First Report of Hemp Fusarium Wilt Caused by Fusarium oxysporum in Croatia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain Collection, Isolation and Growth Conditions
2.2. Fungal Strain Molecular Isolation
2.2.1. Internal Transcribed Spacer Region Analysis
2.2.2. Translation Elongation Factor 1-α Gene Analysis
2.3. Phylogenetic Analysis
2.4. Pathogenicity Test
3. Results
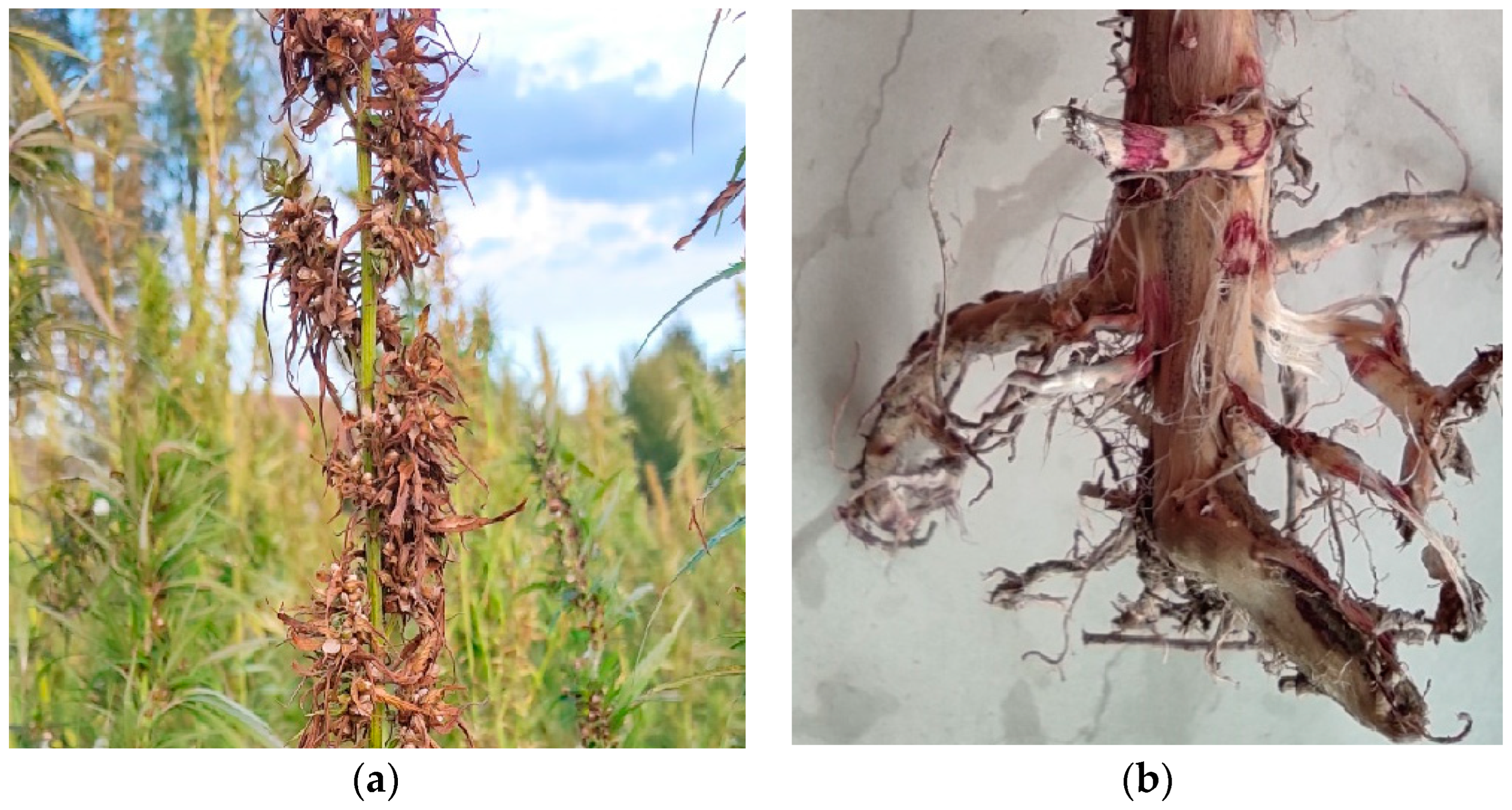
3.1. Fungal Isolate and Identification
3.2. Molecular Identification
3.3. Artificial Inoculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Bot. Mus. Leafl. Harv. Univ. 1974, 23, 337–367. [Google Scholar] [CrossRef]
- McPartland, J.M.; Guy, G. The evolution of Cannabis and coevolution with the cannabinoid receptor—A hypothesis. In The Medicinal Use of Cannabis; Guy, G., Robson, R., Strong, K., Whittle, B., Eds.; Royal Society of Pharmacists: London, UK, 2004; pp. 71–102. [Google Scholar]
- Thamae, T.; Aghedo, S.; Baillie, C.; Matovic, D. Tensile Properties of Hemp and Agave Americana Fibres. In Handbook of Tensile Properties of Textile and Technical Fibres; Woodhead Publishing: Sawston, UK, 2009; pp. 73–99. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New Developments in Fiber Hemp (Cannabis sativa L.) Breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Composit. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Karche, T.; Singh, M.R. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa Cannabinoids as Functional Ingredients in Snack Foods—Historical and Developmental Aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef] [PubMed]
- Go, M.K.; Zhu, T.; Lim, K.J.H.; Hartono, Y.D.; Xue, B.; Fan, H.; Yew, W.S. Cannabinoid Biosynthesis Using Noncanonical Cannabinoid Synthases. Int. J. Mol. Sci. 2023, 24, 1259. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The Genomics of Cannabis and Its Close Relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Maxwell, A.P.J. Recent advances in cannabis biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lip. Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Rupasinghe, V.H.P.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.Z. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Punja, Z.K. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L., marijuana) plants in commercial greenhouse production. Can. J. Plant Pathol. 2021, 43, 216–235. [Google Scholar] [CrossRef]
- Agrios, G.N. (Ed.) Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Ivić, D.; Domijan, A.M.; Peraica, M.; Miličević, T.; Cvjetković, B. Fusarium spp. on wheat, maize, soybean and pea. Arh. Hig. Rada. Toksikol. 2009, 60, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, T.; Sudaric, A.; Matosa Kocar, M.; Cosic, J.; Vrandecic, K. First report of soybean fusarium wilt caused by Fusarium oxysporum in Croatia. Plant Dis. 2017, 101, 249. [Google Scholar] [CrossRef]
