Salicylic Acid- and Potassium-Enhanced Resilience of Quinoa (Chenopodium quinoa Willd.) against Salinity and Cadmium Stress through Mitigating Ionic and Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. Plant Growth
2.2. Stomatal Conductance and Pigment Contents
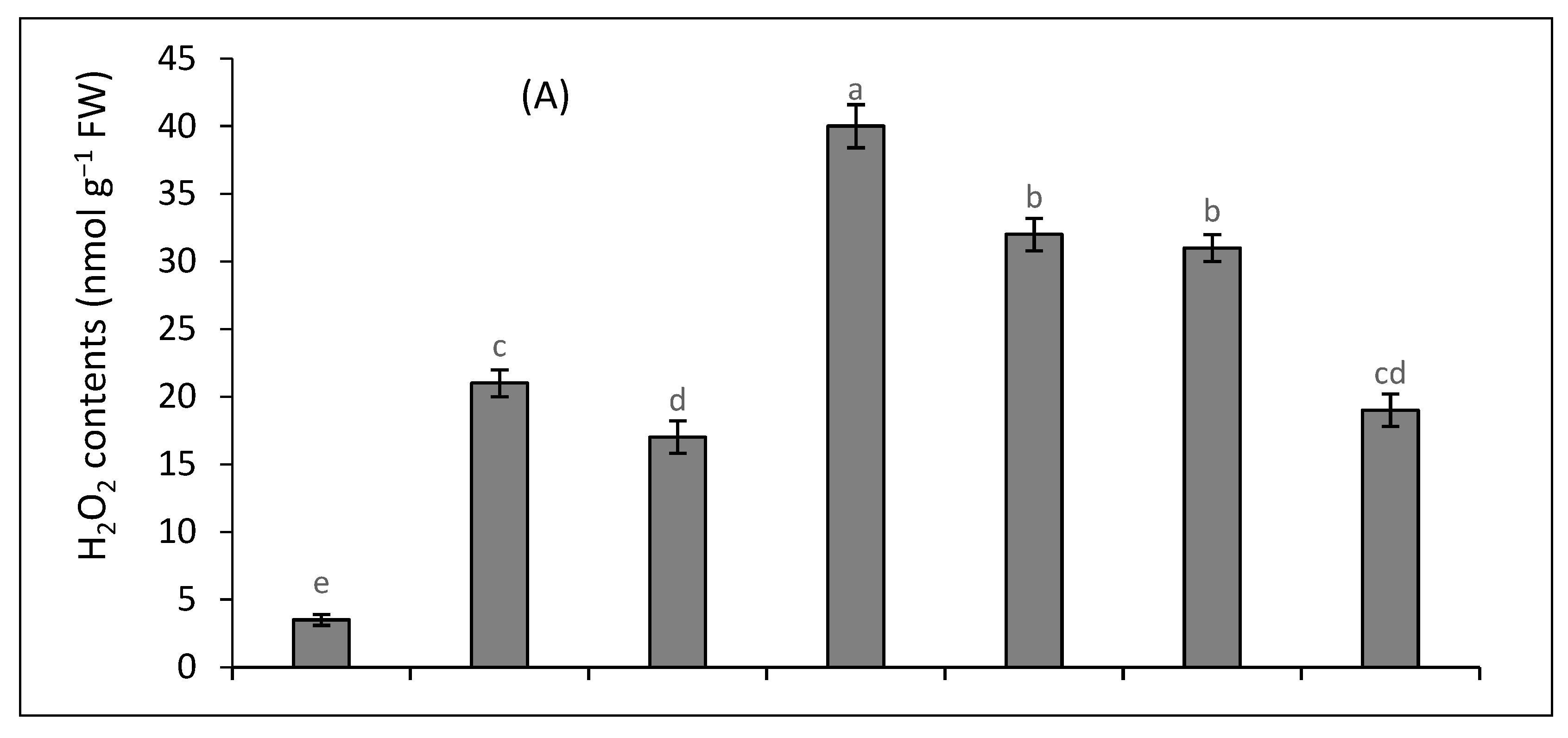
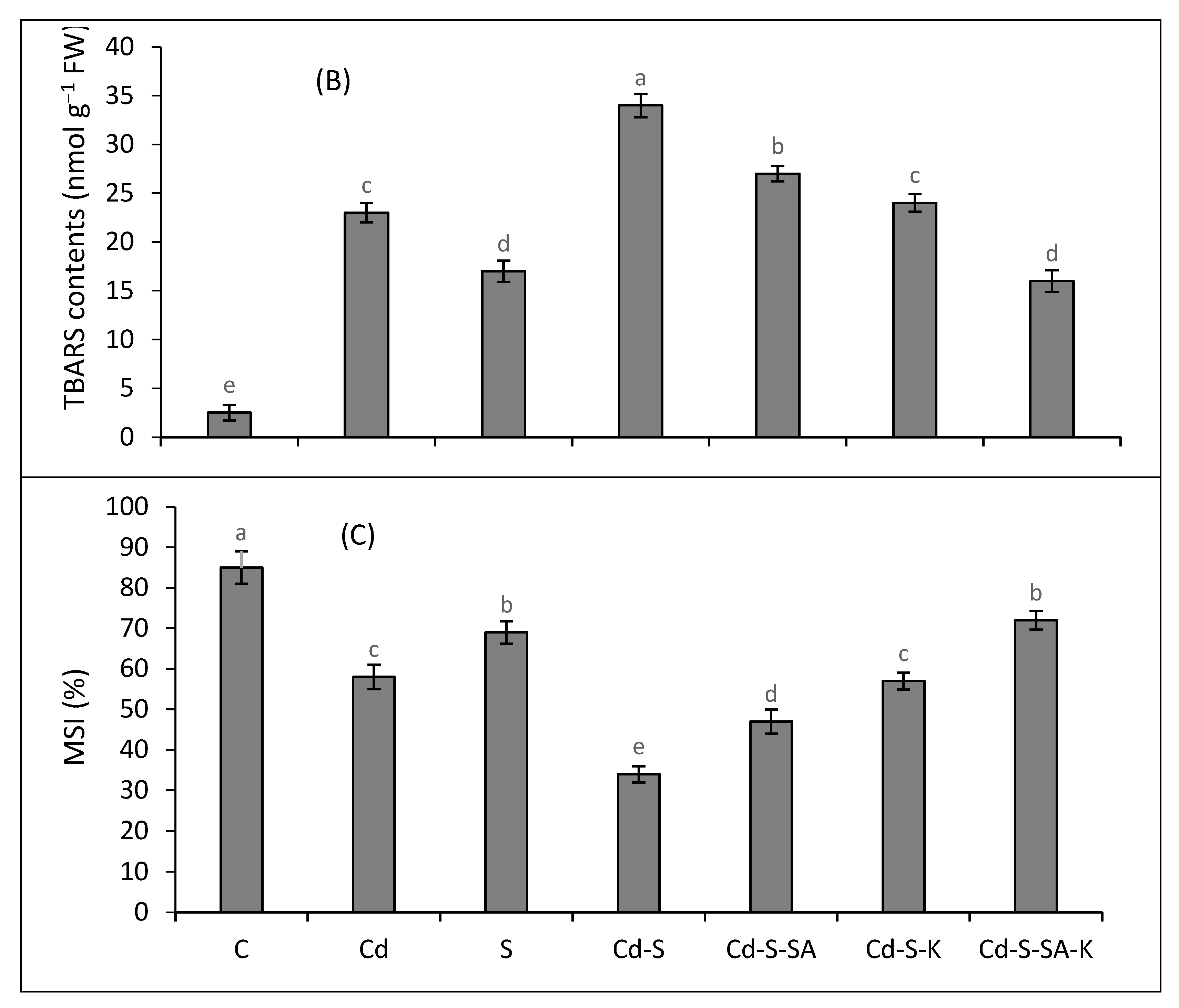
2.3. Oxidative Stress Attributes
2.4. Antioxidant Enzymes
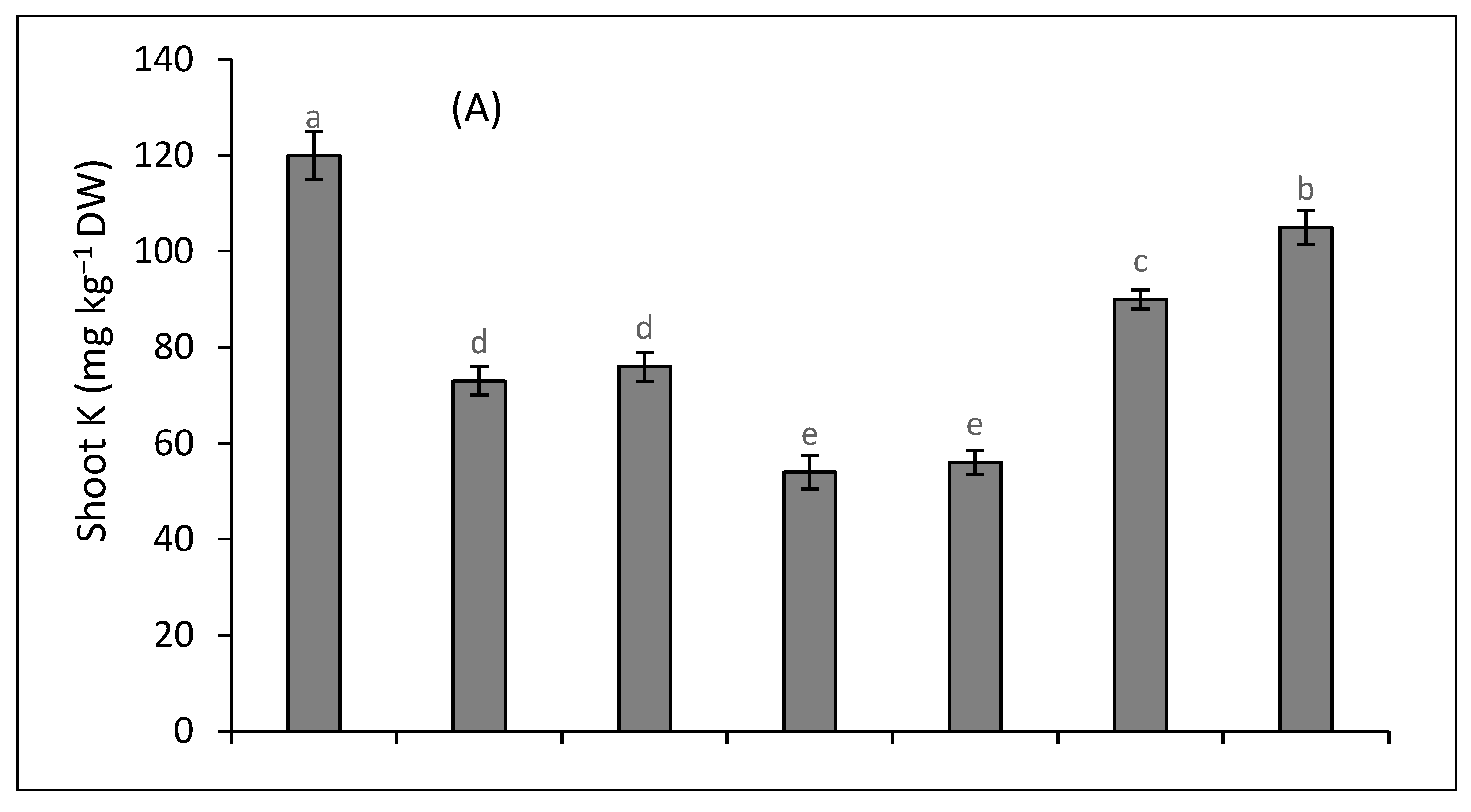
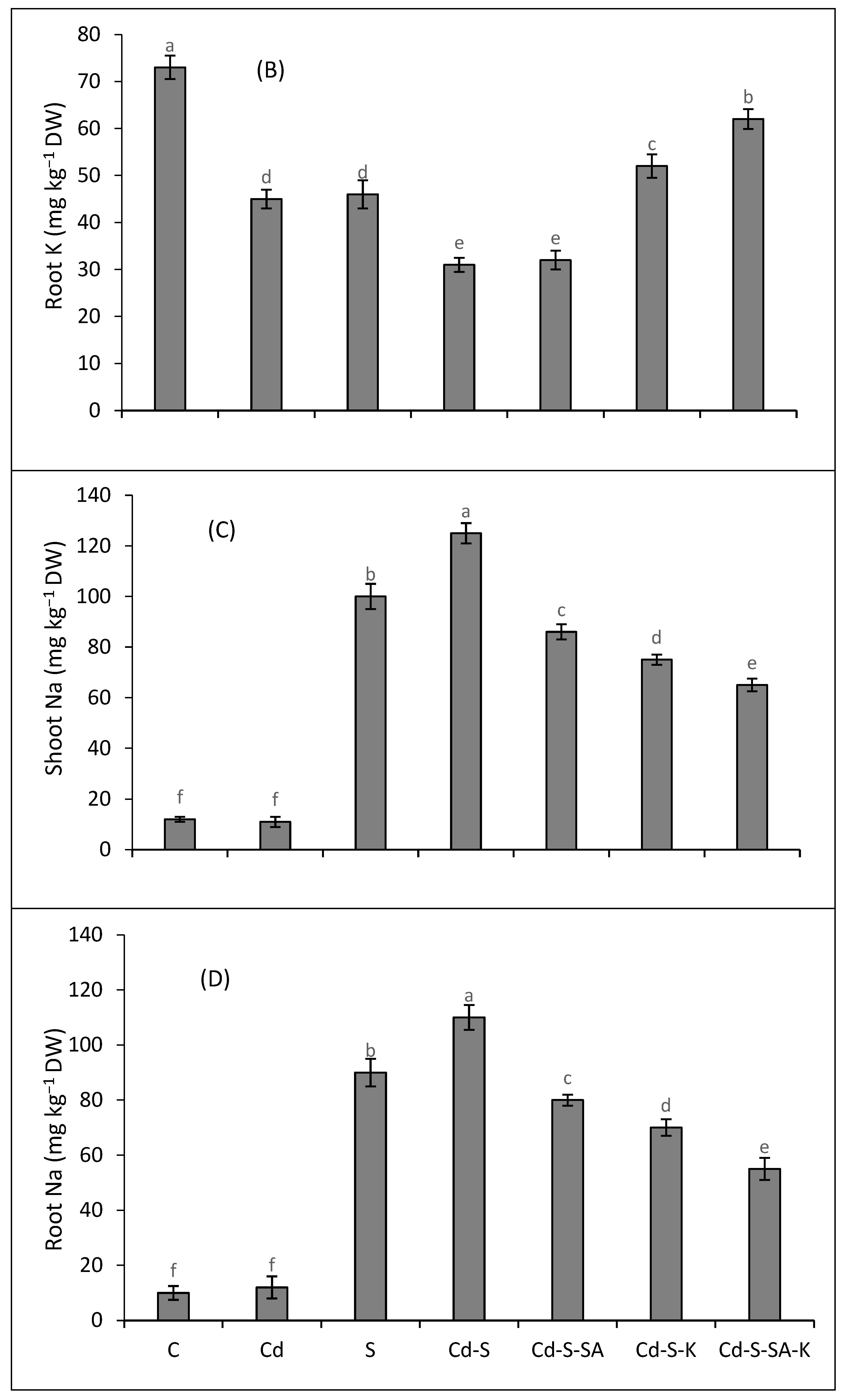
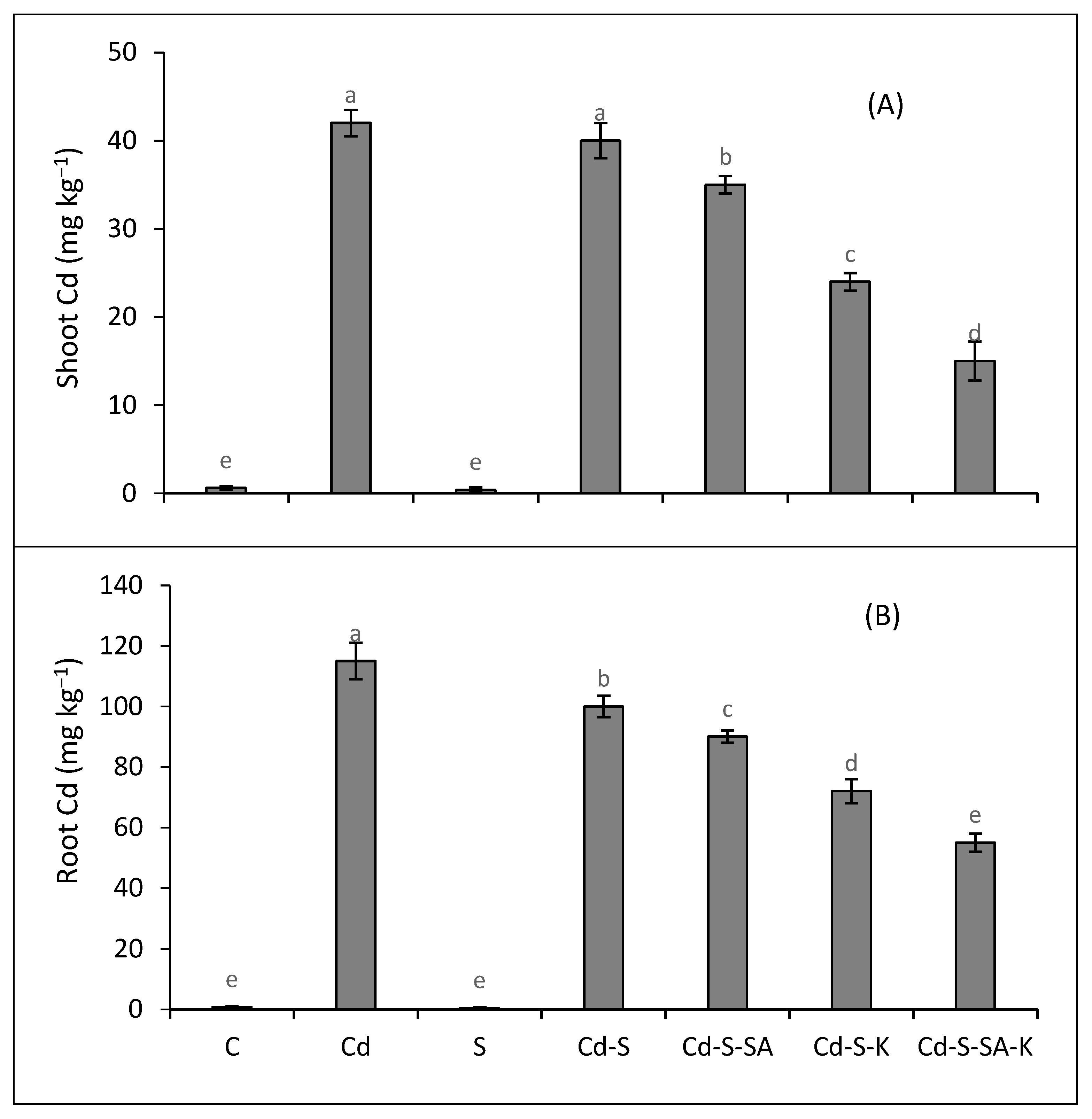
2.5. Metal Accumulation and Translocation
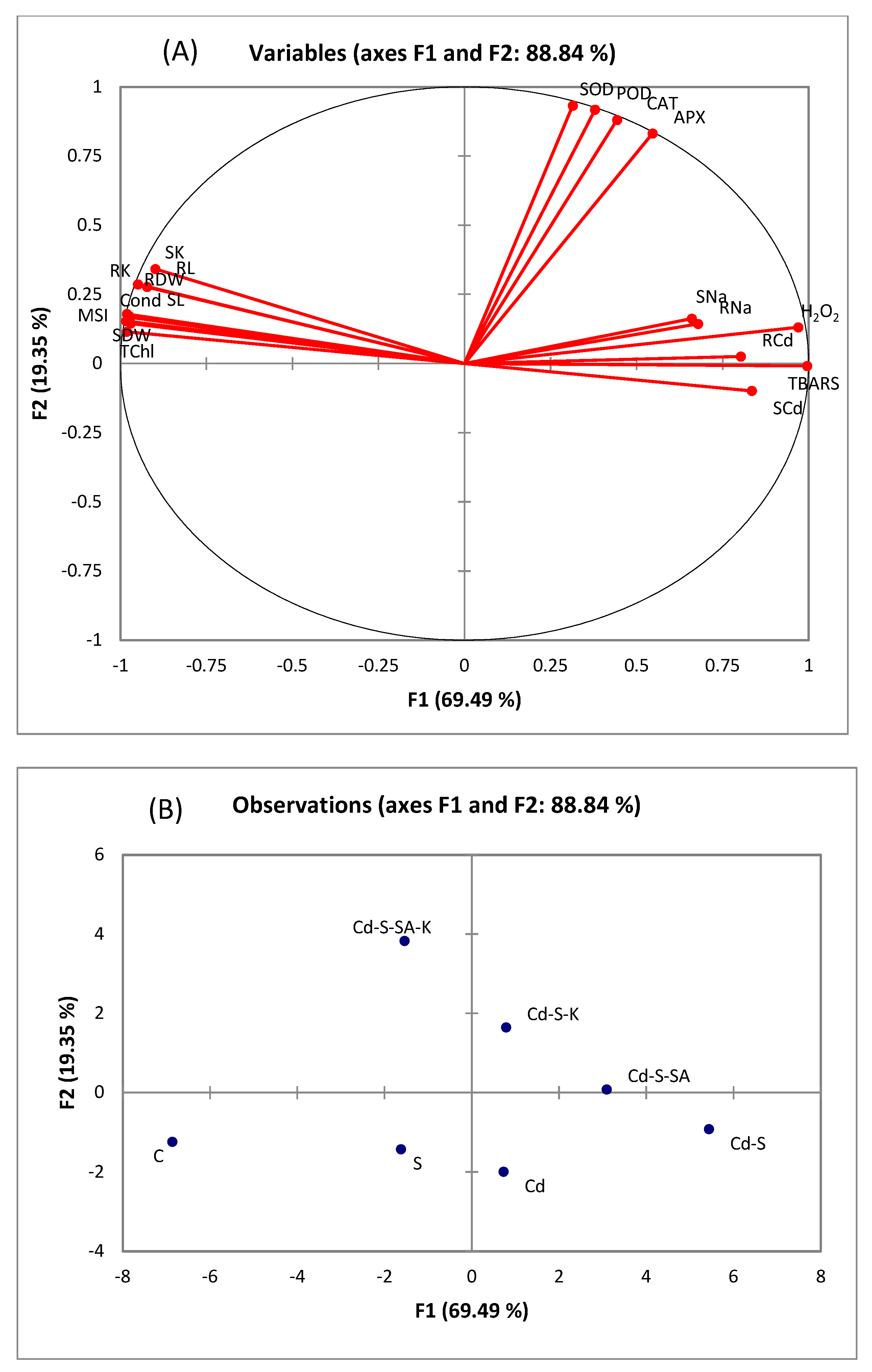
2.6. Multivariate Analyses
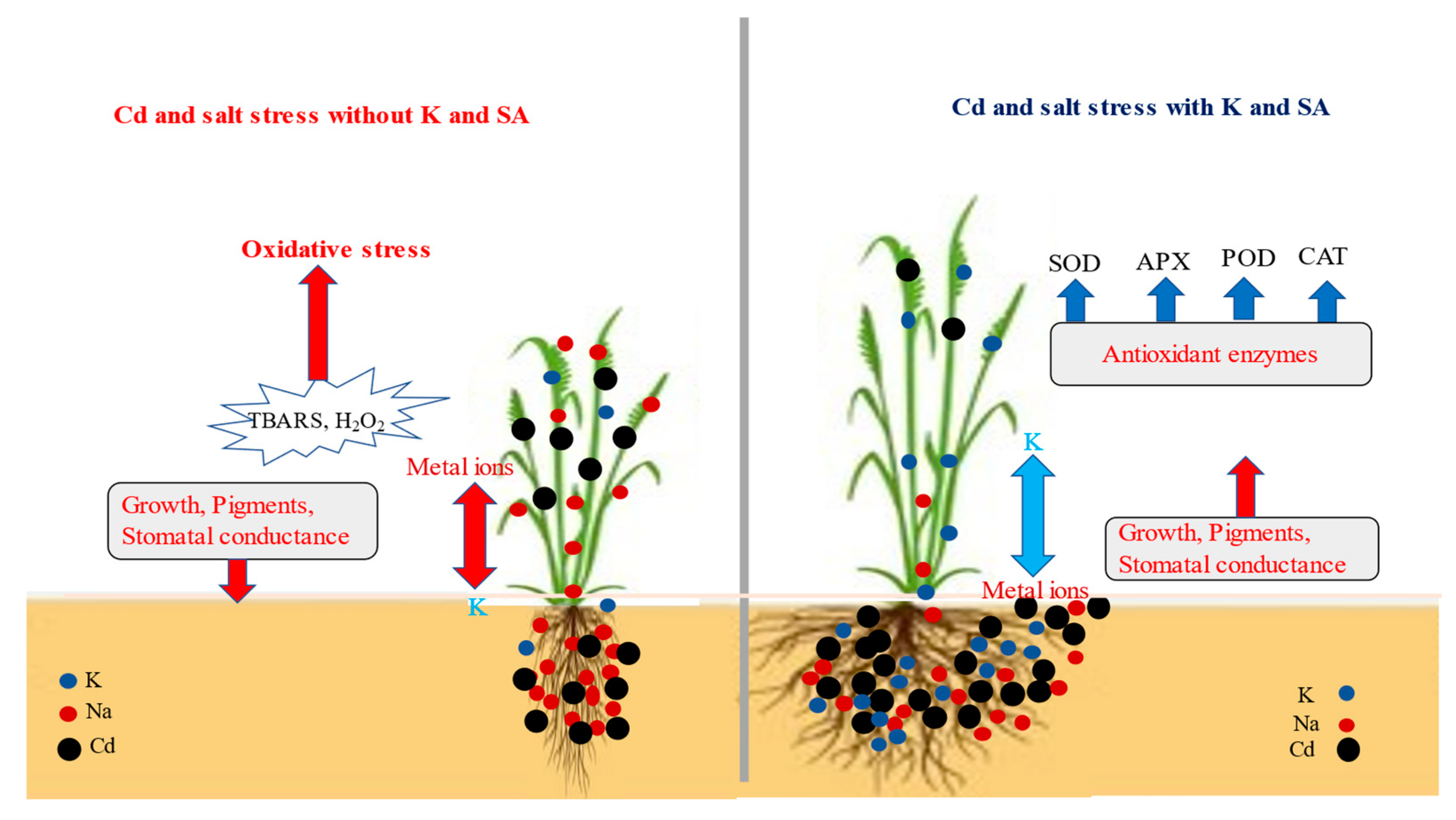
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Treatments
4.2. Plant Growth Measurements
4.3. Metal Analyses
4.4. Leaf Pigments and Stomatal Conductance
