Metarhizium carneum Formulations: A Promising New Biological Control to Be Incorporated in the Integrated Management of Meloidogyne enterolobii on Tomato Plants
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Liquid vs. Powdered Formulation of Metarhizium carneum against Meloidogyne enterolobii in Greenhouse Tomato Plants
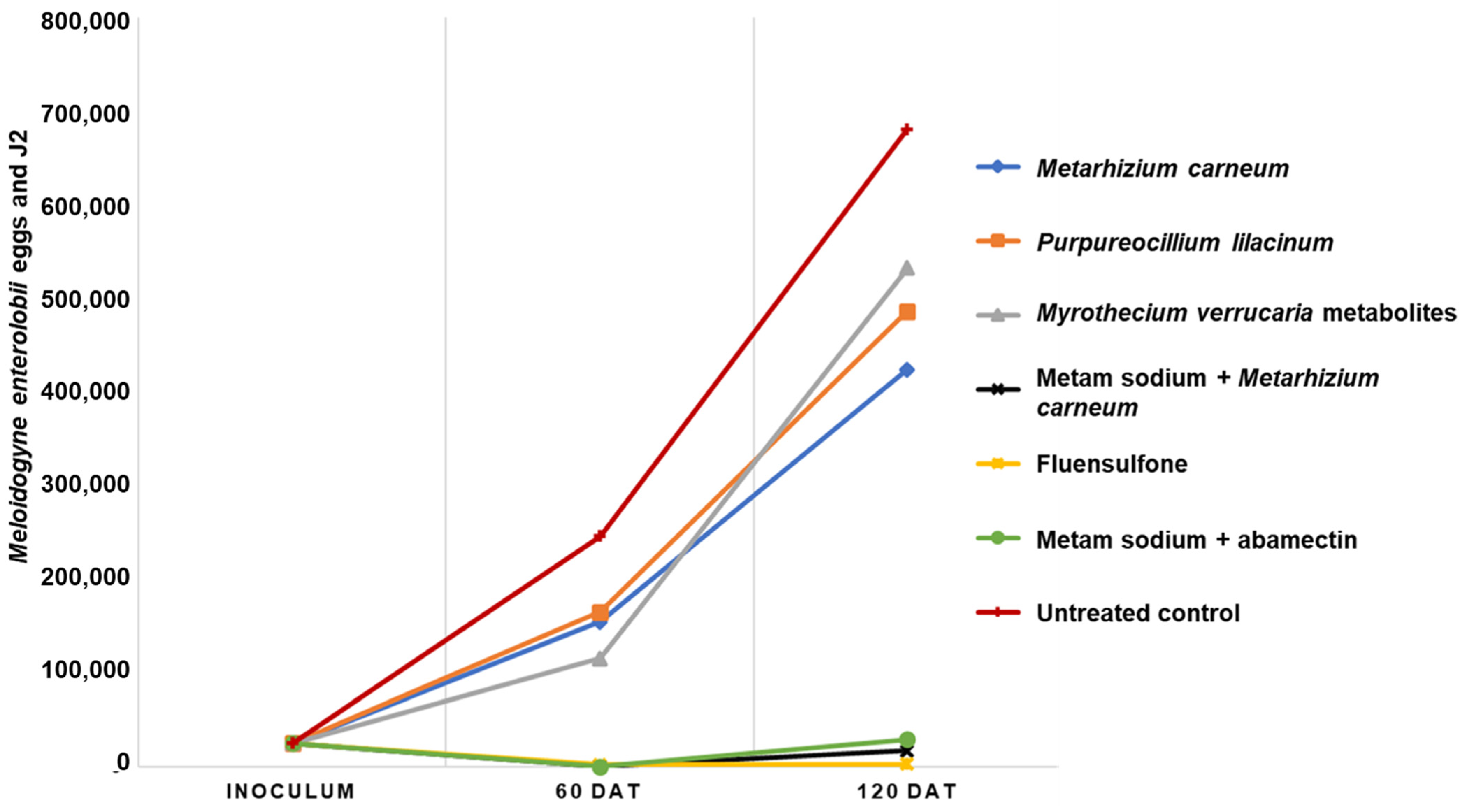
2.2. Effectiveness of M. carneum Liquid Formulation Compared to Commercial Nematicide Products
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Growth Conditions
4.2. Preparation of Metarhizium carneum Formulations
4.3. Obtaining Meloidogyne enterolobii Inoculum
4.4. Evaluation of Metarhizium carneum Formulations for Controlling Meloidogyne enterolobii in Tomato
4.5. Evaluation of Metarhizium carneum Liquid Formulation Compared to Different Commercial Nematicide Products for Controlling Meloidogyne enterolobii in Tomato
4.6. Greenhouse Conditions
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Wyss, U.; Grundler, F.; Munch, A. The parasitic behavior of second-stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica 1992, 38, 98–111. [Google Scholar] [CrossRef]
- Bartlem, D.G.; Jones, M.G.; Hammes, U.Z. Vascularization and nutrient delivery at root-knot nematode feeding sites in host roots. J. Exp. Bot. 2014, 65, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef]
- Ramírez-Suárez, A.; Rosas-Hernández, L.; Alcasio-Rangel, S.; Pérez Valenzuela, G.; Powers, T.O. First report of the root-knot nematode Meloidogyne enterolobii parasitizing watermelon from Veracruz, Mexico. Plant Dis. 2014, 98, 428. [Google Scholar] [CrossRef]
- Martínez-Gallardo, J.Á.; Díaz-Valdés, T.; Allende-Molar, R.; Ortiz-Meza, J.A.; García-Estrada, R.S.; Carrillo-Fasio, J.A. Nematodos fitoparásitos asociados al cultivo de papaya (Carica papaya L.) en Colima, México. Rev. Mex. De Cienc. Agrícolas 2014, 5, 317–323. [Google Scholar]
- Martínez-Gallardo, J.Á.; Díaz-Valdés, T.; Allende-Molar, R.; Retes-Manjarrez, J.E.; Carrillo-Fasio, J.A. Identificación y distribución de Meloidogyne spp. en tomate de Sinaloa México. Rev. Mex. De Cienc. Agrícolas 2019, 10, 453–459. [Google Scholar] [CrossRef]
- FAOSTAT. Cultivos y Productos de Ganadería. Available online: https://www.fao.org/faostat/es/#data/QCL. (accessed on 11 October 2022).