- Gwinn, K.D.; Hansen, Z.; Kelly, H.; Ownley, B.H. Diseases of Cannabis sativa caused by diverse Fusarium species. Front. Agron. 2022, 3, 796062. [Google Scholar] [CrossRef]
- Patschke, K.; Gottwald, R.; Muller, R. Erste Ergebnisse phytopathologischer Beobachtungen im Hanfanbau im Land Brandenburg. Nach. Des Deut. Pflan. 1997, 49, 286–289. [Google Scholar]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N. American weed: A history of Cannabis cultivation in the United States. EchoGéo 2019, 48, 17650. [Google Scholar] [CrossRef]
- McCain, A.H.; Noviello, C. Biological control of Cannabis sativa. In Proceedings of the VI International Symposium on Biological Control of Weeds, Ottawa, ON, Canada, 19–25 August 1985; pp. 635–642. [Google Scholar]
- Noviello, C.; McCain, A.H.; Aloj, B.; Scalcione, M.; Marziano, F. Lotta biologica contro Cannabis sativa medlante l’impiego di Fusarium oxysporum f. sp. cannabis. Annali delta Facolta di Scienze Agrarie della Universita degli Studi di Napoli. Portici 1990, 24, 33–44. [Google Scholar]
- Rataj, K. Skodivi cinitele pradnych rostlin. Pram. Lit. 1957, 2, 1–123. [Google Scholar]
- McPartland, J.M.; Clarke, R.C.; Watson, D.P. (Eds.) Fungal Diseases. In Hemp Diseases and Pests, Management and Biological Control; CABI Publishing: Wallingford, UK, 2000; pp. 93–135. [Google Scholar]
- McPartland, J.M.; Hillig, K.W. Cannabis clinic Fusarium Wilt. J. Ind. Hemp. 2004, 2, 67–77. [Google Scholar] [CrossRef]
- Noviello, C.; Snyder, W.C. Fusarium wilt of hemp. Phytopathology 1962, 52, 1315–1317. [Google Scholar]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.M.; Thompson, I.A. Nitrogen and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D.M., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2009; pp. 31–44. [Google Scholar]
- Lombard, L.; Sandoval-Denis, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, G.W. Soilborne Plant Pathogens; Macmillan Pub. Co.: New York, NY, USA, 1987. [Google Scholar]
- Tiourebaev, K.S.; Pilgeram, A.L.; Anderson, T.W.; Sands, D.C. Soil penetration of a mycoherbicide facilitated by carrier seedlings. Phytopathology 1997, 87 (Suppl. 6), 57. [Google Scholar]
- Agostinelli, A.M.; Clark, A.J.; Brown-Guedira, G.; Van Sanford, D.A. Optimizing phenotypic and genotipis selection for Fusatium head blight resistance in wheat. Euphytica 2012, 186, 115–126. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; p. 315. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.K.; Steinberg, C.; Persson, P.; Friberg, H. Genus-Specific Primers for Study of Fusarium Communities in Field Samples. Appl. Environ. Microbiol. 2015, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Fredrik Ronquist, R.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v.1.4.4. 2018. Available online: https://www.researchgate.net/figure/FigTree-v-144-Rambaut-2018-Genetic-variation-parameters-such-as-observed-alleles_fig1_354454604 (accessed on 11 September 2023).
