Enhancing Sugarcane Yield and Sugar Quality through Optimal Application of Polymer-Coated Single Super Phosphate and Irrigation Management
Abstract
:1. Introduction
2. Results
2.1. Description of Main Effects
2.2. Interactive Effects of Significant Traits
2.2.1. Millable Canes and Stripped Cane Yield
2.2.2. Unstripped Cane Yield and °Brix
2.2.3. Cane Juice Purity % and Cane Sugar Recovery %
2.3. Correlation Explanation
2.4. Biplot Analysis
3. Discussion
4. Materials and Methods
4.1. Field Preparation and Trial Execution
4.2. Data Collection
Sugarcane Juice Extraction
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GOP. Pakistan Economic Survey 2022–23; Finance and Economic Affairs Division, Ministry of Finance, Government of Pakistan: Islamabad, Pakistan, 2023. [Google Scholar]
- Sajid, M.; Munir, H. Agronomic Responses of Sugarcane (Saccharum officinarum L.) Ratoon to Natural and Synthetic Supplements under Water Deficit Conditions. J. Agric. Food 2020, 1, 67–81. [Google Scholar]
- Zafar, S.; Afzal, H.; Ijaz, A.; Mahmood, A.; Ayub, A.; Nayab, A.; Hussain, S.; Maqsood, U.H.; Sabir, M.A.; Zulfiqar, U.; et al. Cotton and drought stress: An updated overview for improving stress tolerance. South Afr. J. Bot. 2023, 161, 258–268. [Google Scholar] [CrossRef]
- Jangpromma, N.; Thammasirirak, S.; Jaisil, P.; Songsri, P. Effects of drought and recovery from drought stress on above ground and root growth, and water use efficiency in sugarcane (Saccharum officinarum L.). Aust. J. Crop. Sci. 2012, 6, 1298–1304. [Google Scholar]
- Liu, X.; Zhang, R.; Ou, H.; Gui, Y.; Wei, J.; Zhou, H.; Tan, H.; Li, Y. Comprehensive transcriptome analysis reveals genes in response to water deficit in the leaves of Saccharum narenga (Nees ex Steud.) hack. BMC Plant Biol. 2018, 18, 250. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1313. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Brestic, M.; Landi, M.; Skalicky, M. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci. Rep. 2020, 10, 10427. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, A.; Pandey, N.; Singh, S. Assessment of waterlogging induced physio-biochemical changes in sugarcane varieties and its association with waterlogging tolerance. J. Environ. Biol. 2019, 40, 384–392. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Afzal, I.; Sabagh, A.E.; Ali, A.; Vicente, O.; Chen, P. Modern biotechnologies: Innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 2021, 11, 1042. [Google Scholar] [CrossRef]
