [CrossRef]
- Tao, J.; Lu, L. Advances in Genes-Encoding Transporters for Cd Uptake, Translocation, and Accumulation in Plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Gai, K.K.; Raz, Z.A.; Saman, T.K.; Ku, M.M.; Govin, D.V. Improving Zinc and Iron Biofortification in Wheat through Genomics Approaches. Mol. Biol. Rep. 2022, 49, 8007–8023. [Google Scholar] [CrossRef]
- Chowdhury, R.; Nallusamy, S.; Shanmugam, V.; Loganathan, A.; Muthurajan, R.; Sivathapandian, S.K.; Paramasivam, J.; Duraialagaraja, S. Genome-wide understanding of evolutionary and functional relationships of rice Yellow Stripe-Like (YSL) transporter family in comparison with other plant species. Biologia 2022, 77, 39–53. [Google Scholar] [CrossRef]
- Lee, G.; Ah, M.H.; Quinta, N.J.; Syll, W.L.; Jani, N.N.; Pre, I.V.; Anderson, J.E.; Piet, Z.B.; Krämer, U. Constitutively enhanced genome integrity maintenance and direct stress mitigation characterize transcriptome of extreme stress-adapted Arabidopsis halleri. Plant J. 2021, 108, 896–911. [Google Scholar] [CrossRef]
- Fu, Y.; Zha, T.H.; Li, Y.; Liu, Q.; Trot, S.V.; Li, C. Physiological and Transcriptomic Comparison of Two Sunflower (Helianthus annuus L.) Cultivars With High/Low Cadmium Accumulation. Front. Plant Sci. 2022, 13, 854386. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Cassin, G.; Couch, D.; Di, V.F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef]
- Koi, K.S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Nie, X.; Qu, M.; Zheng, L.; Tan, Z.; Zhao, H.; Huo, L.; Liu, S.; Zhang, B.; et al. Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. New Phytol. 2015, 207, 692–709. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Kosobryukhov, A.A.; Ivanov, A.A.; Kreslavskii, V.D. The effect of Cd on CO2 exchange, variable fluorescence of chlorophyll, and the level of antioxidant enzymes in pea leaves. Russ. J. Plant Physiol. 2005, 52, 15–20. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Huo, Y.; Guo, K.; Wang, Y.; He, G.; Sun, H.; Li, M.; Li, X.; Xu, N.; et al. Overexpression of Trx CDSP32 gene promotes chlorophyll synthesis and photosynthetic electron transfer and alleviates Cd-induced photoinhibition of PSII and PSI in tobacco leaves. J. Hazard. Mater. 2020, 398, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ling, Q.; Dong, F.; de Dios, V.R.; Zhan, X. Iron and copper mi-cronutrients influences cadmium accumulation in rice grains by altering its transport and allocation. Sci. Total Environ. 2021, 777, 146118. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, D.; Xu, C.; Zhu, H.; Feng, R.W.; Zhu, Q. Fe fortification limits rice Cd accumulation by promoting root cell wall chelation and reducing the mobility of Cd in xylem. Ecotoxicol. Environ. Saf. 2022, 240, 113700. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Shi, G. Effects of iron deficiency on subcellular dis-tribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Sie, D.A. Interaction between Cd and iron and its effects on photosynthetic capacity of primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem. 1996, 34, 833–841. [Google Scholar] [CrossRef]