- European Commission EU Plant Variety Database (v.3.2). Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=240&variety_name=&listed_in=0&show_current=on&show_deleted= (accessed on 15 March 2020).
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjøller, R.; Kõljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers: A user’s guide. N. Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; Jimenez-Gasco, M.D.; Kang, S.C.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant. Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C.; et al. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fun. Gen. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Ilić, J.; Ćosić, J.; Jurković, D.; Vrandečić, K. Vegetative compatibility of Fusarium oxysporum isolated from weeds in eastern Croatia. Poljoprivreda 2013, 19, 20–24. [Google Scholar]
- Backhouse, D.; Burgess, L.W.; Summerell, B.A. Biogeography of Fusarium. In Fusarium Paul E. Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2001; pp. 122–137. [Google Scholar]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Langer, I. Impact of tillage on the incidence of Fusarium spp. in soil. Plant Soil 2004, 267, 13–22. [Google Scholar] [CrossRef]
- Palmero, D.; Iglesias, C.; de Cara, M.; Lomas, T.; Santos, M.; Tello, J.C. Species of Fusarium isolated from river and sea water of southeastern spain and pathogenicity on four plant species. Plant Dis. 2009, 93, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Barreto Crespo, M.T.; San Romão, M.V.; Benoliel, M.J.; Samson, R.A.; Pereira, V.J. New insights concerning the occurrence of fungi in water sources and their potential pathogenicity. Water Res. 2013, 47, 6338–6347. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Laurent, J.; Edel-Hermann, V.; Barbezant, M.; Sixt, N.; Dalle, F.; Aho, S.; Bonnin, A.; Hartemann, P.; Sautour, M. Adaptation of Fusarium oxysporum and F. dimerum to the specific aquatic environment provided by the water systems of hospitals. Water Res. 2015, 76, 53–65. [Google Scholar] [CrossRef]
- Khoa, L.V.; Hatai, K.; Aoki, T. Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J. Fish. Dis. 2004, 27, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, J.; Jussila, J.; Koistinen, L.; Paaver, T.; Hurt, M.; Kokko, H. Fusarium avenaceum causes burn spot disease syndrome in noble crayfish (Astacus astacus). J. Invertebr. Pathol. 2013, 113, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Grant, G.B.; O’Donnell, K.; Wannemuehler, K.A.; Noble-Wang, J.; Rao, C.Y.; Jacobson, L.M.; Crowell, C.S.; Sneed, R.S.; Lewis, F.M.T.; et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 2006, 296, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Saric, G.K.; Milakovic, Z.; Krstanovic, V. Toxicity of Fusarium Toxins. Croat. J. Food Technol. Biotech. Nut. 2011, 6, 112–116. [Google Scholar]
- Pellegrini, M.; Ercole, C.; Gianchino, C.; Bernardi, M.; Pace, L.; Del Gallo, M. Fusarium oxysporum f. sp. cannabis isolated from Cannabis sativa L.: In vitro and in planta biocontrol by a plant growth promoting-bacteria consortium. Plants 2021, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Herrer, J. The Emperor Wears No Clothes: The Authoritative Historical Record of Cannabis Conspiracy Against Marijuana; Publishers Group West: Berkeley, CA, USA, 1998; pp. 1–181. [Google Scholar]
- Mihelčić, D. Primjena Konoplje u Poljoprivredi i Tržišne Perspektive Njezinih Prerađevina i Proizvoda. Master’s Thesis, Josip Juraj Strossmayer University of Osijek, Faculty of Agriculture, Osijek, Croatia, 2017. Available online: https://urn.nsk.hr/urn:nbn:hr:151:098050 (accessed on 7 April 2022).
- The Government of the Republic of Croatia. Available online: https://vlada.gov.hr/UserDocsImages//2016/Sjednice/2019/O%C5%BEujak/148%20sjednica%20VRH//148%20-%2016.pdf (accessed on 27 April 2022).
- Paying Agency for Agriculture, Fisheries and Rural Development (PAAFRD). Available online: www.apprrr.hr/agronet (accessed on 14 March 2022).