- Khodadadi, D. Effect of superabsorbent polymer on salt and drought resistance of eucalyptus globulus. Appl. Eco Environ. Res. 2017, 15, 1791–1802. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhou, H.; Li, M. Improved Viability of Areca (Areca catechu L.) Seedlings under Drought Stress Using a Superabsorbent Polymer. HortScience 2018, 53, 1872–1876. [Google Scholar] [CrossRef]
- Narayanan, A.; Kartik, R.; Sangeetha, E.; Dhamodharan, R. Super water absorbing polymeric gel from chitosan, citric acid and urea: Synthesis and mechanism of water absorption. Carbohydr. Polym. 2018, 1, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2020, 94, 107021. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J. Appl. Polym. Sci 2020, 137, 48495. [Google Scholar] [CrossRef]
- Nascimento, C.D.V.; Simmons, R.W.; de Andrade Feitosa, J.P.; dos Santos Dias, C.T.; Costa, M.C.G. Potential of superabsorbent hydrogels to improve agriculture under abiotic stresses. J. Arid Environ. 2021, 189, 104496. [Google Scholar] [CrossRef]
- Verma, M.K.; Pandey, P.; De, N. Characterization of water retention and release capacity of innovative nano clay polymer composite superabsorbent. J. Pharmogen. Phytochem. 2017, 6, 42–48. [Google Scholar]
- Mazloom, N.; Khorassani, R.; Zohury, G.H.; Emami, H.; Whalen, J. Lignin-based hydrogel alleviates drought stress in maize. Environ. Exp. Bot. 2020, 175, 104055. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability Micha under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers. 2020, 12, 2425. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B.; Guo, H.; Wei, T. The effect of super absorbent polymers on soil and water conservation on the terraces of the loess plateau. Ecol. Eng. 2017, 102, 270–279. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, T.; Dou, P.; Sheng, J.; Luo, M. Effect of various concentrations of superabsorbent polymers on soil particle-size distribution and evaporation with sand mulching. Sci. Rep. 2019, 9, 3511. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum-based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, W.; Liang, L.; Lei, Z. Effects of eco-friendly carbohydrate-based superabsorbent polymers on seed germination and seedling growth of maize. Royal Soc. Open Sci. 2018, 5, 171184. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Ribeiro, R.; Marchiori, P.; Machado, D.; Machado, E.; Landell, M. Biometric and physiological responses to water deficit in sugarcane at different phenological stages. Pesqui. Agropecuária Bras. 2009, 44, 1575–1582. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genetics. 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Da Graça, J.P.; Rodrigues, F.A.; Farias, J.R.B.; De Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef]
- Inman-Bamber, N.; Lakshmanan, P.; Park, S. Sugarcane for water limited environments: Theoretical assessment of suitable traits. Field Crops Res. 2012, 134, 95–104. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.-J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef]
- Javed, T.; Zhou, J.R.; Li, J.; Hu, Z.T.; Wang, Q.N.; Gao, S.J. Identification and expression profiling of WRKY family genes in sugarcane in response to bacterial pathogen infection and nitrogen implantation dosage. Front. Plant Sci. 2022, 13, 917953. [Google Scholar] [CrossRef]
- Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined abiotic stresses: Challenges and potential for crop improvement. Agronomy 2022, 12, 2795. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Jifon, J.L.; dos Santos, C.M.; Jadoski, C.J.; da Silva, J.A.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Soares, R.A.B.; Landell, M.G.d.A.; Campana, M.P. Agronomic performance of sugarcane families in response to water stress. Bragantia 2008, 67, 655–661. [Google Scholar] [CrossRef]
- da Silva, P.P.; Soares, L.; da Costa, J.G.; Viana, L.D.; Farias de Andrade, J.C.; Goncalves, E.R. Path analysis for selection of drought tolerant sugarcane genotypes through physiological components. Ind. Crops Prod. 2012, 37, 11–19. [Google Scholar] [CrossRef]