4.5. Oxidative Stress Attributes
4.6. Membrane Stability Assay
4.7. Enzymatic Activities
4.8. Bioconcentration Factor (BCF) and Translocation Factor (TF)
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Rehman, S.; Siddiqui, M.H.; Ali, H.M.; Farooq, M.A.; Chen, Y. Potassium and humic acid synergistically increase salt tolerance and nutrient uptake in contrasting wheat genotypes through ionic homeostasis and activation of antioxidant enzymes. Plants 2022, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Abdal, N.; Abbas, G.; Asad, S.A.; Ghfar, A.A.; Shah, G.M.; Rizwan, M.; Ali, S.; Shahbaz, M. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: Implications for phytoremediation. Environ. Geochem. Health 2021, 45, 171–185. [Google Scholar] [CrossRef]
- Iftikhar, A.; Abbas, G.; Saqib, M.; Shabbir, A.; Amjad, M.; Shahid, M.; Ahmad, I.; Iqbal, S.; Qaisrani, S.A. Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: A multivariate comparison of physiological and biochemical attributes. Environ. Geochem. Health 2021, 44, 257–272. [Google Scholar] [CrossRef]
- Amjad, M.; Iqbal, M.M.; Abbas, G.; Farooq, A.B.U.; Naeem, M.A.; Imran, M.; Murtaza, B.; Nadeem, M.; Jacobsen, S.-E. Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environ. Geochem. Health 2021, 44, 1487–1500. [Google Scholar] [CrossRef]
- Qayyum, M.F.; ur Rehman, M.Z.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress. Ecotoxico. Environ. 2019, 171, 146–153. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Al-Huqail, A.A.; Siddiqui, M.H.; Al-Huqail, A.A.; Khan, M.I.R. Hydrogen sulphide and salicylic acid regulate antioxidant pathway and nutrient balance in mustard plants under cadmium