The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 1: Vegetables (Broccoli and Tomato)
Abstract
:1. Introduction
2. Results and Discussion
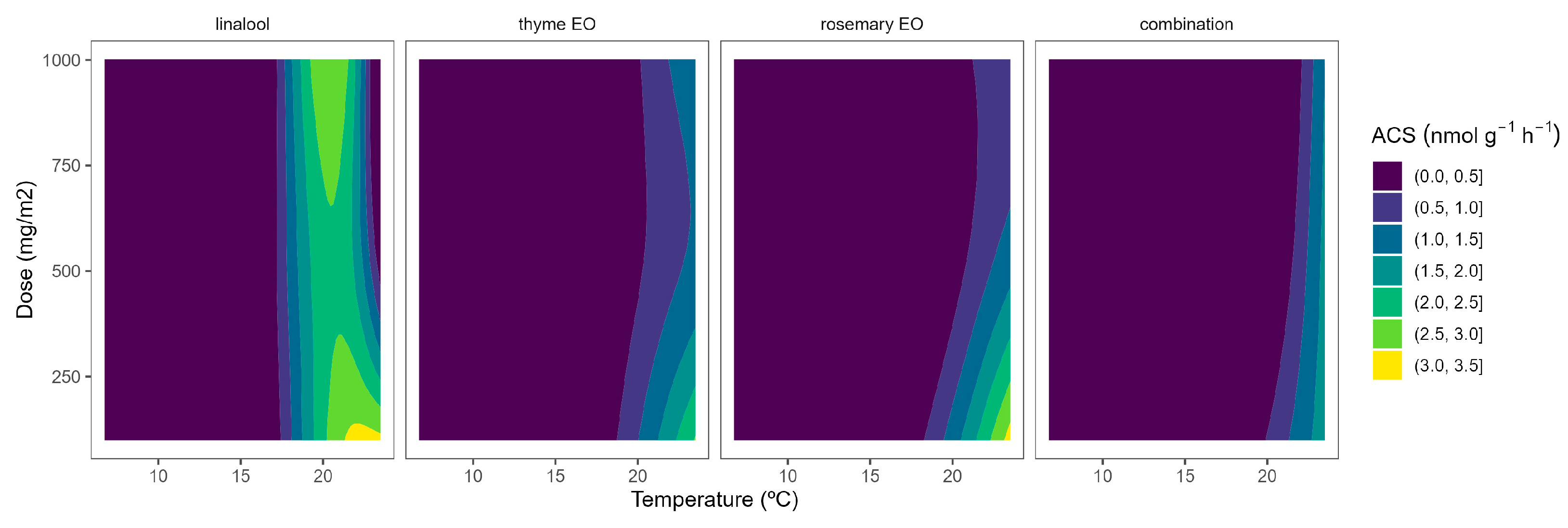
2.1. ACS Activity
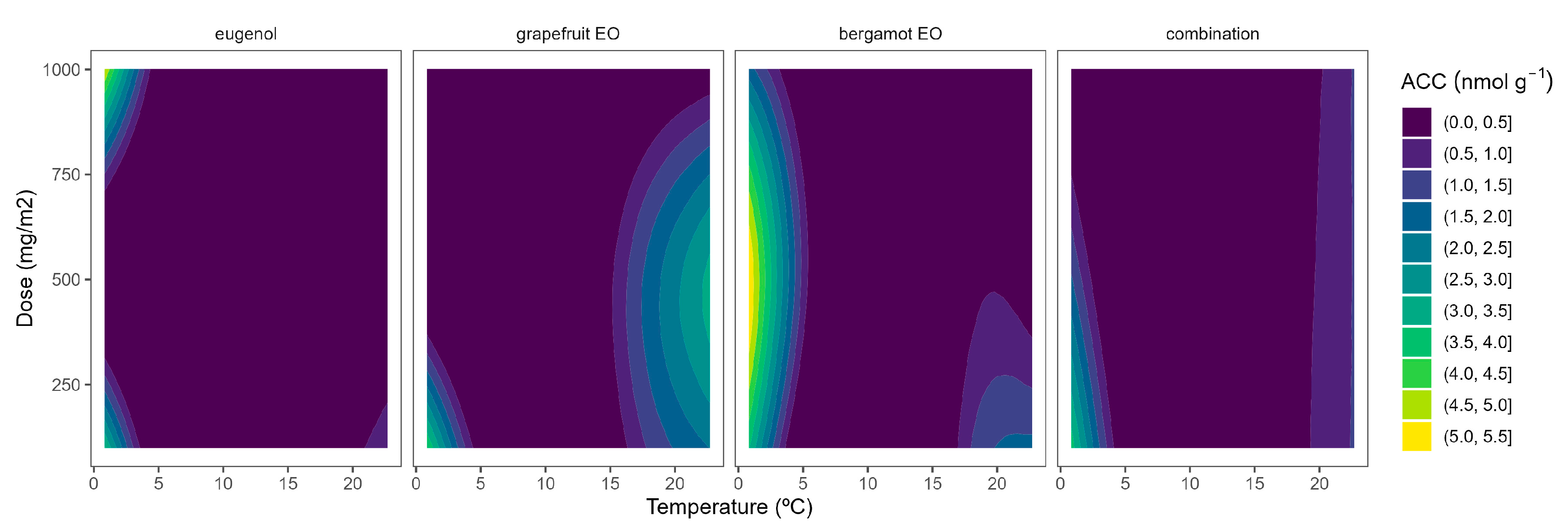
2.2. ACC Content
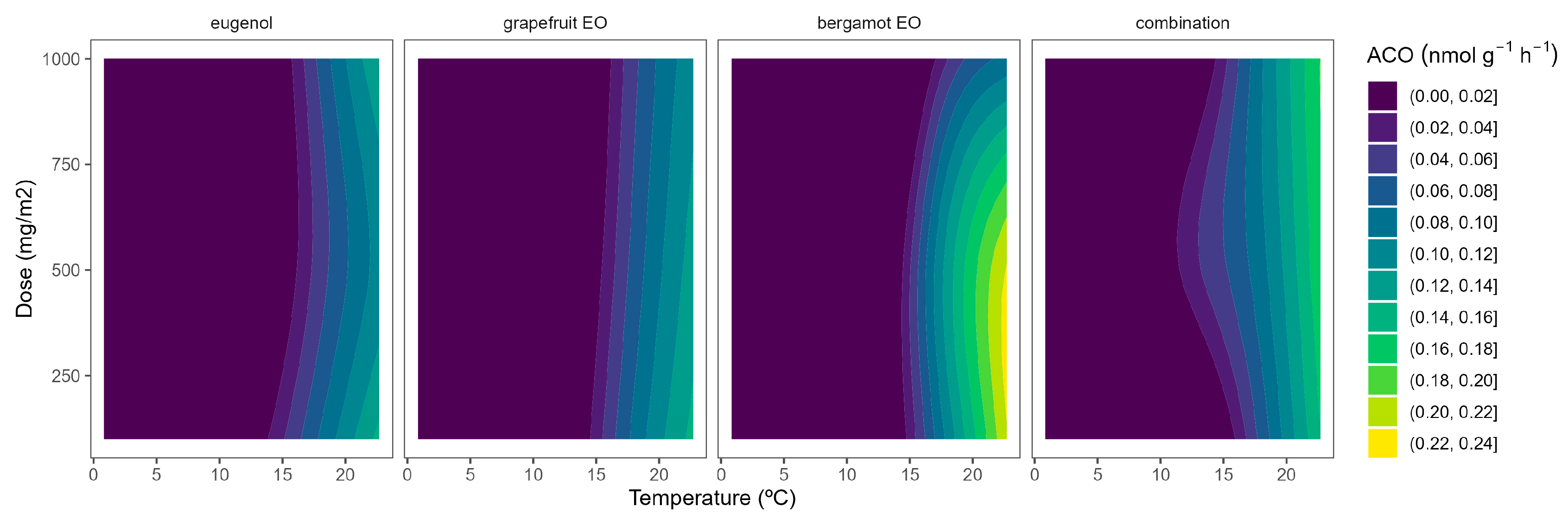
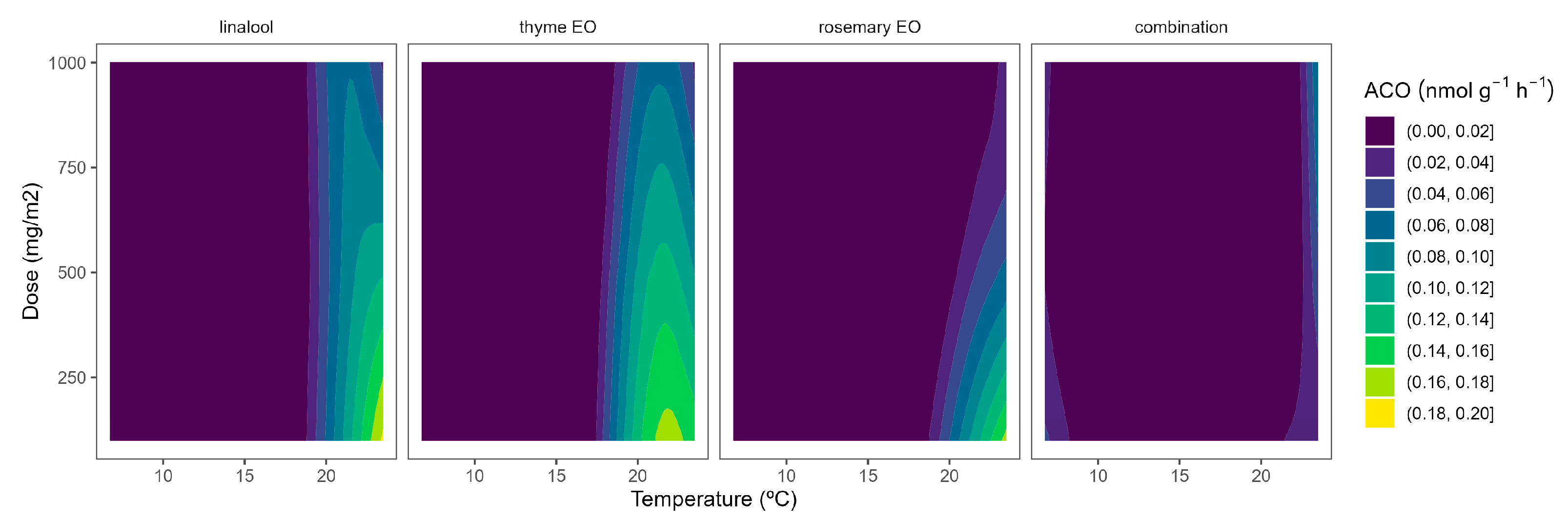
2.3. ACO Activity
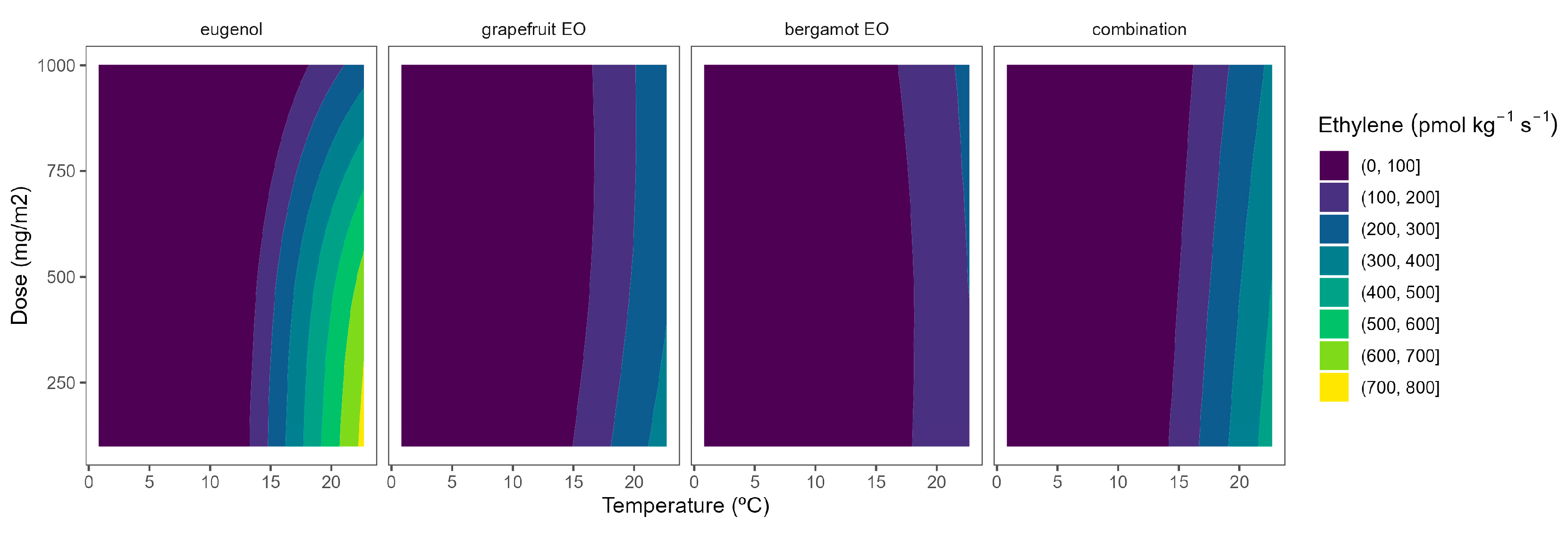
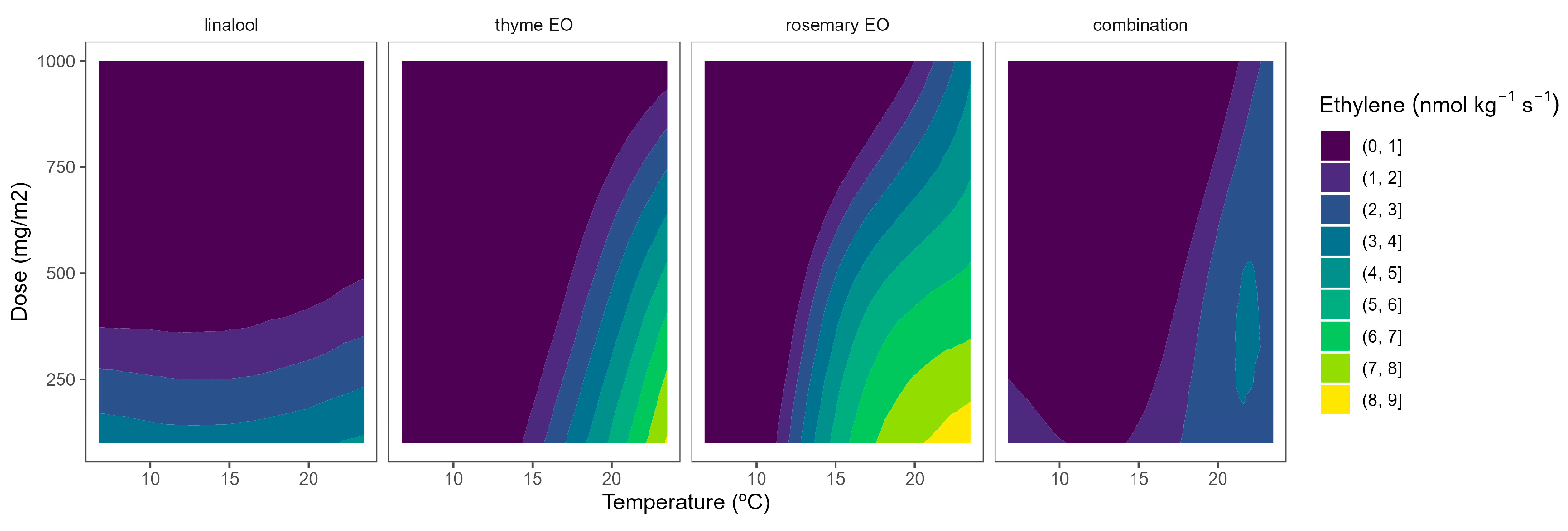
2.4. Ethylene Production
3. Materials and Methods
3.1. Materials
3.2. Encapsulation of Essential Oils and Active Packaging Preparation
3.3. Effect of Active Packaging on the Ethylene Biosynthesis System of Vegetables
3.4. Ethylene Production
3.5. 1-Aminocyclopropane-1-Carboxylic acid (ACC) Content
3.6. ACC Oxidase (ACO) Activity
3.7. ACC Synthase (ACS) Activity
3.8. Data Analysis and Mathematical Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Rome, Italy, 2019; pp. 4–9.
