Primary Screening of Microorganisms against Meloidogyne hapla (Chitwood, 1949) under the Conditions of Laboratory and Vegetative Tests on Tomato
Abstract
:1. Introduction
2. Results
2.1. Identification of Root-Knot Nematode Species
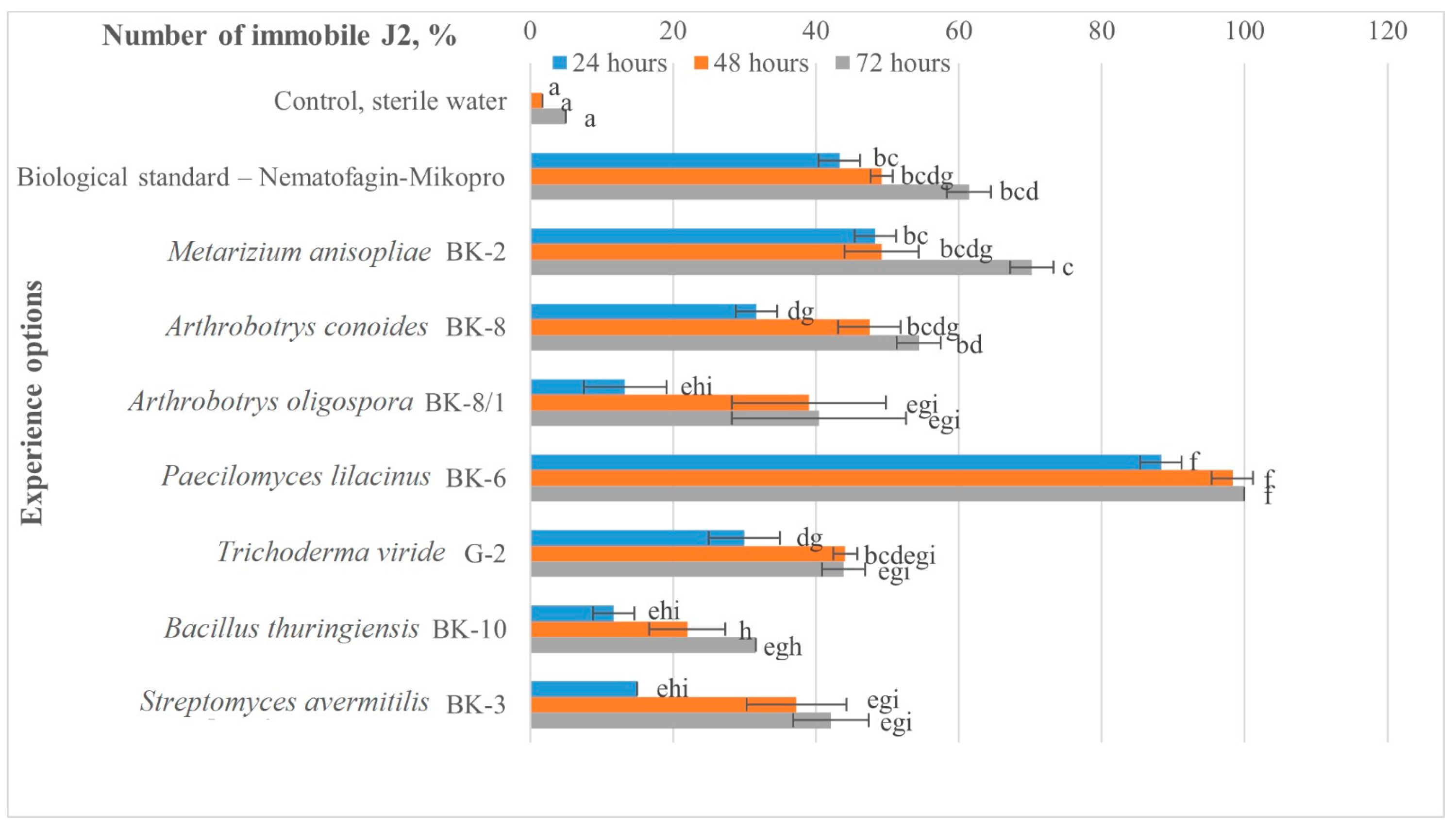
2.2. Effect of Liquid Cultures of Microbial Strains on Second-Stage Juveniles (J2) of M. hapla
2.3. Effect of Liquid Cultures of Microbial Strains on the Presence of Galls on Tomato Plants
3. Discussion
4. Materials and Methods
4.1. Research Location
4.2. Root-Knot Nematode Samples and Their Identification
4.3. Obtaining a Suspension of Juveniles of the Northern Root-Knot Nematode (J2)
4.4. Research Objects
4.5. Effect of Liquid Cultures of Microorganism Strains on M. hapla
4.6. Effect of Liquid Cultures of Microorganism Strains on the Presence of Galls on Tomato Plants
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Churikova, A.K.; Nekoval, S.N. Biological agents and their metabolites to control Meloidogyne spp. when growing vegetables (review). S. Russ. Ecol. Dev. 2022, 17, 175–186. [Google Scholar] [CrossRef]
- Zinovieva, S.V.; Chizhov, V.N.; Pridannikov, M.V.; Subbotin, S.A.; Ryss, A.; Khusainov, R. Phytoparasitic nematodes of Russia; Association of scientific publications of the CMC: Moscow, Russia, 2012; Volume 386. [Google Scholar]
- Zinovieva, S.V.; Udalova, Z.V.; Zayml-Buchinger, V.V.; Khasanov, F.K. Expression of proteinase inhibitor genes in tomato plants during invasion by the root knot nematode Meloidogyne incognita and modulation of their activity by salicylic and jasmonic acids. Izv. Akad. Nauk SSSR Biol. 2021, 2, 126–136. [Google Scholar] [CrossRef]
- Özdemir, F.G.G.; Arici, Ş.E. Effect of culture filtrate concentration of Rhizoctonia solani Kühn against Meloidogyne incognita and Meloidogyne hapla in vitro. Int. J. Agric. For. Life Sci. 2021, 5, 74–79. [Google Scholar]
- Evlice, E.; Toktay, H.; Yatkın, G.; Erdoğuş, F.D.; İmren, M. Population Fluctuations of root-knot nematodes Meloidogyne chitwoodi and M. Hapla under field conditions. Phytoparasitica 2022, 50, 233–242. [Google Scholar] [CrossRef]
- Antil, S.; Kumar, R.; Pathak, D.V.; Kumar, A.; Panwar, A.; Kumari, A.; Kumar, V. Potential of Bacillus altitudinis KMS-6 as a biocontrol agent of Meloidogyne javanica. J. Pest Sci. 2022, 95, 1443–1452. [Google Scholar] [CrossRef]