stress. Plant Biol. 2021, 24, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Hanafy, A.M. 2023. Spermine-salicylic acid interplay restrains salt toxicity in wheat (Triticum aestivum L.). Plants 2023, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Mahmoud, A.W.M.; Hanafy, A.M. Co-application of salicylic acid and spermine alleviates salt stress toxicity in wheat: Growth, nutrient acquisition, osmolytes accumulation, and antioxidant response. Acta Physiol. Plant. 2023, 45, 1. [Google Scholar] [CrossRef]
- Talaat, N.B.; Todorova, D. Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, M.; Li, Y.; Cheng, Z.; Wang, S. Improvement in salt tolerance of Iris pseudacorus L. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, 113703. [Google Scholar] [CrossRef]
- Turcios, E.A.; Papenbrock, J. Potassium, an important element to improve water use efficiency and growth parameters in quinoa (Chenopodium quinoa) under saline conditions. J. Agron. Crop Sci. 2021, 207, 618–630. [Google Scholar] [CrossRef]
- Kausar, A.; Gull, M. Effect of potassium sulphate on the growth and uptake of nutrients in wheat (Triticum aestivum L.) under salt stressed conditions. J. Agric. Sci. 2014, 6, 101. [Google Scholar] [CrossRef][Green Version]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Abbas, G.; Chen, Y.; Khan, F.Y.; Feng, Y.; Palta, J.A.; Siddique, K.H.M. Salinity and Low Phosphorus Differentially Affect Shoot and Root Traits in Two Wheat Cultivars with Contrasting Tolerance to Salt. Agronomy 2018, 8, 155. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J. Cadmium: Uptake in Plants and Its Alleviation Via Crosstalk Between Phytohormones and Sulfur. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Mishra, K., Tandon, P.K., Srivastava, S., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Alharby, H.F.; Abbas, G. Differential uptake and translocation of cadmium and lead by quinoa: A multivariate comparison of physiological and oxidative stress responses. Toxics 2022, 10, 68. [Google Scholar] [CrossRef]
- Ziaf, K.; Talha, H.M.; Ghani, M.A.; Ahmad, I.; Anwar, R.; Ali, B.; Majeed, Y.; Shakeel, A.; Iqbal, M.; Zaid, A. Differential accumulation pattern of cadmium in plant parts of pea varieties in response to varying cadmium levels. S. Afr. J. Bot. 2023, 161, 599–606. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. ATSDR 2015, Agency for Toxic Substances and Disease Registry Toxicological Profile for Lead; U.S. Department of Health and Human Services (P.H.S.): Washington, DC, USA, 2015.