- USDA Tomatoes and Products Anual Report. USDA Foreign Agricultural Sevice. Available online: https://agfstorage.blob.core.windows.net/misc/FP_com/2022/06/17/Ato.pdf (accessed on 9 November 2022).
- Gómez-González, G.; Márquez-Zequera, I.; Cruz-Lachica, I.; Osuna-García, L.A.; García-Estrada, R.S. First report of Meloidogyne enterolobii parasitizing cucumber in Sinaloa, Mexico. Plant Dis. 2020, 104, 1260. [Google Scholar] [CrossRef]
- Carrillo-Fasio, J.A.; Martínez-Gallardo, J.A.; Ayala-Tafoya, F.; López-Orona, C.A.; Allende-Molar, R.; Retes-Manjarrez, J.E. Screening for resistance to Meloidogyne enterolobii in Capsicum annuum landraces from Mexico. Plant Dis. 2020, 104, 817–822. [Google Scholar] [CrossRef]
- Salazar-Mesta, R.J.; Carrillo-Fasio, J.A.; Tovar-Pedraza, J.M.; Garcia-Estrada, R.S.; Mora-Romero, G.A.; Vega-Hernández, R.; Torres-López, J. First report of the root-knot nematode Meloidogyne enterolobii parasitizing eggplant in Mexico. Plant Dis. 2023, 107, 1638. [Google Scholar] [CrossRef]
- Salinas-Castro, A.; Navarro de la Fuente, L.; Tapia-Vázquez, I.; López-Lima, D. First report of Meloidogyne enterolobii on chard (Beta vulgaris subsp. vulgaris) and carrot (Daucus carota) in México. J. Plant Dis. Prot. 2022, 129, 1263–1268. [Google Scholar] [CrossRef]
- Silva, S.D.; Carneiro, R.M.; Faria, M.; Souza, D.A.; Monnerat, R.G.; Lopes, R.B. Evaluation of Pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. J. Nematol. 2017, 49, 77–85. [Google Scholar] [CrossRef]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Haydock, P.P.; Ambrose, E.L.; Wilcox, A.; Deliopoulos, T. Degradation of the nematicide oxamyl under field and laboratory conditions. Nematology 2012, 14, 339–352. [Google Scholar] [CrossRef]
- de Freitas Soares, F.E.; Sufiate, B.L.; de Queiroz, J.H. Nematophagous fungi: Far beyond the endoparasite, predator and ovicidal groups. Agric. Nat. Resour. 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Eapen, S.J.; Beena, B.; Ramana, K.V. Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. J. Invertebr. Pathol. 2005, 88, 218–225. [Google Scholar] [CrossRef]
- Carrión, G.; Hernandez-Leal, T.I.; López-Lima, D.; Núñez-Sánchez, A.E. Uses, Methods and Biological Compositions of the Genus Paecilomyces for the Control, Prevention and Eradication of Phytoparasites in Solanaceae Cultures. WO2012148251 A2. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012148251&tab=PCTBIBLIO (accessed on 8 October 2022).