- Luc, M.; Sikora, R.; Bridge, J. (Eds.) Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Ostasheva, N.A. Root knot nematode Meloidogyne hapla Chitwood—A dangerous parasite of medicinal, fruit and subtropical crops on the black sea coast of Russia and measures to combat it. Subtrop. Decor. Gard. 2011, 44, 236–240. [Google Scholar]
- Perry, R.N.; Moens, M. (Eds.) Plant Nematology, 2nd ed.; CABI Publishing: Wallingford, UK, 2013. [Google Scholar]
- Desaeger, J.; Dickson, D.W.; Locascio, S.J. Methyl bromide alternatives for control of root-knot nematode (spp.) in tomato production in Florida. J. Nematol. 2017, 49, 140–149. [Google Scholar] [CrossRef]
- Jeschke, P.; Witschel, M.; Kramer, W.; Schirmer, U. Modern Crop Protection Compounds, 3rd ed.; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-Vch: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Oka, Y. From Old-Generation to next-Generation Nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Gaberthüel, M.; Slaats, B.; Goll, M. What does it take to develop a nematicide today and for the future? In Integrated Nematode Management: State-of-the-Art and Visions for the Future; CABI: Wallingford, UK, 2021; pp. 439–445. [Google Scholar]
- Desaeger, J.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides—Rationale and review. J. Nematol. 2020, 52, 1–16. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Liu, C.; Schumacher, L.A.; Small, I.M.; Wright, D.L. In-furrow fluopyram nematicide efficacy for Rotylenchulus reniformis management in cotton production. Crop Prot. 2021, 140, 105423. [Google Scholar] [CrossRef]
- Faske, T.R.; Hurd, K. Sensitivity of Meloidogyne incognita and rotylenchulus reniformis to fluopyram. J. Nematol. 2015, 47, 316–321. [Google Scholar]
- Ji, X.; Li, J.; Dong, B.; Zhang, H.; Zhang, S.; Qiao, K. Evaluation of Fluopyram for Southern Root-Knot Nematode Management in Tomato Production in China. Crop Prot. 2019, 122, 84–89. [Google Scholar] [CrossRef]
- Oka, Y.; Saroya, Y. Effect of Fluensulfone and fluopyram on the mobility and infection of second-stage juveniles of Meloidogyne incognita and M. javanica. Pest Manag. Sci. 2019, 75, 2095–2106. [Google Scholar] [CrossRef]
- Kandel, Y.R.; Bradley, C.A.; Chilvers, M.I.; Mathew, F.M.; Tenuta, A.U.; Smith, D.L.; Wise, K.A.; Mueller, D.S. Effect of seed treatment and foliar crop protection products on sudden death syndrome and yield of soybean. Plant Dis. 2019, 103, 1712–1720. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the Russian Federation. State Catalog of Pesticides and Agrochemicals. Available online: https://mcx.gov.ru/upload/iblock/488/2arw7qpik2vey0z6phmgkjntxz9koo9q.zip (accessed on 12 July 2023).