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutr. 2022, 22, 212–269. [Google Scholar]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Natasha Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef]
- Belkadhi, A.; de Haro, A.; Obregon, S.; Chaıbi, W.; Djebali, W. Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat plants. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Zaheer, M.M.; Yasin, N.A.; Ahmad, S.R.; Khan, W.U.; Ahmad, A.; Ali, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2018, 41, 461–476. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-Zahrani, H.S.; Abbas, G. Potassium and Silicon Synergistically Increase Cadmium and Lead Tolerance and Phytostabilization by Quinoa through Modulation of Physiological and Biochemical Attributes. Toxics 2022, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, L.B.; Alansi, S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J. Boil. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, A.S.; El-Maati, M.F.A.; Desoky, E.S.M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef]
- Song, W.Y.; Martinoia, E.; Lee, J.; Kim, D.; Kim, D.Y.; Vogt, E.; Shim, D.; Choi, K.S.; Hwang, I.; Lee, Y. A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 2004, 135, 1027–1039. [Google Scholar] [CrossRef]
- Gómez-Pando, L.R.; Álvarez-Castro, R.; Eguiluz-De La Barra, A. Effect of salt stress on Peruvian germplasm of Chenopodium quinoa Willd.: A promising crop. J. Agron. Crop Sci. 2010, 196, 391–396. [Google Scholar] [CrossRef]
- Takagi, H.; Yamada, S. Roles of enzymes in anti-oxidative response system on three species of chenopodiaceous halophytes under NaCl-stress condition. Soil Sci. Plant Nutr. 2013, 59, 603–611. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Ma, C.; Wang, K.; Hao, Y.; Chen, Q.; Mo, Y.; Rui, Y. Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt. Environ. Pollut. 2019, 252, 1087–1096. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Murtaza, B.; Abbas, G.; Jawad, H. Differential accumulation of potassium results in varied salt-toleranceresponse in tomato (Solanum lycopersicum L.) cultivars. Hort. Environ. Biotechnol. 2016, 57, 248–258. [Google Scholar] [CrossRef]
- Zhang, S.; Ni, X.; Arif, M.; Zheng, J.; Stubbs, A.; Li, C. NaCl improved Cd tolerance of the euhalophyte Suaeda glauca but not the recretohalophyte Limonium aureum. Plant Soil. 2020, 449, 303–318. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, G.S.D.; Pinheiro, F.W.A.; Gheyi, H.R.; Soares, L.A.D.A. Gas exchanges, quantum yield and photosynthetic pigments of West Indian cherry under salt stress and potassium fertilization. Rev. Caatinga 2019, 32, 429–439. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 84–85. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Abbas, G.; Areej, F.; Asad, S.A.; Saqib, M.; Anwar-ul-Haq, M.; Afzal, S.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Akram, M.; et al. Differential effect of heat stress on drought and salt tolerance potential of quinoa genotypes: A physiological and biochemical investigation. Plants 2023, 12, 774. [Google Scholar] [CrossRef]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Shabbir, A.; Saqib, M.; Murtaza, G.; Abbas, G.; Imran, M.; Rizwan, M.; Naeem, M.A.; Ali, S.; Javeed, H.M.R. Biochar mitigates arsenic-induced human health risks and phytotoxicity in quinoa under saline conditions by modulating ionic and oxidative stress responses. Environ. Pollut. 2021, 287, 117348. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]


| Treatments | Shoot Length | Root Length | Shoot Dry Weight | Root Dry Weight |
|---|---|---|---|---|
| (cm) | (cm) | (g plant −1) | (g plant −1) | |
| Control | 21 ± 0.5 a | 19 ± 0.8 a | 2.1 ± 0.08 a | 0.70 ± 0.02 a |
| Cd | 12 ± 0.4 d | 11 ± 0.26 d | 1.3 ± 0.04 d | 0.38 ± 0.023 d |
| S | 15 ± 0.5 b | 13.5 ± 0.6 c | 1.7 ± 0.05 b | 0.48 ± 0.025 c |
| Cd-S | 8.2 ± 0.5 e | 8.0 ± 0.5 e | 0.86 ± 0.07 e | 0.23 ± 0.02 e |
| Cd-S-SA | 12 ± 0.6 d | 10 ± 0.3 d | 1.3 ± 0.06 d | 0.34 ± 0.015 d |
| Cd-S-K | 14 ± 0.5 c | 14 ± 0.6 c | 1.5 ± 0.05 c | 0.45 ± 0.02 c |
| Cd-S-SA-K | 16.5 ± 0.6 b | 16 ± 0.6 b | 1.7 ± 0.04 b | 0.53 ± 0.018 b |
| Treatments | Chl a | Chl b | Total Chl | Stomatal Conductance |
|---|---|---|---|---|
