ROS Homeostasis and Antioxidants in the Halophytic Plants and Seeds
Abstract
:1. Introduction
2. Halophytes: Importance, Classification, and Salt Tolerance Mechanisms
- (1)
- Eu-halophytes: Growth of eu-halophytes is stimulated even in moderate salinities (such as Salicornia europaea and Suaeda maritima).
- (2)
- Facultative halophytes: Growth of these halophytes is slightly stimulated at low salinity (such as Plantago maritima and Aster tripolium).
- (3)
- Non-halophytes with low salinity tolerance: These plants are not halophytes and often include salt-tolerant crops and orchards. This group includes a wide range of economic plants such as barley (Hordeum vulgare), sorghum (Sorghum bicolor), cotton (Gossypium spp.), and pistachios (Pistacia vera).
- (4)
- Halophobic: Plants that are sensitive to salinity and even at low salinity levels there is a significant reduction in their growth and yield, such as saffron (Crocus sativus), common bean (Phaseolus vulgaris), and most vegetables.
- Recretohalophytes: Include halophytes that excrete salts on the outer surface (Exo-recretohalophytes) or the inside (Endo-recretohalophytes) of plant tissue.
- Euhalophytes: Or true halophytes with succulent leaves or stems.
- Pseudo-halophytes: Unreal halophytes that store salts in the parenchymal organs of the root.
- Some halophytes have evolved salt glands or bladders that actively excrete sodium ions from their tissues (excretion of sodium ions.) By removing these ions from their cells, they effectively regulate salt concentration and protect themselves against saline stress [35].
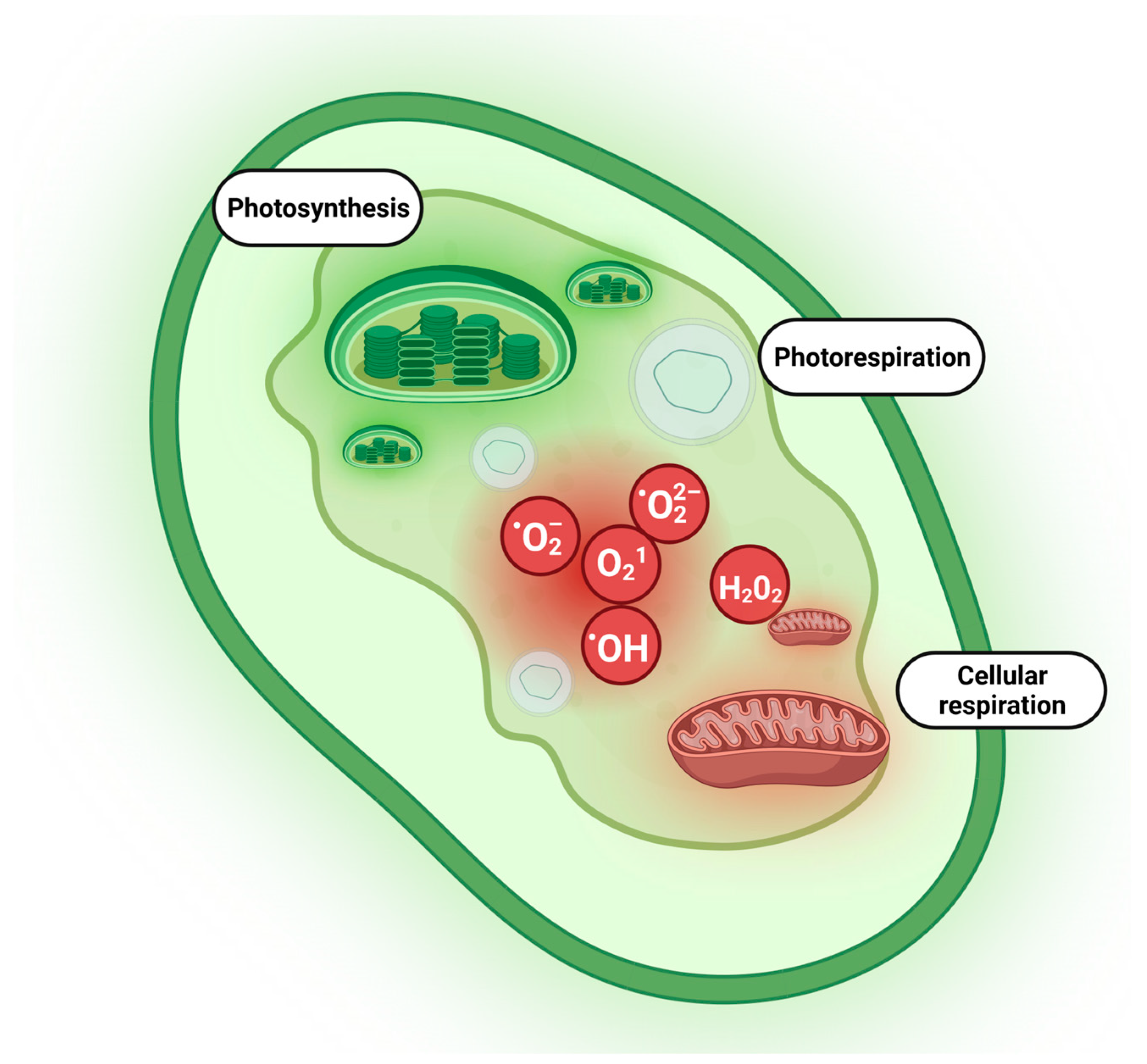
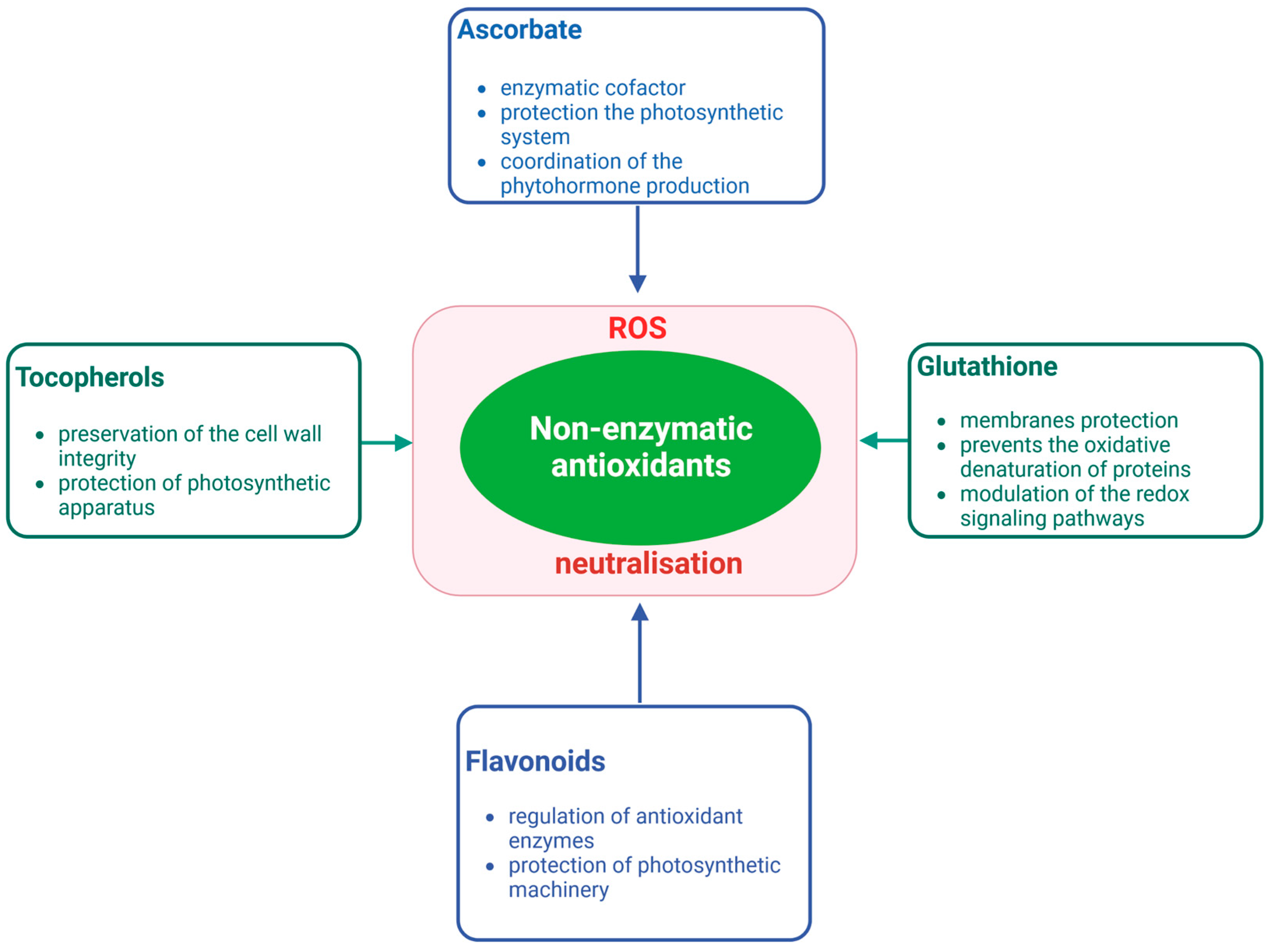
3. ROS and Antioxidants in Halophytes and Their Role in Salinity Tolerance
4. ROS and Antioxidants in Dry Seeds
5. ROS and Antioxidants in Germinating Seeds under Environmental Stresses
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ślesak, I.; Ślesak, H.; Kruk, J. Oxygen and hydrogen peroxide in the early evolution of life on earth: In silico comparative analysis of biochemical pathways. Astrobiology 2012, 12, 775–784. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar GHPakniyat, H.; Emam, Y. Physiological mechanisms of salt stress tolerance in plants: An overview. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons: New York, NY, USA, 2016; pp. 141–160. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Akram, N.A.; Ghafar, M.A.; Panico, A. Forage potential of several halophytic species grown on saline soil in arid environments. Environ. Res. 2023, 219, 114954. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 241–1257. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Pakniyat, H.; Pirasteh-Anosheh, H.; Azooz, M.M. Role of ROS as signaling molecules in plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 585–620. [Google Scholar] [CrossRef]
