Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy
Abstract
1. Introduction
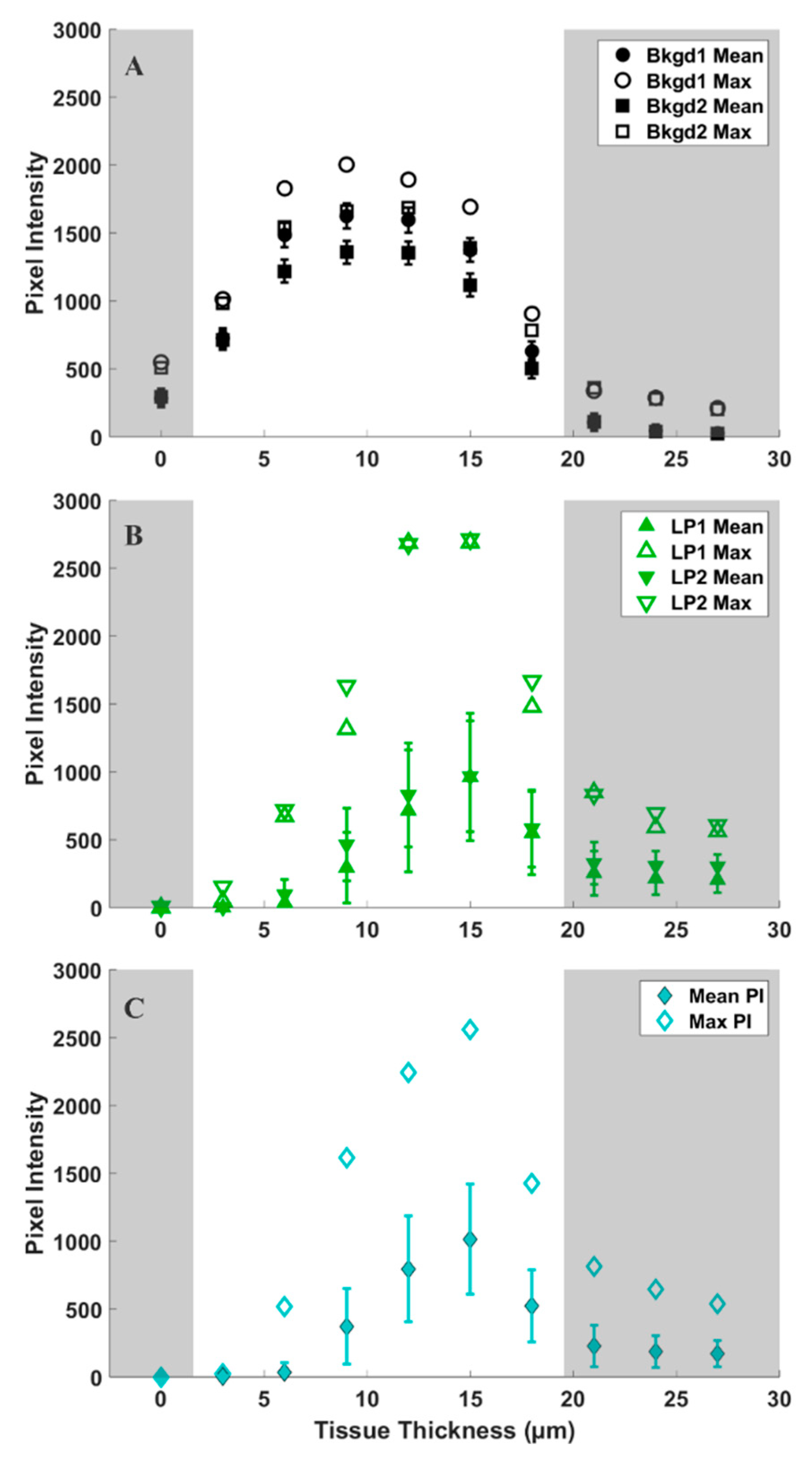
2. Results
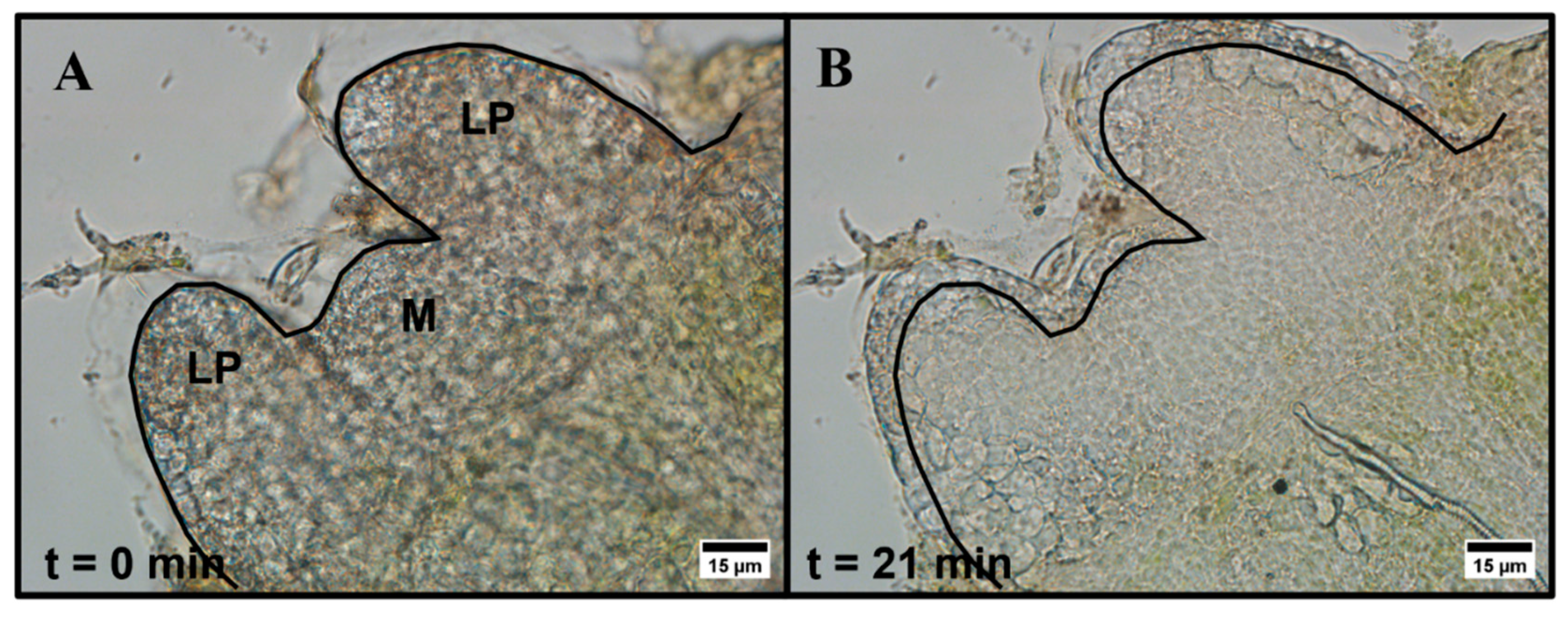
2.1. Brightfield Imaging
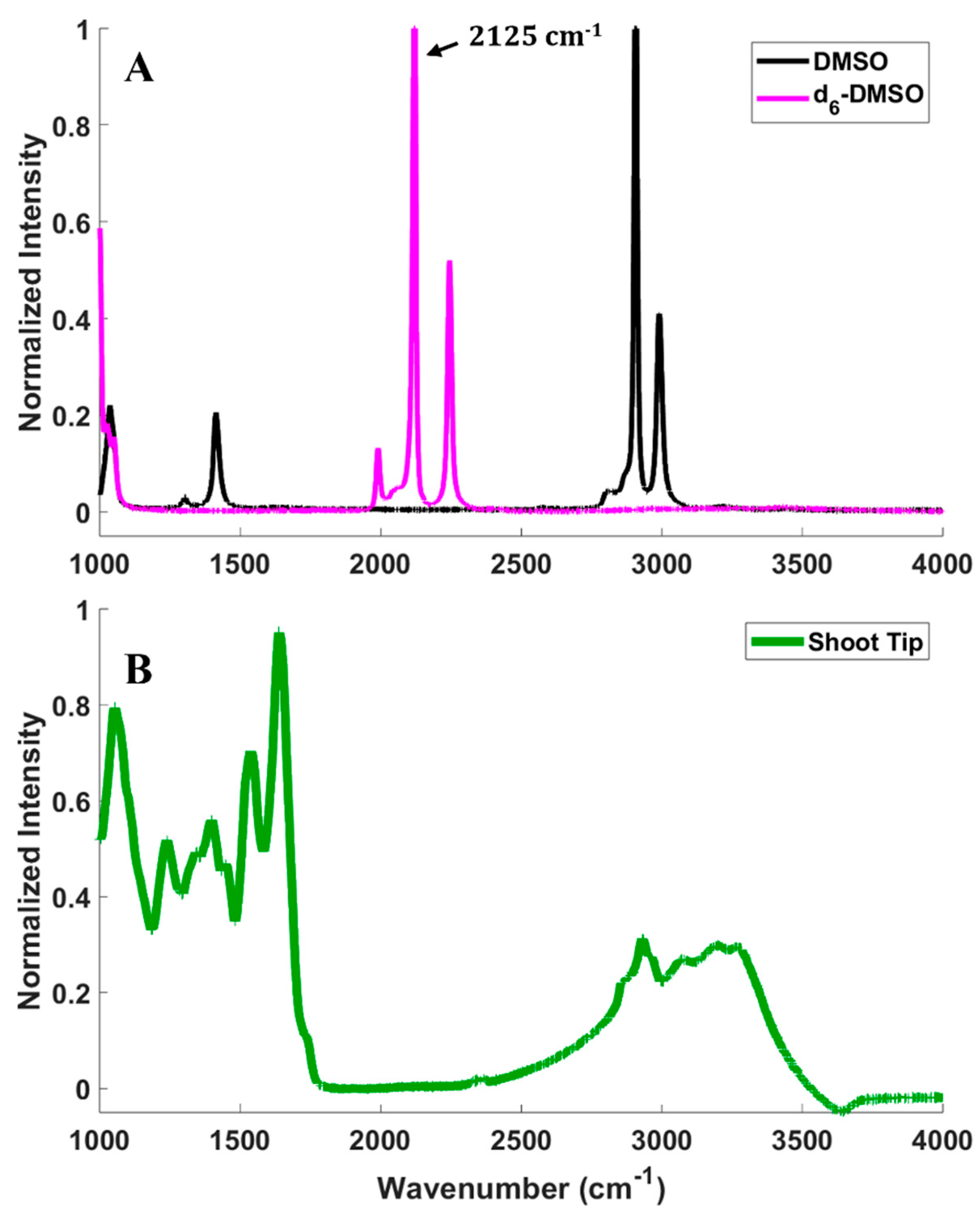
2.2. Vibrational Spectroscopy
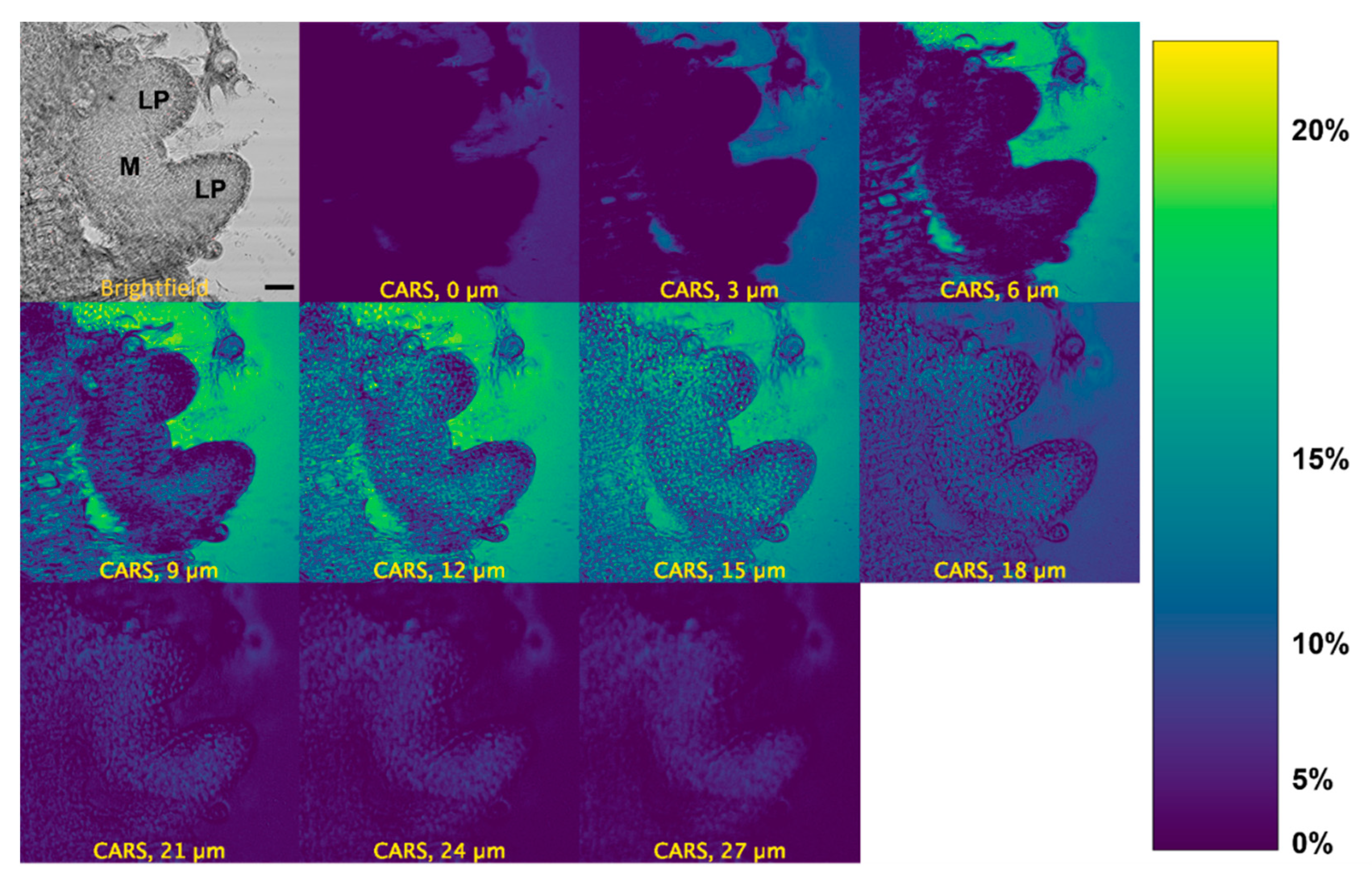