| Pathogen | Isolate | GenBank Accession No. | |
|---|---|---|---|
| ITS | TEF-1α | ||
| F. staphyleae | NRRL 22316 | AF178423 | AF178361 |
| F. illudens | NRRL 22090 | AF178393 | AF178326 |
| Fusarium sp. | NRRL 22163 | AF178394 | AF178328 |
| F. ambrosium | NRRL 20438 | AF178397 | AF178332 |
| F. solani Fusarium sp. | NRRL 22354 | AF178402 | AF178338 |
| F. solani Fusarium sp. | NRRL 22579 | AF178415 | AF178352 |
| F. plagianthi | NRRL 22632 | AF178417 | AF178354 |
| F. solani f. sp. Piperis | NRRL 22570 | AF178422 | AF178360 |
| F. commune | 9312D | DQ016191 | DQ016253 |
| F. cucurbiticola | NRRL 22098 | DQ094301 | AF178327 |
| F. batatas Fusatium sp. | NRRL 22400 | DQ094303 | AF178343 |
| F. mori | NRRL 22230 | DQ094305 | AF178358 |
| F. vanettenii | NRRL 22820 | DQ094310 | AF178355 |
| Fusarium sp. | NRRL 22161 | DQ094311 | AF178330 |
| F. neocosmosporiellum | NRRL 22436 | DQ094317 | AF178348 |
| F. neocosmosporiellum | NRRL 22468 | DQ094318 | AF178349 |
| F. lichenicola | NRRL 28030 | DQ094355 | DQ246877 |
| F. falciforme | NRRL 32721 | DQ094503 | DQ247041 |
| F. tucumaniae | NRRL 31096 | EF408523 | GU170636 |
| F. virguliformae | NRRL 22825 | EF408542 | GU170635 |
| F. aethiopicum | NRRL 46722 | FJ240308 | GU170635 |
| F. vanettenii | MAFF 840047 | AB513852 | AB513842 |
| F. oxysporum f. sp. lycopersici | KoChi-1 | AB675383 | LC648711 |
| F. oxysporum f.sp. melonis | ISPaVe1018 | FR852562 | HE585984 |
| F. euwallaceae | NRRL 62626 | KC691560 | KC691532 |
| F. oligoseptatum | NRRL 62578 | KC691565 | KC691537 |
| F. pseudensiformae | NRRL 46517 | KC691584 | KC691555 |
| F. oxysporum | AMFOWY137HQ43 | KR047072 | KR108763 |
| F. verticillioides | CBS 576.78 | KR071630 | AB674287 |
| F. solani | P2-f11 | LC198903 | LC198905 |
| F. oxysporum | LD01 | MH752745 | MN026924 |
| F. nurragi | CBS 393.96 | MH862577 | MW928840 |
| F. begoniae | CBS 452.97 | MH862660 | MN533994 |
| F. staphyleae | CBS 125482 | MH863592 | MW834282 |
| F. oxysporum | MY | MG564287 | MG674219 |
| F. solani | Fs_HCI_16 | MK393907 | MK814525 |
| F. solani | Fs_HCI_18 | MK393909 | MK814527 |
| F. solani | Fs_HCI_23 | MK393910 | MK814528 |
| F. oxysporum | AG6 | MG564289 | MG677117 |
| F. oxysporum | KB | MG564290 | MG677118 |
| F. oxysporum | AG4 | MG564293 | MG677121 |
| F. oxysporum | EW | MG564296 | MG696756 |
| F. fujikuroi | CBS 221.76 | MW827608 | MN534010 |
| F. proliferatum | F81 | MW995674 | KU974246 |
| F. oxysporum | gss142 | MH290453 | MH341211 |
| F. oxysporum | HFox1 | OM475708 | OR149071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duvnjak, T.; Vrandecic, K.; Sudaric, A.; Cosic, J.; Siber, T.; Matosa Kocar, M. First Report of Hemp Fusarium Wilt Caused by Fusarium oxysporum in Croatia. Plants 2023, 12, 3305. https://doi.org/10.3390/plants12183305
Duvnjak T, Vrandecic K, Sudaric A, Cosic J, Siber T, Matosa Kocar M. First Report of Hemp Fusarium Wilt Caused by Fusarium oxysporum in Croatia. Plants. 2023; 12(18):3305. https://doi.org/10.3390/plants12183305
Chicago/Turabian StyleDuvnjak, Tomislav, Karolina Vrandecic, Aleksandra Sudaric, Jasenka Cosic, Tamara Siber, and Maja Matosa Kocar. 2023. "First Report of Hemp Fusarium Wilt Caused by Fusarium oxysporum in Croatia" Plants 12, no. 18: 3305. https://doi.org/10.3390/plants12183305
APA StyleDuvnjak, T., Vrandecic, K., Sudaric, A., Cosic, J., Siber, T., & Matosa Kocar, M. (2023). First Report of Hemp Fusarium Wilt Caused by Fusarium oxysporum in Croatia. Plants, 12(18), 3305. https://doi.org/10.3390/plants12183305