- Lopez-Llorca, L.V.; Duncan, G.H. A study of fungal endoparasitism of the cereal cyst nematode (Heterodera avenae) by scanning electron microscopy. Can. J. Microbiol. 1988, 34, 613–619. [Google Scholar] [CrossRef]
- Boag, B.; Lopez Llorca, L.V. Nematodes and nematophagous fungi associated with cereal fields and permanent pasture in eastern Scotland. Crop Res. 1989, 29, 1–10. [Google Scholar]
- Sikandar, A.; Jia, L.; Wu, H.; Yang, S. Meloidogyne enterolobii risk to agriculture, its present status and future prospective for management. Front. Plant Sci. 2023, 13, 1093657. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Understanding molecular plant–nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Udo, I.A.; Osai, E.O.; Ukeh, D.A. Management of root-knot disease on tomato with bioformulated Paecilomyces lilacinus and leaf extract of Lantana camara. Braz. Arch. Biol. Technol. 2014, 57, 486–492. [Google Scholar] [CrossRef]
- Nagachandrabose, S. Liquid bioformulations for the management of root-knot nematode, Meloidogyne hapla that infects carrot. Crop Prot. 2018, 114, 155–161. [Google Scholar] [CrossRef]
- Sánchez, J.Y.; Cardona, N.L. Evaluación del bioformulado y del filtrado crudo de Purpureocillium sp. cepa Udea0106 sobre nemátodos fitopatógenos en crisantemo (Dendranthema grandiflora). Biotecnol. Apl. 2018, 35, 2221–2227. [Google Scholar]
- Oclarit, E.; Cumagun, C. Evaluation of efficacy of Paecilomyces lilacinus as biological control agent of Meloidogyne incognita attacking tomato. J. Plant Prot. Res. 2009, 49, 337–340. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jackson, T.A. Progress in the commercialization of bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
- Huang, W.K.; Cui, J.K.; Liu, S.M.; Kong, L.A.; Wu, Q.S.; Peng, H.; He, W.T.; Sun, J.H.; Peng, D.L. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalastrum racemosum and Paecilomyces lilacinus as being most effective. Biol. Control 2016, 92, 31–37. [Google Scholar] [CrossRef]
- Hore, J.; Roy, K.; Maiti, A.K. Evaluation of bio-nematon (Purpureocillium lilacinum 1.15%WP) against root-knot nematode (Meloidogyne incognita) in tomato. J. Entomol. Zool. Stud. 2018, 6, 1700–1704. [Google Scholar]
- Das, M.M.; Rodríguez-Herrera, R.; Haridas, M.; Sabu, A. Purpureocillium lilacinum: A promising bionematicide for sustainable agriculture. In Biocontrol Systems and Plant Physiology in Modern Agriculture: Processes, Strategies, Innovations, 1st ed.; Rojas, R., Martínez Ávila, G.C., Vidales Contreras, J.A., Aguilar, C.N., Eds.; Apple Academic Press: New York, NY, USA, 2022; ISBN 9781003277118. [Google Scholar] [CrossRef]
- de Sequeira, D.C.; Menezes, R.C.; Oliveira, M.M.; Antas, P.R.; De Luca, P.M.; De Oliveira-Ferreira, J.D.; Borba, C.D. Experimental hyalohyphomycosis by Purpureocillium lilacinum: Outcome of the infection in C57BL/6 murine models. Front. Microbiol. 2017, 8, 617. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yeh, L.K.; Ma, D.H.; Lin, H.C.; Sun, C.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Sun, P.L.; Hsiao, C.H. Paecilomyces/Purpureocillium keratitis: A consecutive study with a case series and literature review. Med. Mycol. 2020, 58, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kassam, R.; Yadav, J.; Jaiswal, N.; Chatterjee, M.; Hada, A.; Chawla, G.; Kamil, D.; Rao, U. Identification and potential utility of Metarhizium anisopliae (ITCC9014) for the management of root-knot nematode. Meloidogyne Incognita. Indian Phytopathol. 2022, 75, 875–881. [Google Scholar] [CrossRef]