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020.
- University of Hertfordshire. Fluensulfone (Ref: BYI 01921). Herts.ac.uk. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/2895.htm (accessed on 12 July 2023).
- University of Hertfordshire. Fluopyram. Herts.ac.uk. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1362.htm (accessed on 12 July 2023).
- Nekoval, S.N.; Belyaeva, A.V.; Moskalenko, O.A.; Churikova, A.K.; Lukina, A.E.; Gorlo, V.E. Prospects of organic production in Russia. Agrochem. Bull. 2019, 5, 77–82. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, K.; Yu, Z.; Li, G. Microbial control of phytopathogenic nematodes. In Principles of Plant-Microbe Interactions; Springer International Publishing: Cham, Switzerland, 2015; pp. 155–164. [Google Scholar]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Shailendra Singh, G.G. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Asaturova, A.M.; Bugaeva, L.N.; Homyak, A.I.; Slobodyanyuk, G.A.; Kashutina, E.V.; Yasyuk, L.V.; Sidorov, N.M.; Nadykta, V.D.; Garkovenko, A.V. Bacillusvelezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne Incognita in a Greenhouse. Plants 2022, 11, 275. [Google Scholar] [CrossRef]
- Nekoval, S.N.; Chernyakovich, M.N.; Churikova, A.K. Nematicidal fungi and their mechanism of action against root-knot nematodes (Meloidogyne spp.) (review). Achi. Sci. Tech. Agric. 2023, in press. [Google Scholar]
- Shesteperov, A. Epiphytotiology of Nematode Diseases of Plants; Arisov, M., Indyuhova, E., Eds.; VNIIP—FSC VIEV, Nauka Publishing House: Moscow, Russia, 2021. [Google Scholar] [CrossRef]
- Tazi, H.; Hamza, M.A.; Hallouti, A.; Benjlil, H.; Idhmida, A.; Furze, J.N.; Paulitz, T.C.; Mayad, E.H.; Boubaker, H.; El Mousadik, A. Biocontrol potential of nematophagous fungi against Meloidogyne spp. Infect. Tomato. Org. Agric. 2021, 11, 63–71. [Google Scholar] [CrossRef]
- Peiris, P.U.S.; Li, Y.; Brown, P.; Xu, C. Fungal biocontrol against Meloidogyne spp. in agricultural crops: A systematic review and meta-analysis. Biol. Control 2020, 144, 104235. [Google Scholar] [CrossRef]
- Pestsov, G.V.; Lushnikov, O.V.; Glazunova, A.V. Nematogenic fungi as the basis of a biological method of combating root knot nematodes. Agric. Sci. 2019, 2, 122–125. [Google Scholar]
- Youssef, M.M.A.; El-Nagdi, W.M.A.; Lotfy, D.E.M. Evaluation of the fungal activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as biocontrol agents against root-knot nematode, Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2020, 44, 112. [Google Scholar] [CrossRef]
- Pestsov, G.V.; Glazunova, A.V. Biotechnological utilization of organic waste and production of new products. In Science—Practice; Klimuk, V.V., Gorbach, Y.E., Degil, N.I., Pradun, A.V., Prudnikova, A.N., Eds.; Educational institution “Baranovichi State University”: Baranovichi, Republic of Belarus, 2021; pp. 141–143. [Google Scholar]
- Kiewnick, S.; Sikora, R.A. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol. Control 2006, 38, 179–187. [Google Scholar] [CrossRef]
- El-Marzoky, A.M.; Elnahal, A.S.M.; Jghef, M.M.; Abourehab, M.A.S.; El-Tarabily, K.A.; Ali, M.A.M.S. Purpureocillium lilacinum strain AUMC 10620 as a biocontrol agent against the citrus nematode Tylenchulus semipenetrans under laboratory and field conditions. Eur. J. Plant Pathol. 2023, 167, 59–76. [Google Scholar] [CrossRef]