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- FAO. Transforming Our World: The 2030 Agenda for Sustainable Development; FAO: Rome, Italy, 2023. [Google Scholar]
- Postharvest Technology of Horticultural Crops; Kader, A.A. (Ed.) University of California, Agriculture and Natural Resources: Richmond, CA, USA, 2002. [Google Scholar]
- Reid, M.S. Ethylene in postharvest technology. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Richmond, CA, USA, 2002; p. 149. ISBN 9781879906518. [Google Scholar]
- Adams, D.O.; Yang, S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Adams, D.O.; Yang, S.F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch. Biochem. Biophys. 1979, 198, 280–286. [Google Scholar] [CrossRef]
- Van de Poel, B.; Van Der Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, G.B.; Artés-Hernández, F.; Gómez, P.A.; Artés, F. Comparative behaviour between kailan-hybrid and conventional fresh-cut broccoli throughout shelf-life. LWT-Food Sci. Technol. 2013, 50, 298–305. [Google Scholar] [CrossRef]
- Navarro-Martínez, A.; López-Gómez, A.; Martínez-Hernández, G.B. Potential of essential oils from active packaging to highly reduce ethylene biosynthesis in broccoli and apples. ACS Food Sci. Technol. 2021, 1, 1050–1058. [Google Scholar] [CrossRef]
- Tian, M.S.; Downs, C.G.; Lill, R.; King, G.A. A role for ethylene in the yellowing of broccoli after harvest. J. Am. Soc. Hortic. Sci. 1994, 119, 276–281. [Google Scholar] [CrossRef]
- Taye, A.M.; Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae 2019, 5, 29. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium permanganate-based ethylene scavengers for fresh horticultural produce as an active packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- AECOSAN Las Tendencias del Consumo y del Consumidor en el Siglo XXI. Available online: https://www.consumo.gob.es/es/consumo/las-tendencias-del-consumo-y-del-consumidor-en-el-siglo-xxi (accessed on 10 August 2023).