- Medeiros, D.B.; da Silva, E.C.; Mansur Custodio Nogueira, R.J.; Teixeira, M.M.; Buckeridge, M.S. Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theor. Exp. Plant Physiol. 2013, 25, 213–222. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Robinson, N. Stress physiology: Abiotic stresses. In Sugarcane: Physiology, Biochemistry, and Functional Biology; Moore, P.H., Botha, F.C., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2014; pp. 411–434. [Google Scholar]
- Lembke, C.; Nishiyama, M.Y.; Sato, P.M.; de Andrade, R.F.; Souza, G.M. Identification of sense and antisense transcripts regulated by drought in sugarcane. Plant Mol. Biol. 2012, 79, 461–477. [Google Scholar] [CrossRef]
- Robertson, M.J.; Inman-Bamber, N.G.; Muchow, R.C.; Wood, A.W. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crop. Res. 1999, 64, 211–227. [Google Scholar] [CrossRef]
- Gentile, A.; Dias, L.I.; Mattos, R.S.; Ferreira, T.H.; Menossi, M. MicroRNAs and drought responses in sugarcane. Front. Plant Sci. 2015, 6, 58. [Google Scholar] [CrossRef]
- Gomathi, R.; Gururaja Rao, P.N.; Chandran, K. Adaptive Responses of Sugarcane to Flooding Stress: An Over View. Sugar Tech 2015, 17, 325–338. [Google Scholar] [CrossRef]
- Gomathi, R.; Chandran, K. Effect of flooding on growth and yield of sugarcane clones. Sugarcane Breeding Institute (SBI-ICAR). Q. News Lett. 2009, 29, 1–2. [Google Scholar]
- Million, F.; Hussein, M.; Esayas, T. Correlation of Traits among cane yield and its component in Sugarcane (Saccharum spp.) Genotypes at Metahara Sugar Estate. Int. J. Adv. Res. Biol. Sci. 2018, 5, 56–61. [Google Scholar]
- Hemaprabha, G.; Simon, S.; Leena, L.; Sajitha, B.; Venkataramana, S. Evaluation of Drought Tolerance Potential of Elite Genotypes and Progenies of Sugarcane (Saccharum sp. Hybrids). Sugar Tech 2013, 15, 9–16. [Google Scholar] [CrossRef]
- Gomathi, R.; Vasantha, S.; Hemaprabha, G.; Alarmelu, S.; Rajagopalan, M.S. Evaluation of Elite Sugarcane Clones for Drought Tolerance. J. Sugarcane Res. 2011, 1, 55–62. [Google Scholar]
- Zingaretti, S.M.; Rodrigues, F.A.; da Graça, J.P.; de Matos Pereira, L.; Lourenço, M.V. Sugarcane Responses at Water Deficit Conditions. In Water Stress; Mofizur Rahman, I.M., Hasegawa, H., Eds.; InTech: Rijeka, Croatia, 2011; pp. 255–267. [Google Scholar]