- Öztürk, M.; Altay, V.; Güvensen, A. Sustainable Use of Halophytic Taxa as Food and Fodder: An Important Genetic Resource in Southwest Asia. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Guvensen, A.; Gork, G.; Ozturk, M. An overview of the halophytes in Turkey. In Sabkha Ecosystems; Khan, M.A., Böer, B., Kust, G.S., Barth, H.J., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2006; Volume 42. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Parvizi, H.; Parnian, A.; Esfahan, E.Z.; Ranjbar, G.; Bhardwaj, A.K. Relationship between soil salinity and alkalinity with Alhagi camelorum growth in hypersaline and hyperarid environments. J. Arid Environ. 2022, 206, 104830. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Shah, W.H.; Saleem, S.; Mushtaq, N.U.; Rasool, A.; Tahir, I.; Rehman, R.U. C4 and CAM Plants with Better Resilience to Environmental Stresses. In Photosynthesis and Respiratory Cycles during Environmental Stress Response in Plants; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 163–191. [Google Scholar]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons: London, UK, 2016; pp. 24–40. [Google Scholar] [CrossRef]
- Pehlivan, F.E. Free radicals and antioxidant system in seed biology. In Advances in Seed Biology; InTech: London, UK, 2017; pp. 167–175. [Google Scholar]
- Pirasteh-Anosheh, H.; Parnian, A.; Spasiano, D.; Race, M.; Ashraf, M. Haloculture: A system to mitigate the negative impacts of pandemics on the environment, society and economy, emphasizing COVID-19. Environ. Res. 2021, 198, 111228. [Google Scholar] [CrossRef]
- FAO. The Statistical Year Book; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Solis, C.A.; Yong, M.T.; Vinarao, R.; Jena, K.; Holford, P.; Shabala, L.; Zhou, M.; Shabala, S.; Chen, Z.H. Back to the wild: On a quest for donors toward salinity tolerant rice. Front. Plant Sci. 2020, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, G.; Pirasteh-Anosheh, H.; Dehghanie, F.; Keshtkar, S.; Race, M. Feasibility of growing Salicornia species in a coastal environment through planting date and density management in a direct seawater irrigation system. Environ. Sci. Pollut. Res. 2022, 29, 47800–47809. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, G.; Pirasteh-Anosheh, H. A glance at the salinity research in Iran with emphasis on the improvement of field crop production. Iran. J. Crop Sci. 2015, 17, 165–178. [Google Scholar]
- Pirasteh-Anosheh, H.; Mirhosseini, A.; Akram, N.A.; Hasanuzzaman, M. Forage potential of Salsola species in arid-saline rangelands. Turk. J. Bot. 2021, 45, 203–215. [Google Scholar] [CrossRef]