- Rabbany, A.B.M.G.; Mizutani, F. Effect of essential oils on ethylene production and ACC content in apple fruit and peach seed tissues. J. Jpn. Soc. Hortic. Sci. 1996, 65, 7–13. [Google Scholar] [CrossRef]
- López-Gómez, A.; Navarro-Martínez, A.; Martínez-Hernández, G.B. Active paper sheets including nanoencapsulated essential oils: A green packaging technique to control ethylene production and maintain quality in fresh horticultural products. A case study in flat peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, G.B.; López-Gómez, A. Potential of Released Essential Oils from Active Packaging to Reduce Refrigeration Needs of Fruit and Vegetables. Clean Technol. 2022, 4, 1255–1268. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; Da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2013, 26, 34–40. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. Technol. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Zapata, P.J.; Castillo, S.; Valero, D. The addition of essential oils to MAP as a tool to maintain the overall quality of fruits. Trends Food Sci. Technol. 2008, 19, 464–471. [Google Scholar] [CrossRef]
- EU Regulation (EC) no 1334/2008 of the European Parliament and of the council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC). Off. J. Eur. Union 2008, 354, 34–50.
- EU Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334. Off. J. Eur. Union 2012, 267, 1–161.
- MAPA Lista Comunitaria de Sustancias Activas Aprobadas, Excluidas y en Evaluación Comunitaria, Sustancias de bajo Riesgo, Sustancias Candidatas a la Sustitución y Lista de Sustancias Básicas. Available online: https://www.mapa.gob.es/agricultura/pags/fitos/registro/fichas/pdf/Lista_Sustancias_activas_aceptadas_excluidas.pdf (accessed on 9 October 2019).