- Cardona, N.L.; Borrego, D.A.; Fernández, E.P.; Sánchez, J.; Cardona, V.; Montoya, G. Microbiological evaluation and pathogenicity of a liquid bioformulation of the fungus Purpureocillium sp. (strain UdeA 0109) on Meloidogyne incognita-javanica stages. Biotecnol. Apl. 2014, 31, 210–215. [Google Scholar]
- Mishra, J.; Arora, N.K. Bioformulations for plant growth promotion and combating phytopathogens: A sustainable approach. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; ISBN 978-81-322-2779-3. [Google Scholar] [CrossRef]
- Bawa, N.; Kaur, S.; Dhillon, N.K. Efficacy of Purpureocillium lilacinum, Trichoderma harzianum and T. viride bio-formulations against Meloidogyne incognita. Indian Phytopathol. 2020, 73, 799–804. [Google Scholar] [CrossRef]
- Song, Z.; Shen, L.; Zhong, Q.; Yin, Y.; Wang, Z. Liquid culture production of microsclerotia of Purpureocillium lilacinum for use as bionematicide. Nematology 2016, 18, 719–726. [Google Scholar] [CrossRef]
- Butt, T.M.; Greenfield, B.P.; Greig, C.; Maffeis, T.G.; Taylor, J.W.; Piasecka, J.; Dudley, E.; Abdulla, A.; Dubovskiy, I.M.; Garrido-Jurado, I.; et al. Metarhizium anisopliae pathogenesis of mosquito larvae: A verdict of accidental death. PLoS ONE 2013, 8, e81686. [Google Scholar] [CrossRef]
- Sikandar, A.; Gao, F.; Mo, Y.; Chen, Q.; Ullah, R.M.K.; Wu, H. Efficacy of Aspergillus tubingensis GX30 Fermentation against Meloidogyne enterolobii in Tomato (Solanum lycopersicum L.). Plants 2023, 12, 2724. [Google Scholar] [CrossRef]
- Natarajan, A.; Selvam, D.; Palaniappan, K.; Subbaiah Balamurali, A.; Perumal, C.; Durai, R.; Sadasivam, S.; Asokan, A.; Sivalingam, R.; Subiramaniyan, A. Standardization of the Optimum Effects of Indole 3-Butyric Acid (IBA) to Control Root Knot Nematode, Meloidogyne enterolobii, in Guava (Psidium guajava L.). Molecules 2023, 28, 1839. [Google Scholar] [CrossRef]
- Moosavi, M.R.; Zare, R. Fungi as biological control agents of plant-parasitic nematodes. In Plant Defence: Biological Control. Progress in Biological Control; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2020; Volume 22, ISBN 978-3-030-51034-3. [Google Scholar] [CrossRef]
- Khalil, A.E.; El-Sherif, A.G.; Bekhiet, M.A.; Kella, A.M. New trend for Meloidogyne javanica management by Myrothecium verrucaria (Ditera) as promising biological agent. J. Plant Prot. Pathol. 2010, 1, 539–558. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Jang, J.Y.; Kim, T.Y.; Yu, N.H.; Park, A.R.; Lee, S.; Bae, C.H.; Yeo, J.H.; Hur, J.S.; Park, H.W.; et al. Nematicidal activity of verrucarin A and roridin A isolated from Myrothecium verrucaria against Meloidogyne incognita. Pestic. Biochem. Physiol. 2018, 148, 133–143. [Google Scholar] [CrossRef]
- Hagag, E.S. Evaluation of metabolites of Myrothecium verrucaria as biological nematicide against root-knot nematode, Meloidogyne incognita in vitro and in vivo on sugar beet plants. J. Plant Prot. Pathol. 2021, 12, 47–53. [Google Scholar] [CrossRef]