- De Silva, A.L.C.; De Costa, W.A.J.M.; Bandara, D.M.U.S. Growth of Root System and the Patterns of Soil Moisture Utilization in Sugarcane under Rain-Fed and Irrigated Conditions in Sri Lanka. Sugar Tech 2011, 13, 198–205. [Google Scholar] [CrossRef]
- Avivi, S.; Soeparjono, S.; Slameto; Ramadhan, R.A. Physiological Characters of Sugarcane after Flooding Stress. Agric. Agric. Sci. 2016, 9, 31–39. [Google Scholar] [CrossRef]
- Zhao, D.; Glaz, M.; Comstock, J.C. Sugarcane response to water-deficit stress during early growth on organic and sand soils. Am. J. Agric. Biol. Sci. 2010, 5, 403–414. [Google Scholar] [CrossRef]
- Ali, A.; Chu, N.; Ma, P.; Javed, T.; Zaheer, U.; Huang, M.T.; Fu, H.Y.; Gao, S.J. Genome-wide analysis of mitogen-activated protein (MAP) kinase gene family expression in response to biotic and abiotic stresses in sugarcane. Physiol. Plant. 2021, 171, 86–107. [Google Scholar] [CrossRef]
- Zhou, J.R.; Sun, H.D.; Ali, A.; Rott, P.C.; Javed, T.; Fu, H.Y.; Gao, S.J. Quantitative proteomic analysis of the sugarcane defense responses incited by Acidovorax avenae subsp. avenae causing red stripe. Ind. Crops Prod. 2021, 162, 113275. [Google Scholar] [CrossRef]
- Ali, A.; Javed, T.; Zaheer, U.; Zhou, J.R.; Huang, M.T.; Fu, H.Y.; Gao, S.J. Genome-wide identification and expression profiling of the bHLH transcription factor gene family in Saccharum spontaneum under bacterial pathogen stimuli. Trop. Plant Biol. 2021, 14, 283–294. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Hussain, S.; Naseer, M.A.; Ejaz, I.; Ali, M.M.; Ahmar, S.; Yousef, A.F. Nanotechnology for endorsing abiotic stresses: A review on the role of nanoparticles and nanocompositions. Funct. Plant Biol. 2022, 1–19. [Google Scholar] [CrossRef]
- Hong, D.K.; Javed, T.; Yao, Y.; Zou, Z.Y.; Fu, H.Y.; Gao, S.J.; Xie, Y.; Wang, J. Silicon enhancement for endorsement of Xanthomonas albilineans infection in sugarcane. Ecotoxicol. Environ. Safety. 2021, 220, 112380. [Google Scholar] [CrossRef]