- Erdoğuş, F.D.; Erdurmuş, D.; Yağci, M.; Bayraktar, H. Effects of some Metarhizium anisopliae (Metschn.) Sorokin, 1883 (Hypocreales: Clavicipitaceae) isolates on root-knot nematodes under laboratory conditions. Turk. Entomol. Derg. 2021, 45, 183–191. [Google Scholar] [CrossRef]
- Kassam, R.; Yadav, J.; Jaiswal, N.; Chatterjee, M.; Hada, A.; Chawla, G.; Kamil, D.; Rao, U. Identification and potential utility of Metarhizium anisopliae (ITCC9014) for the management of root-knot nematode, Meloidogyne incognita. Indian Phytopathol. 2022, 75, 875–881. [Google Scholar] [CrossRef]
- Soliman, M.S.; El-Deriny, M.M.; Ibrahim, D.S.S.; Zakaria, H.; Ahmed, Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021, 131, 2402–2415. [Google Scholar] [CrossRef]
- Men, A.E.; Wilson, P.; Siemering, K.; Forrest, S. Sanger DNA sequencing. In Next Generation Genome Sequencing; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–11. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Konrat, A.N.; Lychagina, S.V.; Shesteperov, A.A. Methodological instructions “methodology for in vitro screening of strains, isolates of bacteria with parasitic and nonmaticidal properties”. Theory Pract. Parasit. Dis. Control. 2021, 22, 575–590. [Google Scholar] [CrossRef]
- Bocharnikova, N.I. Genetic Collection of Tomato Mutant forms and Use in the Breeding and Genetic Research; VNIISSOK: Moscow, Russia, 2011. [Google Scholar]
- Lemanova, N.; Poyraz, L. Nematicidal properties of rhizospheric bacteria. In Biotehnologia Microbiologică-Domeniu Scientointensiv al Ştiinţei Contemporane; Ch.: “Elena-V.I.” SRL: Chisinau, Moldova, 2011; pp. 178–179. [Google Scholar]
- Abbott, W.S. A Method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [PubMed]
- Padilla, I.W. Methods for determining the biological activity of nematicidal products. Biotecnol. Appl. 2017, 34, 4301–4304. [Google Scholar]


| M_hapla_1 | M_hapla_2 | M_hapla_3 | M_hapla_4 | M_hapla _MH128580 | M_exigua _MH128478 | M_incognita _MH128456 | M_javanica_ MH128449 | M_arenaria _MH128444 | M_floridensis _MH128431 | |
|---|---|---|---|---|---|---|---|---|---|---|
| M_hapla_1 | ||||||||||
| M_hapla_2 | 0.00137931 | |||||||||
| M_hapla_3 | 0.00137931 | 0.00000000 | ||||||||
| M_hapla_4 | 0.00275862 | 0.00137931 | 0.00137931 | |||||||
| M_hapla_MH128580 | 0.00690608 | 0.00828729 | 0.00828729 | 0.00966851 | ||||||
| M_exigua_MH128478 | 0.14364641 | 0.14502762 | 0.14502761 | 0.14502762 | 0.13674033 | |||||
| M_incognita_MH128456 | 0.14583333 | 0.14722222 | 0.14722222 | 0.14861111 | 0.13888889 | 0.14305556 | ||||
| M_javanica_MH128449 | 0.14722222 | 0.14861111 | 0.14861111 | 0.15000000 | 0.14027778 | 0.14444444 | 0.00277778 | |||
| M_arenaria_MH128444 | 0.14722222 | 0.14861111 | 0.14861111 | 0.15000000 | 0.14027778 | 0.14444444 | 0.00277778 | 0.00000000 | ||
| M_floridensis_MH128431 | 0.14722222 | 0.14861111 | 0.14861111 | 0.15000000 | 0.14027778 | 0.14444444 | 0.00277778 | 0.00000000 | 0.00000000 |
| M_incognita_1 | M_incognita _MH743219 | M_hapla _MH128580 | M_luci _MG969511 | M_javanica _OR038715 | M_inornata _ku372168 | M_ethiopica _ku372162 | M_paranaensis _OQ750577 | M_hispanica _OQ842292 | |
|---|---|---|---|---|---|---|---|---|---|
| M_incognita_1 | |||||||||
| M_incognita_MH743219 | 0.00000000 | ||||||||