- López-Lima, D.; Carrión, G.; Sánchez-Nava, P.; Desgarennes, D.; Villain, L. Fungal diversity and Fusarium oxysporum pathogenicity associated with coffee corky-root disease in Mexico. Rev. De La Fac. De Cienc. Agrar. UNCuyo 2020, 52, 276–292. [Google Scholar]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Talavera-Rubia, M.; Vela-Delgado, M.D.; Verdejo-Lucas, S. A cost-benefit analysis of soil disinfestation methods against root-knot nematodes in mediterranean intensive horticulture. Plants 2022, 11, 2774. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, B.B. Scenarios for sustainable management of plant parasitic nematodes. Indian Phytopathol. 2021, 74, 469–475. [Google Scholar] [CrossRef]
- Li, J.; Huang, B.; Wang, Q.; Li, Y.; Fang, W.; Han, D.; Yan, D.; Guo, M.; Cao, A. Effects of fumigation with metam-sodium on soil microbial biomass, respiration, nitrogen transformation, bacterial community diversity and genes encoding key enzymes involved in nitrogen cycling. Sci. Total Environ. 2017, 598, 1027–1036. [Google Scholar] [CrossRef]
- Sederholm, M.R.; Schmitz, B.W.; Barberán, A.; Pepper, I.L. Effects of metam sodium fumigation on the abundance, activity, and diversity of soil bacterial communities. Appl. Soil Ecol. 2018, 124, 27–33. [Google Scholar] [CrossRef]
- Alam, M.S.; Khanal, C.; Rutter, W.; Roberts, J. Non-fumigant Nematicides are Promising Alternatives to Fumigants for the Management of Meloidogyne enterolobii in Tobacco. J. Nematol. 2022, 54, 3922. [Google Scholar] [CrossRef]
- Watson, T.T. Sensitivity of Meloidogyne enterolobii and M. incognita to fluorinated nematicides. Pest Manag. Sci. 2022, 78, 1398–1406. [Google Scholar] [CrossRef]
- Giannakou, I.O.; Panopoulou, S. The use of fluensulfone for the control of root-knot nematodes in greenhouse cultivated crops: Efficacy and phytotoxicity effects. Cogent Food Agric. 2019, 5, 1643819. [Google Scholar] [CrossRef]
- Tigano, M.; De Siqueira, K.; Castagnone-Sereno, P.; Mulet, K.; Queiroz, P.; Dos Santos, M.; Teixeira, C.; Almeida, M.; Silva, J.; Carneiro, R. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava damaging species. Plant Pathol. 2010, 59, 1054–1061. [Google Scholar] [CrossRef]
- Coyne, D.L.; Ross, J.L. Protocol for Nematode Resistance Screening: Root Knot Nematodes, Meloidogyne spp.; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2014; ISBN 978-978-8444-43-5. [Google Scholar]
- Carneiro, R.; Tigano, M.; Randig, O.; Almeida, M.R.; Sarah, J.L. Identification and genetic diversity of Meloidogyne spp. (Tylenchida: Meloidogynidae) on coffee from Brazil, Central America, and Hawaii. Nematology 2004, 6, 287–298. [Google Scholar] [CrossRef]
- van Bezooijen, J. Methods and Techniques for Nematology. Available online: https://www.wur.nl/upload_mm/f/9/3/10aac0cb-8289-400a-a6e5-c4687461d138_MethodsandTechniquesforNematology.pdf (accessed on 12 August 2023).
- Carrillo-Fasio, J.A.; Angúlo-Castro, A.; Martínez-Gallardo, J.Á.; Ayala-Tafoya, F.; Yáñez-Juárez, M.G.; Retes-Manjarrez, J.E. Distribution and incidence of root-knot nematodes (Meloidogyne spp.) on pepper in Sinaloa, Mexico. Trop. Plant Pathol. 2021, 46, 195–200. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Optimizing sampling and extraction methods for plant-parasitic and entomopathogenic nematodes. Plants 2021, 10, 629. [Google Scholar] [CrossRef]


| Treatments | Galls Number per Root | Galling Index | Eggs and J2 g Root−1 | J2 g−1 Rhizospheric Soil | Total Nematode Population |
|---|---|---|---|---|---|
| Liquid | 145 ± 110 | 1.86 ± 0.4 | 14,481 ± 11,938 a 1 | 12 ± 17 a | 85,005 ± 75,363 a |
| Powder | 156 ± 124 | 1.93 ± 0.26 | 22,207 ± 13,505 a | 11 ± 12 a | 74,678 ± 57,624 a |