| (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (mmol m−2 s−1) | |
| Control | 460 ± 10 a | 210 ± 5 a | 670 ± 20 a | 480 ± 10 a |
| Cd | 320 ± 15 c | 125 ± 8 d | 445 ± 25 d | 260 ± 20 d |
| S | 400 ± 12 b | 152 ± 5 c | 552 ± 15 c | 340 ± 14 c |
| Cd-S | 230 ± 5 e | 85 ± 6 e | 315 ± 15 f | 140 ± 18 f |
| Cd-S-SA | 280 ± 15 d | 127 ± 5 d | 400 ± 22 e | 215 ± 12 e |
| Cd-S-K | 310 ± 8 c,d | 142 ± 4 c | 452 ± 20 d,e | 280 ± 15 d |
| Cd-S-SA-K | 400 ± 10 b | 170 ± 6 b | 590 ± 10 b | 390 ± 10 b |
| Treatment | BCF | TF | TI |
|---|---|---|---|
| Cd | 3.74 ± 0.20 a | 0.37 ± 0.04 b | 62 ± 2.0 c |
| S | - | - | 81 ± 3.0 b |
| Cd-S | 3.56 ± 0.30 b | 0.4 ± 0.02 a | 41 ± 2.5 d |
| Cd-S-SA | 3.11 ± 0.26 c | 0.39 ± 0.01 a,b | 62 ± 1.9 c |
| Cd-S-K | 2.13 ± 0.15 d | 0.33 ± 0.01 c | 71 ± 2.0 b |
| Cd-S-SA-K | 1.33 ± 0.17 e | 0.27 ± 0.03 d | 81 ± 2.1 a |
| Variables | SL | RL | RDW | SDW | TChl | Cond | SOD | CAT | POD | APX | H2O2 | TBARS | MSI | RCd | SCd | SK | RK | SNa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL | 0.980 | |||||||||||||||||
| RDW | 0.996 | 0.987 | ||||||||||||||||
| SDW | 0.987 | 0.961 | 0.984 | |||||||||||||||
| TChl | 0.971 | 0.955 | 0.973 | 0.974 | ||||||||||||||
| Cond | 0.985 | 0.976 | 0.989 | 0.977 | 0.995 | |||||||||||||
| SOD | −0.139 | −0.048 | −0.169 | −0.155 | −0.139 | −0.137 | ||||||||||||
| CAT | −0.281 | −0.173 | −0.302 | −0.321 | −0.290 | −0.277 | 0.970 | |||||||||||
| POD | −0.220 | −0.091 | −0.232 | −0.234 | −0.218 | −0.210 | 0.962 | 0.963 | ||||||||||
| APX | −0.394 | −0.289 | −0.414 | −0.416 | −0.390 | −0.385 | 0.958 | 0.987 | 0.965 | |||||||||
| H2O2 | −0.909 | −0.858 | −0.918 | −0.913 | −0.944 | −0.938 | 0.403 | 0.529 | 0.496 | 0.621 | ||||||||
| TBARS | −0.978 | −0.942 | −0.981 | −0.967 | −0.975 | −0.981 | 0.296 | 0.426 | 0.380 | 0.534 | 0.971 | |||||||
| MSI | 0.965 | 0.950 | 0.973 | 0.975 | 0.991 | 0.989 | −0.188 | −0.338 | −0.262 | −0.433 | −0.959 | −0.975 | ||||||
| RCd | −0.797 | −0.762 | −0.793 | −0.836 | −0.819 | −0.795 | 0.305 | 0.465 | 0.315 | 0.507 | 0.740 | 0.800 | −0.784 | |||||
| SCd | −0.841 | −0.825 | −0.839 | −0.879 | −0.869 | −0.848 | 0.198 | 0.365 | 0.208 | 0.416 | 0.763 | 0.828 | −0.838 | 0.987 | ||||
| SK | 0.924 | 0.975 | 0.942 | 0.882 | 0.901 | 0.933 | 0.002 | −0.080 | −0.018 | −0.207 | −0.803 | −0.888 | 0.898 | −0.635 | −0.712 | |||
| RK | 0.932 | 0.974 | 0.952 | 0.892 | 0.917 | 0.946 | −0.062 | −0.145 | −0.087 | −0.271 | −0.844 | −0.912 | 0.919 | −0.646 | −0.718 | 0.996 | ||
| SNa | −0.596 | −0.567 | −0.617 | −0.557 | −0.566 | −0.603 | 0.310 | 0.332 | 0.413 | 0.435 | 0.714 | 0.652 | −0.638 | 0.101 | 0.146 | −0.614 | −0.653 | |
| RNa | −0.614 | −0.588 | −0.635 | −0.570 | −0.590 | −0.626 | 0.302 | 0.322 | 0.404 | 0.429 | 0.733 | 0.672 | −0.657 | 0.120 | 0.166 | −0.641 | −0.680 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.A.; Alharby, H.F.; Abbas, G.; Al-Solami, H.M.; Younas, A.; Aldehri, M.; Alabdallah, N.M.; Chen, Y. Salicylic Acid- and Potassium-Enhanced Resilience of Quinoa (Chenopodium quinoa Willd.) against Salinity and Cadmium Stress through Mitigating Ionic and Oxidative Stress. Plants 2023, 12, 3450. https://doi.org/10.3390/plants12193450
Alghamdi SA, Alharby HF, Abbas G, Al-Solami HM, Younas A, Aldehri M, Alabdallah NM, Chen Y. Salicylic Acid- and Potassium-Enhanced Resilience of Quinoa (Chenopodium quinoa Willd.) against Salinity and Cadmium Stress through Mitigating Ionic and Oxidative Stress. Plants. 2023; 12(19):3450. https://doi.org/10.3390/plants12193450
Chicago/Turabian StyleAlghamdi, Sameera A., Hesham F. Alharby, Ghulam Abbas, Habeeb M. Al-Solami, Afshan Younas, Majed Aldehri, Nadiyah M. Alabdallah, and Yinglong Chen. 2023. "Salicylic Acid- and Potassium-Enhanced Resilience of Quinoa (Chenopodium quinoa Willd.) against Salinity and Cadmium Stress through Mitigating Ionic and Oxidative Stress" Plants 12, no. 19: 3450. https://doi.org/10.3390/plants12193450
APA StyleAlghamdi, S. A., Alharby, H. F., Abbas, G., Al-Solami, H. M., Younas, A., Aldehri, M., Alabdallah, N. M., & Chen, Y. (2023). Salicylic Acid- and Potassium-Enhanced Resilience of Quinoa (Chenopodium quinoa Willd.) against Salinity and Cadmium Stress through Mitigating Ionic and Oxidative Stress. Plants, 12(19), 3450. https://doi.org/10.3390/plants12193450