- Sajid, M.; Amjid, M.; Munir, H.; Ahmad, M.; Zulfiqar, U.; Ali, M.F.; Abul Farah, M.; Ahmed, M.A.A.; Artyszak, A. Comparative Analysis of Growth and Physiological Responses of Sugarcane Elite Genotypes to Water Stress and Sandy Loam Soils. Plants 2023, 12, 2759. [Google Scholar] [CrossRef]
- Hu, Z.T.; Ntambo, M.S.; Zhao, J.Y.; Javed, T.; Shi, Y.; Fu, H.Y.; Huang, M.T.; Gao, S.J. Genetic Divergence and Population Structure of Xanthomonas albilineans Strains Infecting Saccharum spp. Hybrid and Saccharum officinarum. Plants 2023, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Spancer, G.L.; Meade, G.P. Cane Sugar Hand Book, 9th ed.; Meade, G.P., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1963; p. 17. [Google Scholar]
- Panigrahi, C.; Shaikh, A.E.Y.; Bag, B.B.; Mishra, H.N.; De, S. A technological review on processing of sugarcane juice: Spoilage, preservation, storage, and packaging aspects. J. Food Process Eng. 2021, 44, e13706. [Google Scholar] [CrossRef]
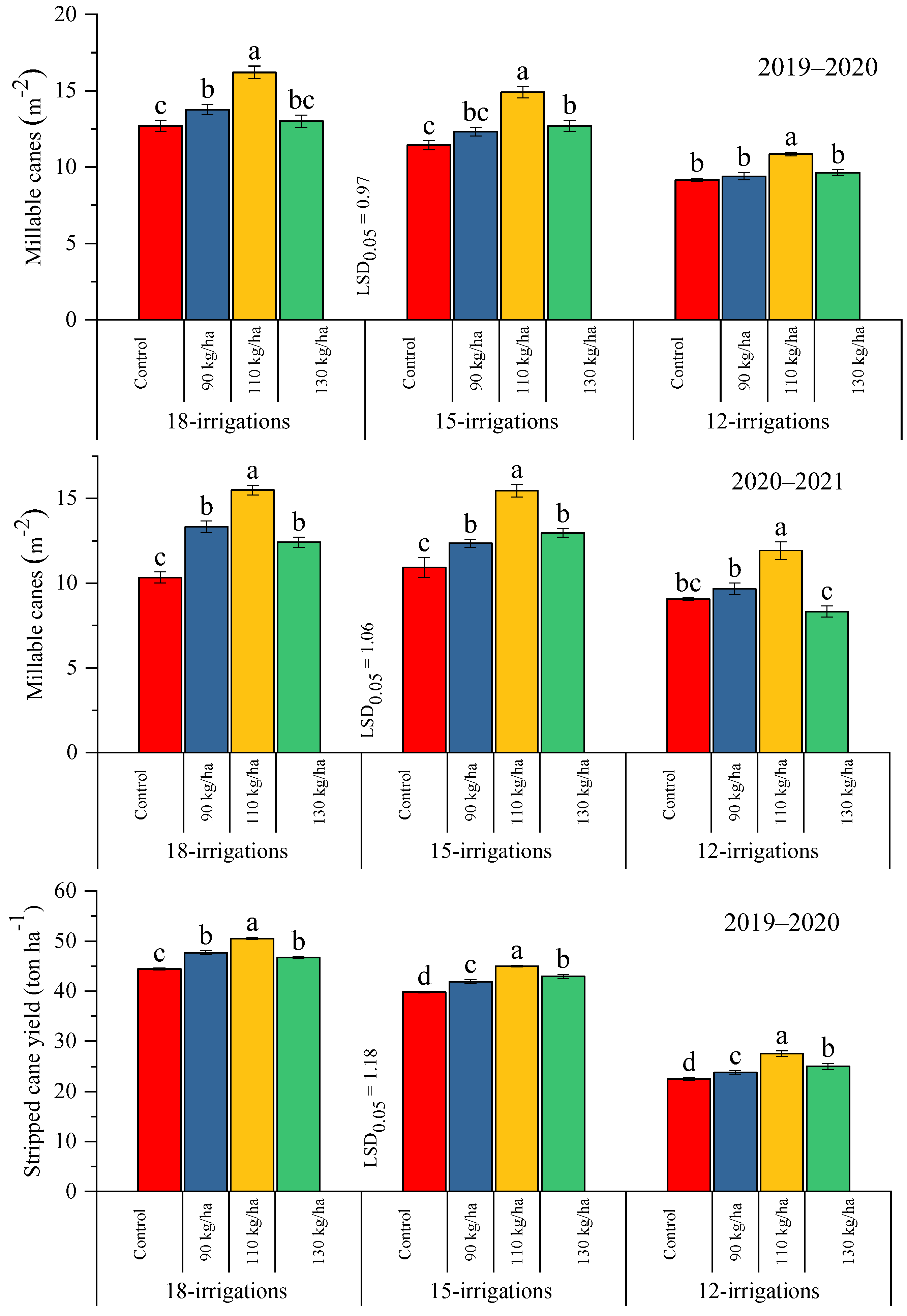
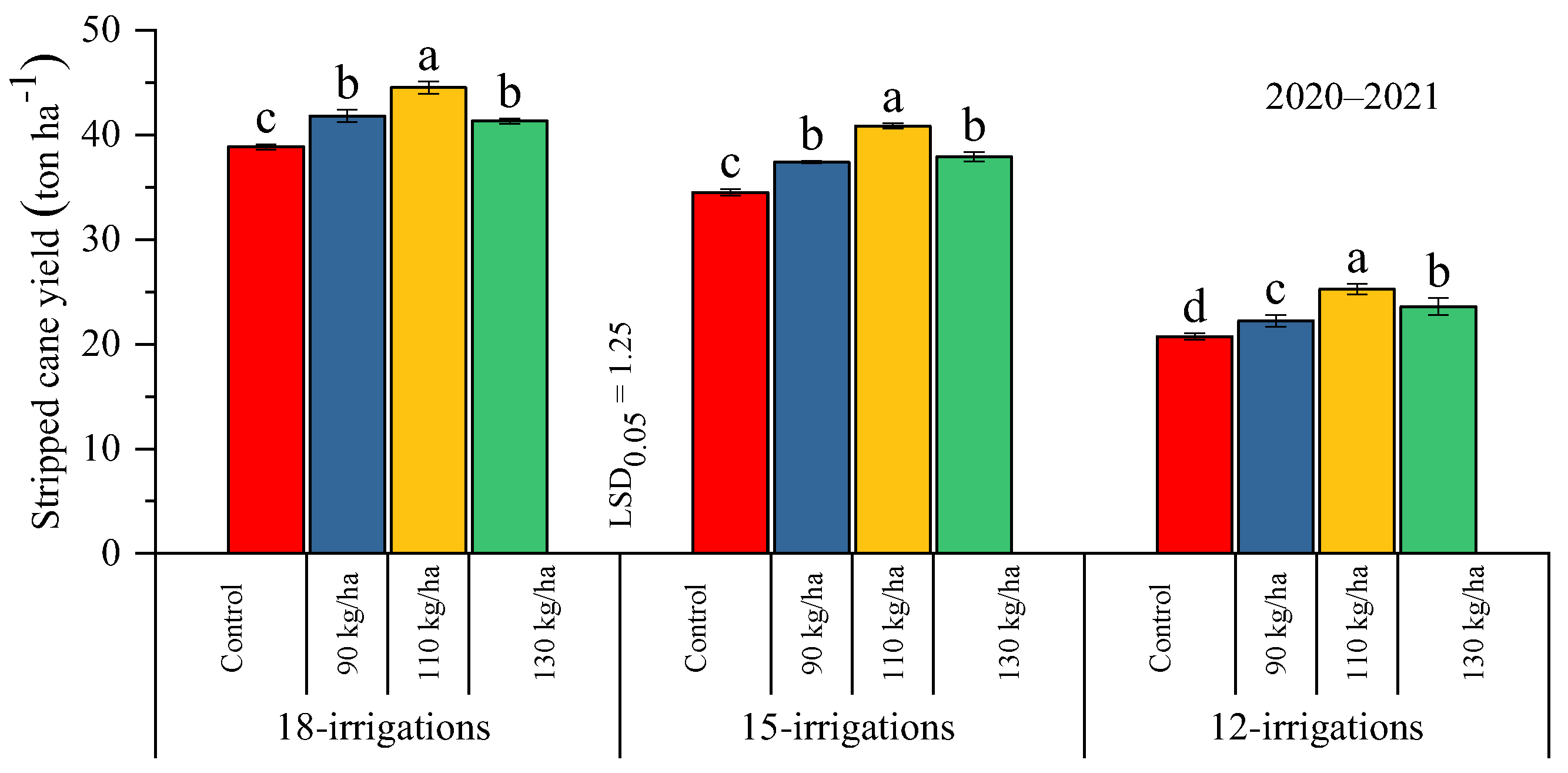
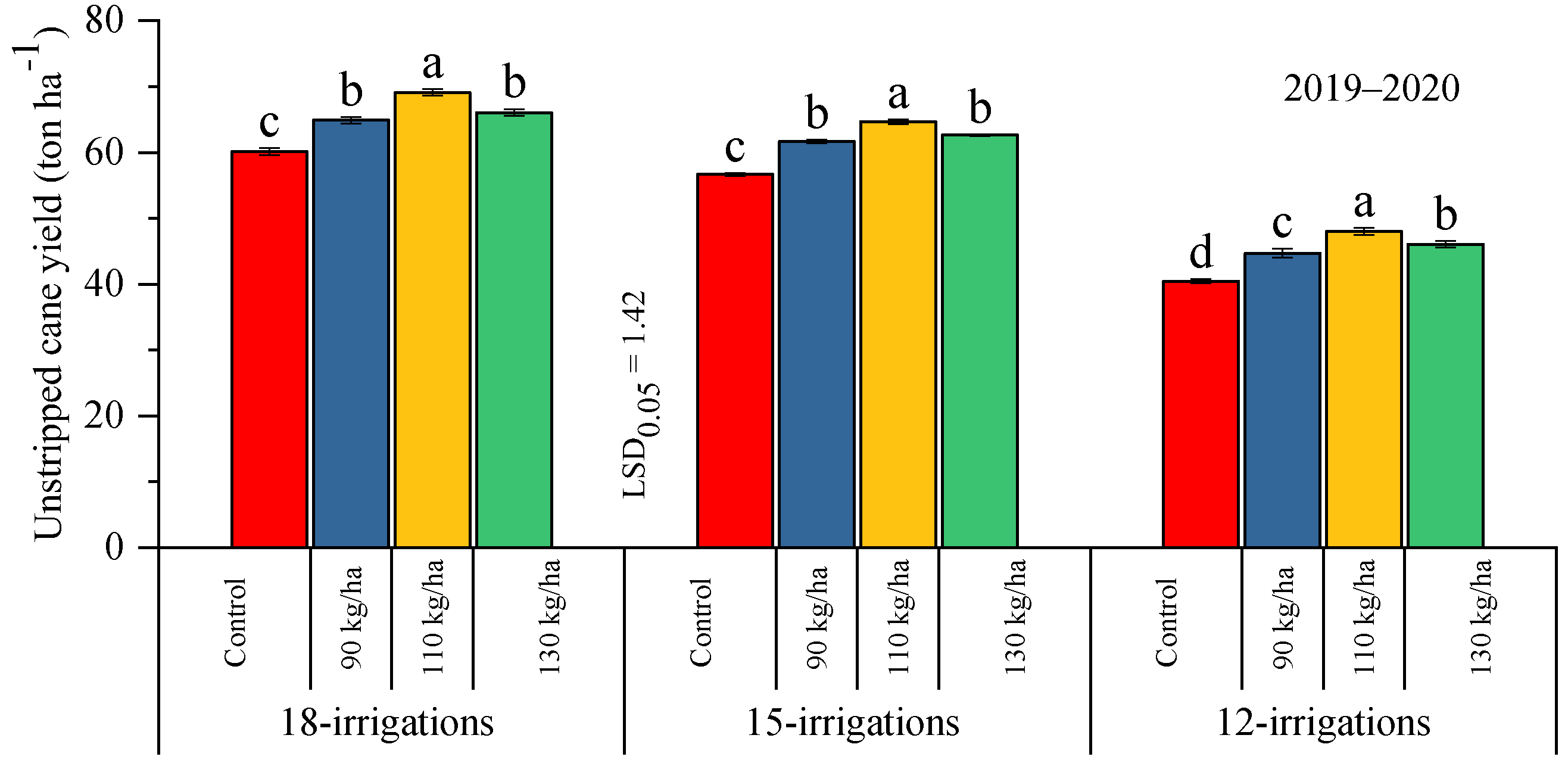

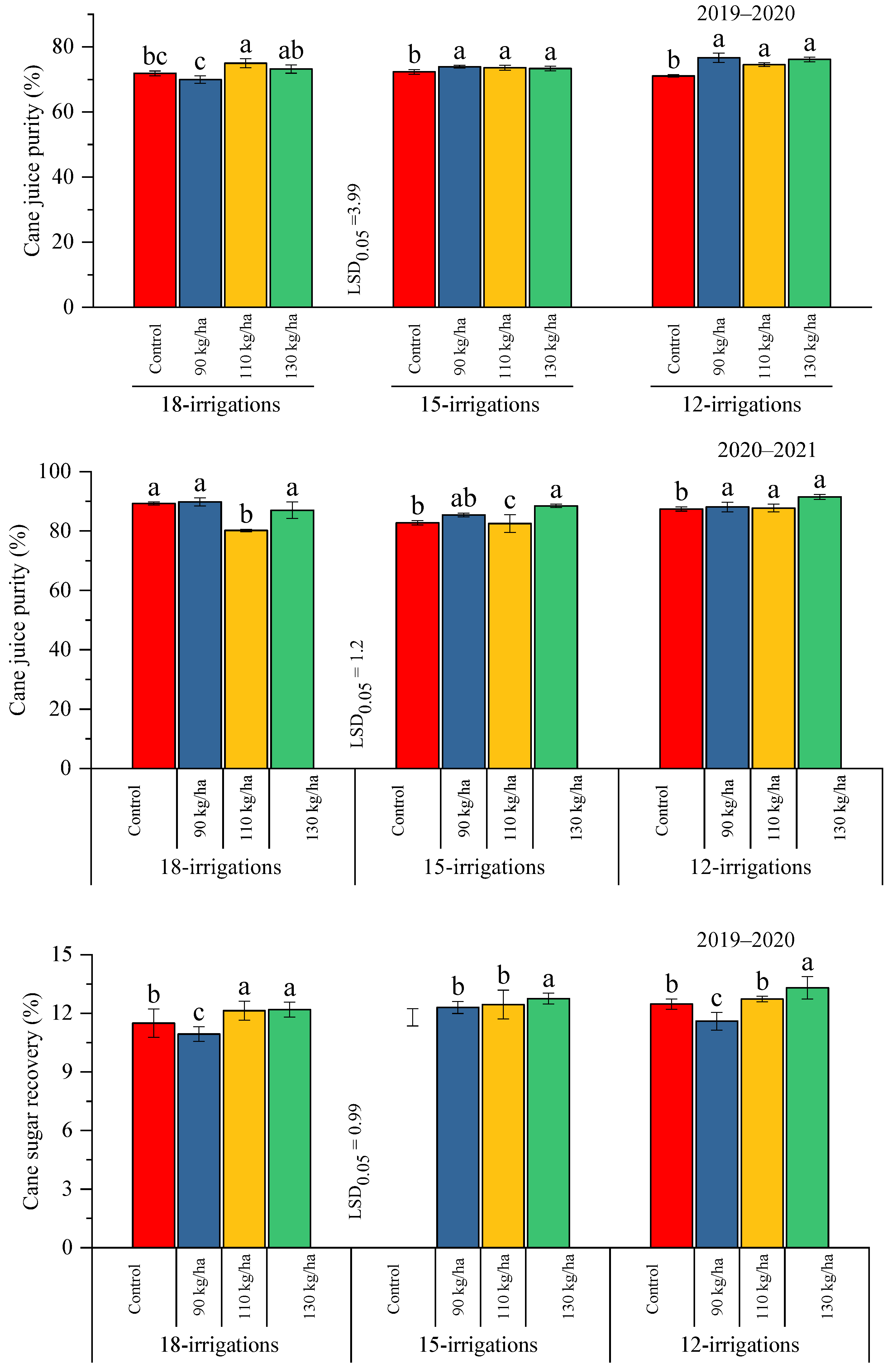

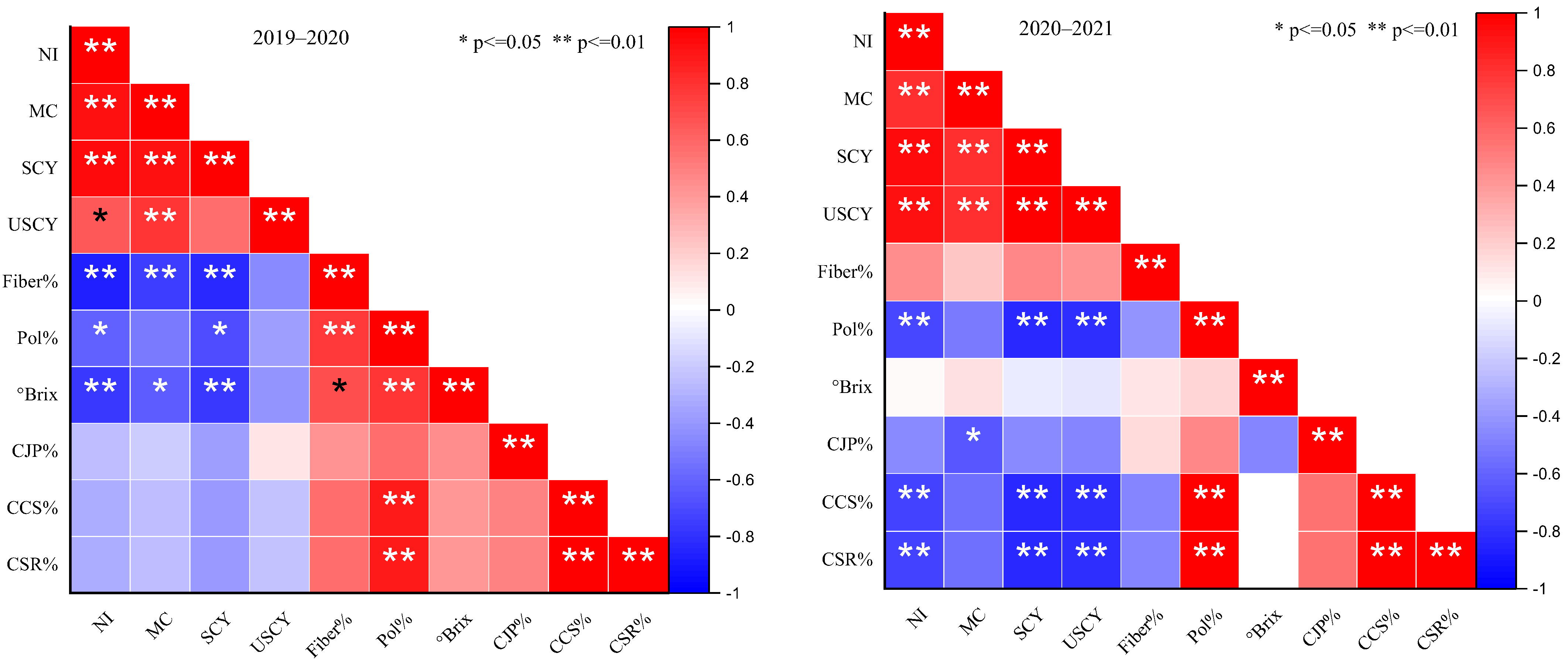
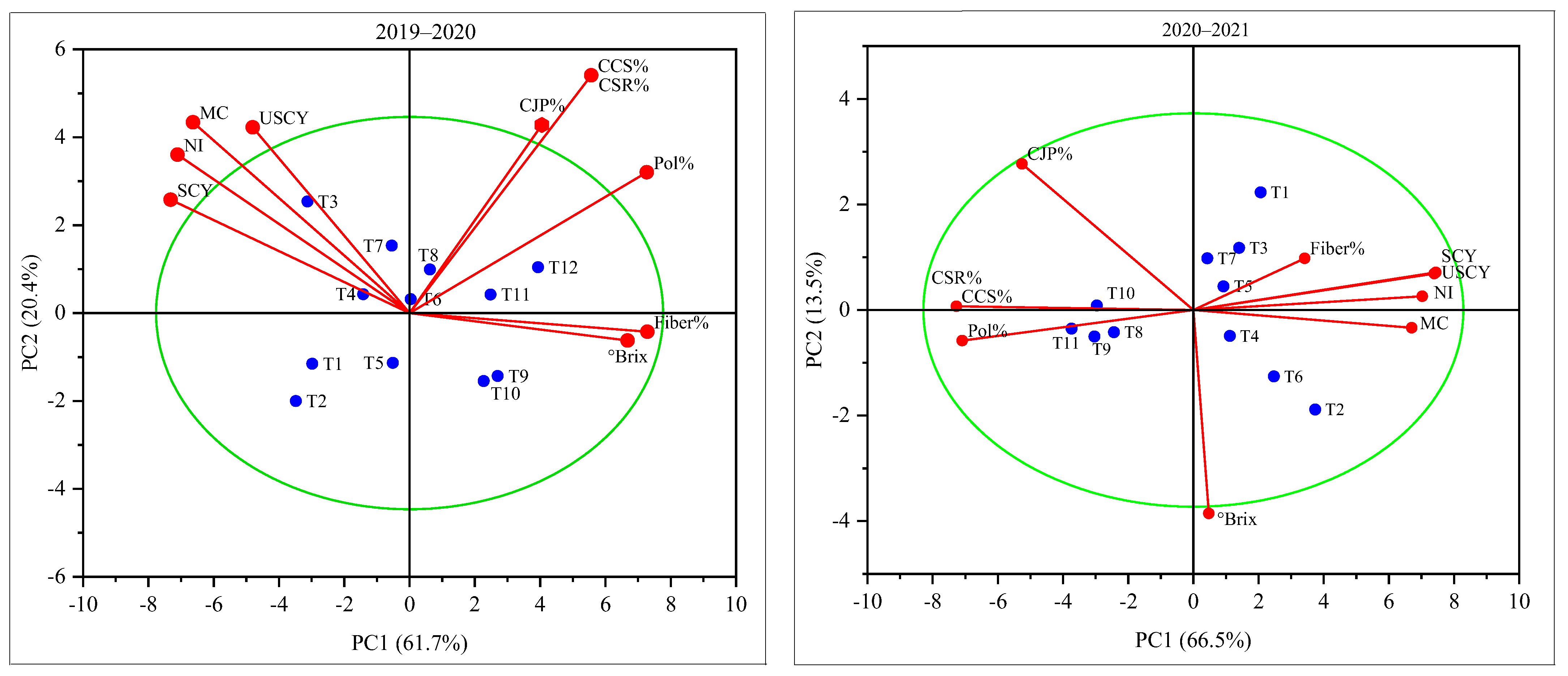
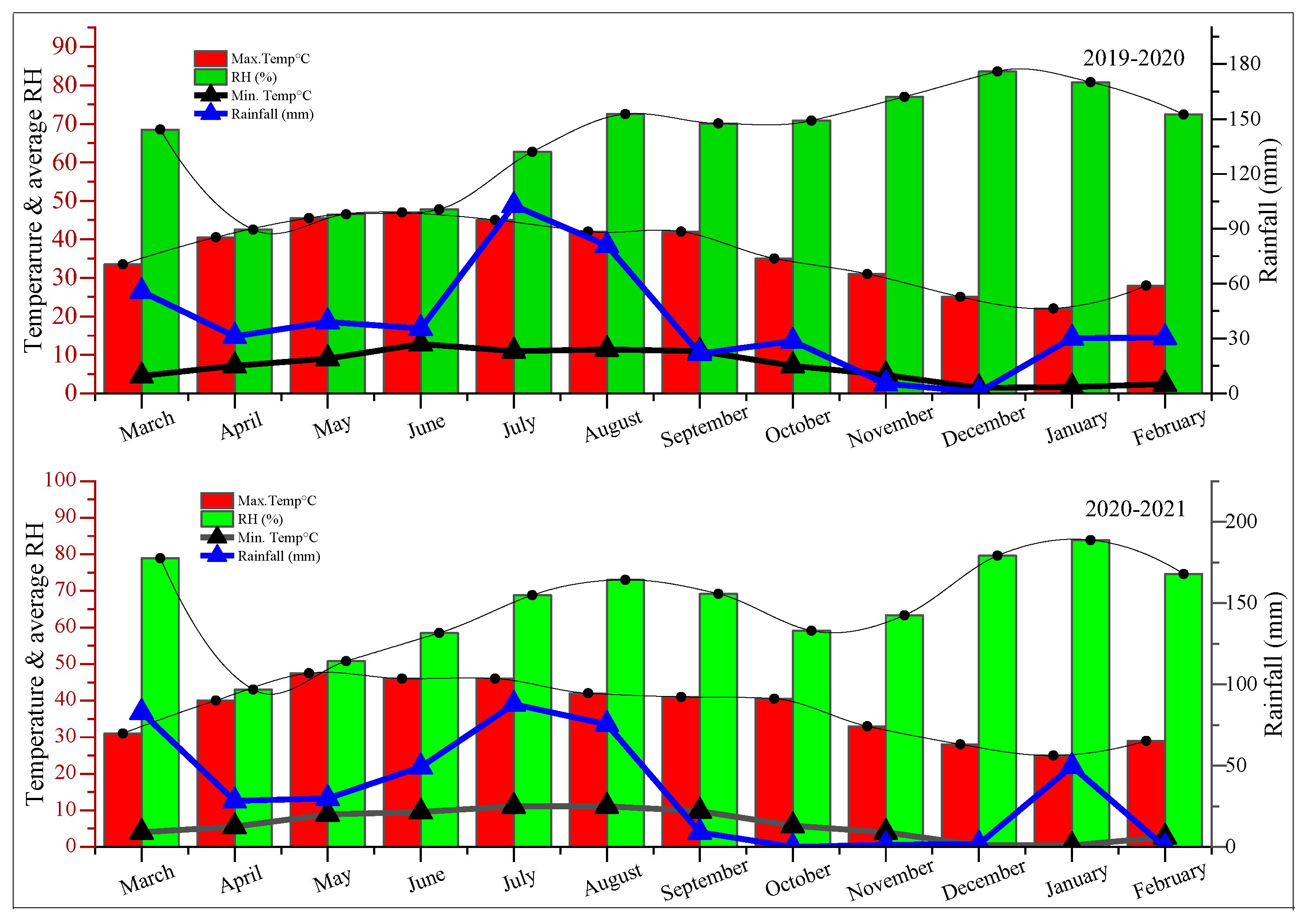
| Treatments | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Internodes Cane−1 | Millable Canes m−2 | Stripped Cane Yield t ha−1 | Unstripped Cane Yield t ha−1 | Fiber % | ||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| W1 = 18 irrigations | 16.78 ± 0.25 a | 15.00 ± 0.55 a | 13.92 ± 0.37 a | 12.90 ± 0.31 a | 47.34 ± 0.25 a | 41.65 ± 0.42 a | 65.05 ± 0.50 a | 59.26 ± 0.53 a | 11.96 ± 0.11 | 12.97 ± 0.38 |
| W2 = 15 irrigations | 14.49 ± 0.34 b | 13.02 ± 0.62 b | 12.84 ± 0.32 b | 12.93 ± 0.35 a | 42.42 ± 0.27 b | 37.68 ± 0.28 b | 61.42 ± 0.24 b | 56.32 ± 0.27 b | 12.49 ± 0.30 | 12.59 ± 0.24 |
| W3 = 12 irrigations | 10.85 ± 0.16 c | 10.17 ± 0.31 c | 9.76 ± 0.15 c | 9.75 ± 0.31 b | 24.73 ± 0.44 c | 22.96 ± 0.55 c | 44.81 ± 0.51 c | 40.18 ± 0.52 c | 12.80 ± 0.46 | 12.55 ± 0.34 |
| LSD (α 5%) | 0.24 | 0.59 | 0.45 | 0.71 | 0.41 | 1.36 | 0.52 | 0.34 | NS | NS |
| P0 = control | 13.29 ± 0.19 c | 11.96 ± 0.37 c | 11.10 ± 0.24 c | 10.11 ± 0.32 c | 35.59 ± 0.21 c | 31.39 ± 0.29 c | 52.41 ± 0.36 d | 48.84 ± 0.16 c | 12.47 ± 0.33 | 12.68 ± 0.18 |