| M_hapla_MH128580 | 0.12271541 | 0.12271541 | |||||||
| M_luci_MG969511 | 0.01190476 | 0.01190476 | 0.13315927 | ||||||
| M_javanica_OR038715 | 0.01666667 | 0.01666667 | 0.13054830 | 0.02857143 | |||||
| M_inornata_KU372168 | 0.02619048 | 0.02619048 | 0.13577024 | 0.03809524 | 0.03809524 | ||||
| M_ethiopica_KU372162 | 0.02857143 | 0.02857143 | 0.14621410 | 0.03095238 | 0.04047619 | 0.05000000 | |||
| M_paranaensis_OQ750577 | 0.02676399 | 0.02676399 | 0.15223097 | 0.03406326 | 0.04379562 | 0.04866180 | 0.05352798 | ||
| M_hispanica_OQ842292 | 0.02985075 | 0.02985075 | 0.1436031 | 0.04228856 | 0.04228856 | 0.04228856 | 0.05472637 | 0.05750000 |
| Experimental Option | Quantity of the Preparation, mL | Number of Studied Plants | Number of Infected Plants | Number of Galls/Plant (n = 40) | Biological Efficiency, % |
|---|---|---|---|---|---|
| Control (infected plants, water) | - | 40 | 40 | 97.6 ± 6.1 | - |
| Control (uninfected plants, water) | - | 0 | 0 | 0.0 | - |
| Biological standard—Nematofagin-Mikopro | 50 | 40 | 19 | 17.7 ± 1.9 | 82.0 |
| Metarisium anisopliae BK-2 | 50 | 40 | 21 | 23.9 ± 2.3 | 75.7 |
| Arthrobotrys conoides BK-8 | 50 | 40 | 28 | 25.2 ± 1.7 | 74.5 |
| Arthrobotrys oligospora BK-8/1 | 50 | 40 | 31 | 55.7 ± 3.6 | 42.9 |
| Paecilomyces lilacinus BK -6 | 50 | 40 | 19 | 18.5 ± 2.4 | 81.2 |
| Trichoderma viride G-2 | 50 | 40 | 35 | 47.2 ± 3.3 | 51.3 |
| Bacillus thuringiensis BK-10 | 50 | 40 | 38 | 61.9 ± 2.8 | 36.8 |
| Streptomyces avermitilis BK-3 | 50 | 40 | 35 | 58.3 ± 2.3 | 40.4 |
| Type of Microorganism | Strain | Titer, Colony-forming Units (CFU)/mL |
|---|---|---|
| Metarisium anisopliae | BK-2 | (7.0 ± 0.5) × 107 |
| Arthrobotrys conoides | BK-8 | (6.0 ± 0.3) × 106 |
| Arthrobotrys oligospora | BK-8/1 | (5.5 ± 0.1) × 107 |
| Paecilomyces lilacinus | BK-6 | (3.2 ± 0.8) × 107 |
| Trichoderma viride | G-2 | (5.5 ± 0.2) × 106 |
| Bacillus thuringiensis | BK-10 | (5.0 ± 0.2) × 109 |
| Streptomyces avermitilis | BK-3 | (3.0 ± 0.1) × 107 |
| Arthrobotrys oligospora (Nematofagin-Mikopro) | F-1303 | (3.0 ± 0.2) × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekoval, S.N.; Churikova, A.K.; Chernyakovich, M.N.; Pridannikov, M.V. Primary Screening of Microorganisms against Meloidogyne hapla (Chitwood, 1949) under the Conditions of Laboratory and Vegetative Tests on Tomato. Plants 2023, 12, 3323. https://doi.org/10.3390/plants12183323
Nekoval SN, Churikova AK, Chernyakovich MN, Pridannikov MV. Primary Screening of Microorganisms against Meloidogyne hapla (Chitwood, 1949) under the Conditions of Laboratory and Vegetative Tests on Tomato. Plants. 2023; 12(18):3323. https://doi.org/10.3390/plants12183323
Chicago/Turabian StyleNekoval, Svetlana N., Arina K. Churikova, Maxim N. Chernyakovich, and Mikhail V. Pridannikov. 2023. "Primary Screening of Microorganisms against Meloidogyne hapla (Chitwood, 1949) under the Conditions of Laboratory and Vegetative Tests on Tomato" Plants 12, no. 18: 3323. https://doi.org/10.3390/plants12183323
APA StyleNekoval, S. N., Churikova, A. K., Chernyakovich, M. N., & Pridannikov, M. V. (2023). Primary Screening of Microorganisms against Meloidogyne hapla (Chitwood, 1949) under the Conditions of Laboratory and Vegetative Tests on Tomato. Plants, 12(18), 3323. https://doi.org/10.3390/plants12183323