- Öztürk, M.; Altay, V.; Gucel, S.; Guvensen, A. Halophytes in the East Mediterranean—Their Medicinal and Other Economical Values. In Sabkha Ecosystems; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2014; Volume 47. [Google Scholar] [CrossRef]
- Polo-Ávila, A.; Infante-Izquierdo, M.D.; Sánchez-Gullón, E.; Castillo, J.M.; Muñoz-Rodríguez, A.F. Population Dynamic of the Annual Halophyte Salicornia ramosissima in Salt Pans: Towards a Sustainable Exploitation of Its Wild Populations. Plants 2022, 11, 1676. [Google Scholar] [CrossRef]
- Hedayati-Firoozabadi, A.; Kazemeini, S.A.; Pirasteh-Anosheh, H.; Ghadiri, H.; Pessarakli, M. Forage yield and quality as affected by salt stress in different ratios of Sorghum bicolor-Bassia indica intercropping. J. Plant Nutr. 2020, 43, 2579–2589. [Google Scholar] [CrossRef]
- Fekete, R.; Bak, H.; Vincze, O.; Süveges, K.; Molnár, V.A. Road traffic and landscape characteristics predict the occurrence of native halophytes on roadside verges. Sci. Rep. 2022, 12, 1298. [Google Scholar] [CrossRef]
- Ranjbar, G.; Pirasteh-Anosheh, H.; Banakar, M.H.; Miri, H.R. Review on halophytes researches in Iran: Explanation of Challenges and Offer Approaches. J. Plant Ecophysiol. 2018, 10, 117–129. [Google Scholar]
- Prasad, M.N.V. Trace Elements as Contaminants and Nutrients: Consequences in Ecosystems and Human Health; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kue Foka, I.C.; Ketehouli, T.; Zhou, Y.; Li, X.W.; Wang, F.W.; Li, H. The emerging roles of diacylglycerol kinase (DGK) in plant stress tolerance, growth, and development. Agronomy 2020, 10, 1375. [Google Scholar] [CrossRef]
- Kefu, Z.; Hai, F.; Ungar, I.A. Survey of halophyte species in China. Plant Sci. 2002, 163, 491–498. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2020, 72, 842–862. [Google Scholar] [CrossRef]
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of Salt Tolerance in Halophytes: Current Understanding and Recent Advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, C.; Romero-Puertas, M.C.; Gupta, D.K.; del Río, L.A.; Sandalio, L.M.; Abdelly, C. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ. Exp. Bot. 2010, 69, 129–136. [Google Scholar] [CrossRef]
- Reginato, M.; Cenzano, A.M.; Arslan, I.; Furlan, A.; Varela, C.; Cavallin, V.; Papenbrock, J.; Luna, V. Na2SO4 and NaCl salts differentially modulate the antioxidant systems in the highly stress tolerant halophyte Prosopis strombulifera. Plant Physiol. Biochem. 2021, 167, 748–762. [Google Scholar] [CrossRef]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells. Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Pakar, N.; Pirasteh-Anosheh, H.; Emam, Y.; Pessarakli, M. Barley growth, yield, antioxidant enzymes, and ion accumulation affected by PGRs under salinity stress conditions. J. Plant Nutr. 2016, 39, 1372–1379. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. The role of antioxidant responses on the tolerance range of extreme halophyte Salsola crassa grown under toxic salt concentrations. Ecotoxicol. Environ. Saf. 2014, 110, 21–30. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Ciarkowska, A.; Rajabi Dehnavi, A.; Cárdenas-Pérez, S.; Piernik, A. Growth Stage-, Organ- and Time-Dependent Salt Tolerance of Halophyte Tripolium pannonicum (Jacq.) Dobrocz. Life 2023, 13, 462. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Rajabi Dehnavi, A.; Leszczynski, K.; Lubinska-Mielinska, S.; Ludwiczak, A.; Piernik, A. Salicornia europaea L. Functional Traits Indicate Its Optimum Growth. Plants 2022, 11, 1051. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Sofo, A. Catalase and ascorbate peroxidase representative H2O2 detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Shugaev, A.G.; Lashtabega, D.A.; Shugaeva, N.A.; Vyskrebentseva, E.I. Activities of antioxidant enzymes in mitochondria of growing and dormant sugar beet roots. Russ. J. Plant Physiol. 2011, 58, 387–393. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Int. J. Phytoremediat. 2021, 23, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ros and no metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Kumar, N.; Lata, C.; Kumar, A.; Meena, B.L.; Kumar, A. Physiological and differential gene expression reveals a trade-off between antioxidant capacity and salt tolerance in halophytes Urochondra setulosa and Dichanthium annulatum. Plant Growth Regul. 2023. preprint. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Sphaerophysa kotschyana, an endemic species from Central Anatolia: Antioxidant system responses under salt stress. J. Plant Res. 2013, 126, 729–742. [Google Scholar] [CrossRef]
- Hameed, A.; Rasheed, A.; Gul, B.; Khan, M.A. Salinity inhibits seed germination of perennial halophytes Limonium stocksii and Suaeda fruticosa by reducing water uptake and ascorbate dependent antioxidant system. Environ. Exp. Bot. 2014, 107, 32–38. [Google Scholar] [CrossRef]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate–glutathione–α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Magné, C.; Foggi, G.; Conte, G.; Santin, M.; et al. Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae 2022, 8, 828. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Custódio, L.; Mecha, D.; Zengin, G.; Cziáky, Z.; Sotkó, G.; Pereira, C.G. Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants 2022, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Kamińska, I.; Lukasiewicz, A.; Klimek-Chodacka, M.; Długosz-Grochowska, O.; Rutkowska, J.; Szymonik, K.; Baranski, R. Antioxidative and osmoprotecting mechanisms in carrot plants tolerant to soil salinity. Sci. Rep. 2022, 12, 7266. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, S.; Jiang, Y.; Wang, N.; Wang, R.; Shen, W.; Yang, J. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 2013, 370, 47–57. [Google Scholar] [CrossRef]