| Untreated | 189 ± 106 | 2 ± 0 | 64,791 ± 38,944 b | 26 ± 8 b | 257,187 ± 170,414 b |
| p | p = 0.66 | p = 0.72 | p < 0.01 | p < 0.01 | p < 0.01 |
| Treatments | Height cm | Foliage Fresh Weight g | Rooth Length cm | Root Fresh Weight g |
|---|---|---|---|---|
| Liquid | 97 ± 10 | 46 ± 10 a 1 | 19.5 ± 4.1 | 5.2 ± 3.6 |
| Powder | 89 ± 15 | 36 ± 10.9 b | 16.4 ± 4.3 | 3.7 ± 1.9 |
| Control | 90 ± 13 | 40 ± 10 ab | 16.8 ± 3.8 | 4.6 ± 2.6 |
| p | 0.21 | 0.01 | 0.08 | 0.09 |
| Treatments | Galling Index | Eggs and J2 g Root−1 | Final Eggs and J2 | Reproduction Factor |
|---|---|---|---|---|
| Metarhizium carneum | 2.6 ± 0.5 b 1 | 6665 ± 2453 cd | 154,542 ± 37,516 b | 6.2 ± 1.5 b |
| Purpureocillium lilacinum | 2.6 ± 0.5 b | 5626 ± 1045 bc | 164,850 ± 28,727 b | 6.6 ± 1.1 b |
| Metabolites from M. verrucaria fermentation | 2.3 ± 0.5 b | 3553 ± 2020 b | 115,238 ± 58,897 b | 4.6 ± 2.3 b |
| Metam sodium + Metarhizium carneum | 1.0 ± 0 a | 66 ± 19 a | 563 ± 194 a | 0.02 ± 0.007 a |
| Fluensulfone | 1.0 ± 0 a | 158 ± 59 a | 1913 ± 1017 a | 0.1 ± 0.04 a |
| Metam sodium + abamectina | 1.0 ± 0 a | 15 ± 4 a | 128 ± 22.5 a | 0.005 ± 0.0009 a |
| Control | 3.3 ± 0.5 c | 9255 ± 2355 d | 245,625 ± 26,591 c | 9.8 ± 1 c |
| p | <0.01 | <0.01 | <0.01 | <0.01 |
| Treatments | Galling Index | Eggs and J2 g Root−1 | Final Eggs and J2 | Reproduction Factor |
|---|---|---|---|---|
| Metarhizium carneum | 3.4 ± 0.5 b 1 | 2614 ± 1298 ab | 423,825 ± 168,141 b | 17.0 ± 6.7 b |
| Purpureocillium lilacinum | 4.3 ± 0.5 c | 5296 ± 2344 b | 486,300 ± 133,878 b | 19.5 ± 5.4 b |
| Metabolites from M. verrucaria fermentation | 4.7 ± 0.4 c | 9049 ± 3733 c | 532,425 ± 127,316 b | 21.3 ± 5.1 b |
| Metam sodium + Metarhizium carneum | 1.3 ± 0.5 a | 336 ± 12.2 a | 16,980 ± 5087 a | 0.7 ± 0.2 a |
| Fluensulfone | 1.0 ± 0 a | 83 ± 33 a | 2625 ± 1267 a | 0.1 ± 0.05 a |
| Metam sodium + abamectina | 1.3 ± 0.5 a | 312 ± 212 a | 29,175 ± 12,862 a | 1.2 ± 0.5 a |
| Control | 5.0 ± 0 c | 9268 ± 2111 c | 681,000 ± 531,754 b | 27.2 ± 21.3 b |
| p | <0.01 | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Lima, D.; Alarcón-Utrera, D.; Ordáz-Meléndez, J.Á.; Villain, L.; Carrión, G. Metarhizium carneum Formulations: A Promising New Biological Control to Be Incorporated in the Integrated Management of Meloidogyne enterolobii on Tomato Plants. Plants 2023, 12, 3431. https://doi.org/10.3390/plants12193431
López-Lima D, Alarcón-Utrera D, Ordáz-Meléndez JÁ, Villain L, Carrión G. Metarhizium carneum Formulations: A Promising New Biological Control to Be Incorporated in the Integrated Management of Meloidogyne enterolobii on Tomato Plants. Plants. 2023; 12(19):3431. https://doi.org/10.3390/plants12193431
Chicago/Turabian StyleLópez-Lima, Daniel, David Alarcón-Utrera, José Ángel Ordáz-Meléndez, Luc Villain, and Gloria Carrión. 2023. "Metarhizium carneum Formulations: A Promising New Biological Control to Be Incorporated in the Integrated Management of Meloidogyne enterolobii on Tomato Plants" Plants 12, no. 19: 3431. https://doi.org/10.3390/plants12193431
APA StyleLópez-Lima, D., Alarcón-Utrera, D., Ordáz-Meléndez, J. Á., Villain, L., & Carrión, G. (2023). Metarhizium carneum Formulations: A Promising New Biological Control to Be Incorporated in the Integrated Management of Meloidogyne enterolobii on Tomato Plants. Plants, 12(19), 3431. https://doi.org/10.3390/plants12193431