- Mihucz, V.G.; Csog, Á.; Fodor, F.; Tatár, E.; Szoboszlai, N.; Silaghi-Dumitrescu, L.; Záray, G. Impact of two iron (III) chelators on the iron, Cd, lead and nickel accumulation in poplar grown under heavy metal stress in hydroponics. J. Plant Physiol. 2012, 169, 561–566. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, J.; Guan, J.; Tan, Z.; Zhang, Z.; Shi, G. Genome-Wide Identification and Transcript Analysis Reveal Potential Roles of Oligopeptide Transporter Genes in Iron Deficiency Induced Cadmium Accumulation in Peanut. Front. Plant Sci. 2022, 13, 894848. [Google Scholar] [CrossRef] [PubMed]
- Sol, T.A.; Gáspár, L.; Mészáros, I.; Szigeti, Z.; Lévai, L.; Sárvári, E. Impact of iron supply on the kinetics of recovery of photosynthesis in Cd-stressed poplar (Populus glauca). Ann. Bot. 2008, 102, 771–782. [Google Scholar] [CrossRef]
- Mu, N.S.; Jeong, B.R.; Kim, T.H.; Lee, J.H.; Soun, D.P. Transcriptional and physiological changes in relation to Fe uptake under conditions of Fe-deficiency and Cd-toxicity in roots of Vigna radiata L. J. Plant Res. 2014, 127, 731–742. [Google Scholar] [CrossRef]
- Suzu, K.M.; Ura, B.A.; Sasa, K.S.; Tsugawa, R.; Nishio, S.; Mukai, Y.H.; Murata, Y.; Masuda, H.; Aung, M.S.; Mera, A.; et al. Development of a mugineic acid family phytosiderophore analog as an iron fertilizer. Nat. Commun. 2021, 12, 1558. [Google Scholar] [CrossRef]
- Conte, S.S.; Chu, H.H.; Rodriguez, D.C.; Punshon, T.; Vasques, K.A.; Salt, D.E.; Walker, E.L. Arabidopsis thaliana Yellow Stripe1-Like4 and Yellow Stripe1-Like6 localize to internal cellular membranes and are involved in metal ion homeostasis. Front. Plant Sci. 2013, 4, 283. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987, 131, 101–110. [Google Scholar] [CrossRef]
- Reshmi, G.R.; Rajala, K.R. Drought and UV stress response in Spilanthes acmella Murr., (tooth-ache plant). J. Physiol. Biochem. 2012, 8, 110–129. [Google Scholar]
- Zhang, X.; Wang, L.; Meng, H.; Wen, H.; Fan, Y.; Zhao, J. Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol. Biol. 2011, 75, 365–378. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chang, M.-H.; Li, H.; Shu, Y.-J.; Bai, Y.; Gao, J.-Y.; Zhu, J.-X.; Dong, X.-Y.; Guo, D.-L.; Guo, C.-H. MsYSL6, A Metal Transporter Gene of Alfalfa, Increases Iron Accumulation and Benefits Cadmium Resistance. Plants 2023, 12, 3485. https://doi.org/10.3390/plants12193485
Zhang M, Chang M-H, Li H, Shu Y-J, Bai Y, Gao J-Y, Zhu J-X, Dong X-Y, Guo D-L, Guo C-H. MsYSL6, A Metal Transporter Gene of Alfalfa, Increases Iron Accumulation and Benefits Cadmium Resistance. Plants. 2023; 12(19):3485. https://doi.org/10.3390/plants12193485
Chicago/Turabian StyleZhang, Miao, Meng-Han Chang, Hong Li, Yong-Jun Shu, Yan Bai, Jing-Yun Gao, Jing-Xuan Zhu, Xiao-Yu Dong, Dong-Lin Guo, and Chang-Hong Guo. 2023. "MsYSL6, A Metal Transporter Gene of Alfalfa, Increases Iron Accumulation and Benefits Cadmium Resistance" Plants 12, no. 19: 3485. https://doi.org/10.3390/plants12193485
APA StyleZhang, M., Chang, M.-H., Li, H., Shu, Y.-J., Bai, Y., Gao, J.-Y., Zhu, J.-X., Dong, X.-Y., Guo, D.-L., & Guo, C.-H. (2023). MsYSL6, A Metal Transporter Gene of Alfalfa, Increases Iron Accumulation and Benefits Cadmium Resistance. Plants, 12(19), 3485. https://doi.org/10.3390/plants12193485