- López-Gómez, A.; Navarro-Martínez, A.; Garre, A.; Iguaz, A.; Maldonado-Guzmán, P.; Martínez-Hernández, G.B. Kinetics of carvacrol release from active paper packaging for fresh fruits and vegetables under conditions of open and closed package. Food Packag. Shelf Life 2023, 37, 101081. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial packaging systems. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: New York, NY, USA, 2005; pp. 80–107. ISBN 9780123116321. [Google Scholar]
- Kato, M.; Kamo, T.; Wang, R.; Nishikawa, F.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Wound-induced ethylene synthesis in stem tissue of harvested broccoli and its effect on senescence and ethylene synthesis in broccoli florets. Postharvest Biol. Technol. 2002, 24, 69–78. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Shiomi, S.; Kubo, Y.; Inaba, A. Expression and Internal Feedback Regulation of ACC Synthase and ACC Oxidase Genes in Ripening Tomato Fruit. Plant Cell Physiol. 1997, 38, 1103–1110. [Google Scholar] [CrossRef]
- Bulens, I.; Van de Poel, B.; Hertog, M.L.A.T.M.; De Proft, M.P.; Geeraerd, A.H.; Nicolaï, B.M. Protocol: An updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. Plant Methods 2011, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- van de Poel, B.; Bulens, I.; Markoula, A.; Hertog, M.L.A.T.M.; Dreesen, R.; Wirtz, M.; Vandoninck, S.; Oppermann, Y.; Keulemans, J.; Hell, R.; et al. Targeted Systems Biology Profiling of Tomato Fruit Reveals Coordination of the Yang Cycle and a Distinct Regulation of Ethylene Biosynthesis during Postclimacteric Ripening. Plant Physiol. 2012, 160, 1498–1514. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Castillo, S.; Valero, D.; Guillén, F.; Serrano, M.; Díaz-Mula, H.M. The use of alginate as edible coating alone or in combination with essential oils maintained postharvest quality of tomato. Acta Hortic. 2010, 877, 1529–1534. [Google Scholar] [CrossRef]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Serrano, M.; Valero, D.; Rodríguez-López, M.I.; Gabaldón, J.A.; Castillo, S.; Valverde, J.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D. Thymol encapsulated into HP-β-cyclodextrin as an alternative to synthetic fungicides to induce lemon resistance against sour rot decay. Molecules 2020, 25, 4348. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Pérez-Alfonso, C.O.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 2014, 35, 132–136. [Google Scholar] [CrossRef]
- Manolikar, M.K.; Sawant, M.R. Study of solubility of isoproturon by its complexation with β-cyclodextrin. Chemosphere 2003, 51, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Lizada, M.C.C.; Yang, S.F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 1979, 100, 140–145. [Google Scholar] [CrossRef]
- Kato, M.; Hyodo, H. Purification and characterization of ACC oxidase and increase in its activity during ripening of pear fruit. Engei Gakkai Zasshi 1999, 68, 551–557. [Google Scholar] [CrossRef]
- McElreath, R. Statistical Rethinking. A Bayesian Course with Examples in R and Stan, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780367139919. [Google Scholar]
- R-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bürkner, P.C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Wood, S.N.; Scheipl, F.; Faraway, J.J. Straightforward intermediate rank tensor product smoothing in mixed models. Stat. Comput. 2013, 23, 341–360. [Google Scholar] [CrossRef]
- Wood, S.N. Thin plate regression splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.A.; Guo, J.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76, 1–32. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Buendía−Moreno, L.; Soto−Jover, S.; Ros−Chumillas, M.; Antolinos−López, V.; Navarro−Segura, L.; Sánchez−Martínez, M.J.; Martínez−Hernández, G.B.; López−Gómez, A. An innovative active cardboard box for bulk packaging of fresh bell pepper. Postharvest Biol. Technol. 2020, 164, 111171. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, A.; Navarro-Martínez, A.; Garre, A.; Artés-Hernández, F.; Villalba, P.; Martínez-Hernández, G.B. The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 1: Vegetables (Broccoli and Tomato). Plants 2023, 12, 3404. https://doi.org/10.3390/plants12193404
López-Gómez A, Navarro-Martínez A, Garre A, Artés-Hernández F, Villalba P, Martínez-Hernández GB. The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 1: Vegetables (Broccoli and Tomato). Plants. 2023; 12(19):3404. https://doi.org/10.3390/plants12193404
Chicago/Turabian StyleLópez-Gómez, Antonio, Alejandra Navarro-Martínez, Alberto Garre, Francisco Artés-Hernández, Pedro Villalba, and Ginés Benito Martínez-Hernández. 2023. "The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 1: Vegetables (Broccoli and Tomato)" Plants 12, no. 19: 3404. https://doi.org/10.3390/plants12193404
APA StyleLópez-Gómez, A., Navarro-Martínez, A., Garre, A., Artés-Hernández, F., Villalba, P., & Martínez-Hernández, G. B. (2023). The Potential of Essential Oils from Active Packaging to Reduce Ethylene Biosynthesis in Plant Products. Part 1: Vegetables (Broccoli and Tomato). Plants, 12(19), 3404. https://doi.org/10.3390/plants12193404