- Lima, A.R.; Castaneda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Al-Shamsi, N.; Hussain, M.I.; El-Keblawy, A. Physiological responses of the xerohalophyte Suaeda vermiculata to salinity in its hyper-arid environment. Flora 2020, 273, 151705. [Google Scholar] [CrossRef]
- Duan, H.; Ma, Y.; Liu, R.; Li, Q.; Yang, Y.; Song, J. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol. Biochem. 2018, 127, 231–237. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, G.; Gu, H. Physiological and antioxidant responses of three leguminous species to saline environment during seed germination stage. Afr. J. Biotechnol. 2009, 8, 5773–5779. [Google Scholar]
- Rasheed, A.; Hameed, A.; Khan, M.A.; Gul, B. Variation in temperature and light but not salinity invokes antioxidant enzyme activities in germinating seeds of Salsola drummondii. Plant Biosyst. 2016, 150, 1072–1082. [Google Scholar] [CrossRef]
- Chai, Y.Y.; Jiang, C.D.; Shi, L.; Shi, T.S.; Gu, W.B. Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol. Plant. 2010, 54, 145–148. [Google Scholar] [CrossRef]
- Pinheiro, D.T.; Silva, A.L.; Silva, L.J.; Sekita, M.C.; Dias, D.C. Germination and antioxidant action in melon seeds exposed to salt stress. Pesqui. Agropecuária Trop. 2016, 46, 336–342. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014, 6, plu047. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.A.; Zamfirache, M.M.; Grigore, M.N.; Oprica, L. Determination of antioxidant enzymatic activity in several halophytes from Dobrogea area. J. Exp. Mol. Biol. 2012, 13, 47. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirasteh-Anosheh, H.; Samadi, M.; Kazemeini, S.A.; Ozturk, M.; Ludwiczak, A.; Piernik, A. ROS Homeostasis and Antioxidants in the Halophytic Plants and Seeds. Plants 2023, 12, 3023. https://doi.org/10.3390/plants12173023
Pirasteh-Anosheh H, Samadi M, Kazemeini SA, Ozturk M, Ludwiczak A, Piernik A. ROS Homeostasis and Antioxidants in the Halophytic Plants and Seeds. Plants. 2023; 12(17):3023. https://doi.org/10.3390/plants12173023
Chicago/Turabian StylePirasteh-Anosheh, Hadi, Maryam Samadi, Seyed Abdolreza Kazemeini, Munir Ozturk, Agnieszka Ludwiczak, and Agnieszka Piernik. 2023. "ROS Homeostasis and Antioxidants in the Halophytic Plants and Seeds" Plants 12, no. 17: 3023. https://doi.org/10.3390/plants12173023
APA StylePirasteh-Anosheh, H., Samadi, M., Kazemeini, S. A., Ozturk, M., Ludwiczak, A., & Piernik, A. (2023). ROS Homeostasis and Antioxidants in the Halophytic Plants and Seeds. Plants, 12(17), 3023. https://doi.org/10.3390/plants12173023