| P1 = 90 kg ha−1 | 13.18 ± 0.31 c | 11.97 ± 0.48 c | 11.83 ± 0.28 b | 11.79 ± 0.30 b | 37.79 ± 0.38 b | 33.82 ± 0.41 b | 57.09 ± 0.46 c | 51.74 ± 0.50 b | 12.42 ± 0.35 | 12.84 ± 0.49 |
| P2 = 110 kg ha−1 | 15.55 ± 0.31 a | 14.02 ± 0.60 a | 13.99 ± 0.30 a | 14.30 ± 0.39 a | 41.03 ± 0.32 a | 36.89 ± 0.45 a | 60.61 ± 0.47 a | 54.77 ± 0.56 a | 12.31 ± 0.24 | 12.62 ± 0.31 |
| P3 = 130 kg ha−1 | 14.14 ± 0.21 b | 12.97 ± 0.52 b | 11.78 ± 0.31 b | 11.24 ± 0.28 b | 38.24 ± 0.37 b | 34.28 ± 0.52 b | 58.27 ± 0.38 b | 52.32 ± 0.53 b | 12.48 ± 0.23 | 12.66 ± 0.30 |
| LSD (α 5%) | 0.53 | 0.89 | 0.56 | 0.61 | 0.68 | 0.72 | 0.82 | 0.86 | NS | NS |
| Interaction | NS | NS | * | ** | * | ** | * | * | NS | NS |
| Treatments | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pol% | °Brix | CCS% | CJP% | CSR% | ||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| W1 = 18 irrigations | 16.15 ± 0.30 c | 16.08 ± 0.18 b | 18.05 ± 0.40 c | 19.01 ± 0.34 | 12.44 ± 0.52 b | 11.71 ± 0.25 b | 72.48 ± 1.13 | 86.56 ± 1.26 ab | 11.69 ± 0.49 b | 11.00 ± 0.23 b |
| W2 = 15 irrigations | 17.13 ± 0.36 b | 16.32 ± 0.13 b | 19.13 ± 0.15 b | 19.27 ± 0.24 | 13.12 ± 0.46 ab | 12.04 ± 0.16 b | 73.28 ± 0.63 | 84.77 ± 1.22 b | 12.33 ± 0.44 ab | 11.32 ± 0.15 b |
| W3 = 12 irrigations | 17.80 ± 0.28 a | 17.83 ± 0.14 a | 20.47 ± 0.35 a | 19.74 ± 0.35 | 13.33 ± 0.38 a | 13.85 ± 0.18 a | 74.60 ± 0.78 | 88.67 ± 1.13 a | 12.53 ± 0.36 a | 13.02 ± 0.17 a |
| LSD (α 5%) | 0.60 | 0.39 | 0.40 | NS | 0.80 | 0.60 | NS | 3.62 | 0.75 | 0.56 |
| P0 = control | 16.60 ± 0.34 b | 16.26 ± 0.20 c | 18.59 ± 0.25 b | 19.13 ± 0.28 b | 12.69 ± 0.50 a,b | 11.96 ± 0.19 c | 71.74 ± 0.61 b | 86.45 ± 0.64 b | 11.92 ± 0.47 a,b | 11.25 ± 0.18 c |
| P1 = 90 kg ha−1 | 16.64 ± 0.34 b | 16.70 ± 0.10 b | 19.52 ± 0.24 a | 19.12 ± 0.30 b | 12.36 ± 0.40 b | 12.55 ± 0.12 b | 73.49 ± 0.98 a | 87.77 ± 1.20 a,b | 11.62 ± 0.37 b | 11.80 ± 0.11 b |
| P2 = 110 kg ha−1 | 17.32 ± 0.30 a | 16.84 ± 0.13 b | 19.48 ± 0.41 a | 19.90 ± 0.46 a | 13.24 ± 0.48 a,b | 12.53 ± 0.21 b | 74.36 ± 0.90 a | 83.46 ± 1.57 c | 12.44 ± 0.45 a,b | 11.78 ± 0.20 b |
| P3 = 130 kg ha−1 | 17.54 ± 0.26 a | 17.16 ± 0.16 a | 19.28 ± 0.28 a | 19.21 ± 0.21 b | 13.57 ± 0.43 a | 13.09 ± 0.27 a | 74.21 ± 0.90 a | 88.98 ± 1.40 a | 12.75 ± 0.41 a | 12.30 ± 0.25 a |
| LSD (α 5%) | 0.61 | 0.27 | 0.62 | 0.52 | 0.89 | 0.37 | 1.45 | 2.44 | 0.84 | 0.34 |
| Interaction | NS | NS | * | * | NS | NS | ** | * | * | * |
| Soil Characters | 2019–2020 | 2020–2021 | ||
|---|---|---|---|---|
| Units | Value | Units | Value | |
| Texture | Loam | – | – | – |
| pH | – | 7.5 ± 0.1 | – | 7.54 ± 0.1 |
| EC | d S L–1 | 1.87 ± 0.02 | d S L–1 | 1.93 ± 0.04 |
| Organic matter | µg g–1 | 6.19 ± 0.19 | µg g–1 | 6.87 ± 0.14 |
| Nitrogen | µg g–1 | 292 ± 8.16 | µg g–1 | 324 ± 11.13 |
| Phosphorus | µg g–1 | 7.89 ± 0.28 | µg g–1 | 8.45 ± 0.31 |
| Potassium | µg g–1 | 235 ± 17 | µg g–1 | 256 ± 10.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, M.; Amjid, M.; Munir, H.; Valipour, M.; Rasul, F.; Khil, A.; Alqahtani, M.D.; Ahmad, M.; Zulfiqar, U.; Iqbal, R.; et al. Enhancing Sugarcane Yield and Sugar Quality through Optimal Application of Polymer-Coated Single Super Phosphate and Irrigation Management. Plants 2023, 12, 3432. https://doi.org/10.3390/plants12193432
Sajid M, Amjid M, Munir H, Valipour M, Rasul F, Khil A, Alqahtani MD, Ahmad M, Zulfiqar U, Iqbal R, et al. Enhancing Sugarcane Yield and Sugar Quality through Optimal Application of Polymer-Coated Single Super Phosphate and Irrigation Management. Plants. 2023; 12(19):3432. https://doi.org/10.3390/plants12193432
Chicago/Turabian StyleSajid, Muhammad, Muhammad Amjid, Hassan Munir, Mohammad Valipour, Fahd Rasul, Aka Khil, Mashael Daghash Alqahtani, Muhammad Ahmad, Usman Zulfiqar, Rashid Iqbal, and et al. 2023. "Enhancing Sugarcane Yield and Sugar Quality through Optimal Application of Polymer-Coated Single Super Phosphate and Irrigation Management" Plants 12, no. 19: 3432. https://doi.org/10.3390/plants12193432
APA StyleSajid, M., Amjid, M., Munir, H., Valipour, M., Rasul, F., Khil, A., Alqahtani, M. D., Ahmad, M., Zulfiqar, U., Iqbal, R., Ali, M. F., & Ibtahaj, I. (2023). Enhancing Sugarcane Yield and Sugar Quality through Optimal Application of Polymer-Coated Single Super Phosphate and Irrigation Management. Plants, 12(19), 3432. https://doi.org/10.3390/plants12193432