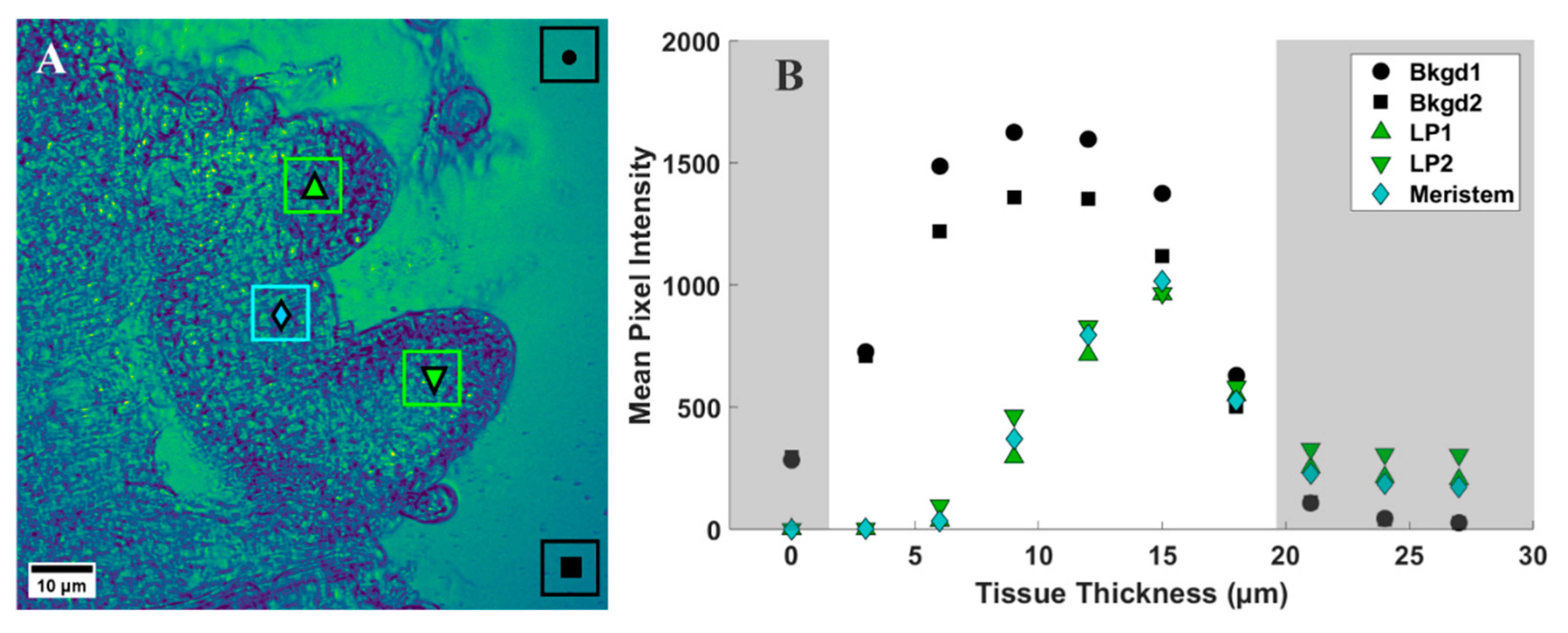
2.3. CARS Imaging
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.1.1. Mentha × piperita Cultures
4.1.2. Cryoprotectant Solutions
4.1.3. Shoot Tip Excision and Sample Preparation
4.2. Microscopy and Spectroscopy Methods
4.2.1. Brightfield Microscopy with Flow
4.2.2. Steady-State Raman and IR Spectroscopy
4.2.3. CARS Microscopy Studies
4.3. Image and Data Analysis
4.3.1. CARS z-Stack Processing
4.3.2. Region of Interest Analysis
4.3.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitro Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Li, D.-Z.; Pritchard, H.W. The Science and Economics of Ex Situ Plant Conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Jenderek, M.M.; Reed, B.M. Cryopreserved Storage of Clonal Germplasm in the USDA National Plant Germplasm System. Vitro Cell. Dev. Biol. Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Engelmann, F. Plant Cryopreservation: Progress and Prospects. Vitro Cell. Dev. Biol. Plant 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in Implementing Plant Shoot Tip Cryopreservation Technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Ogur, E.; Adanacioglu, N.; Galatali, S.; Ceylan, M.; Kaya, E. Cryopreservation of Mentha piperita L. Germplasm and Confirmation of Genetic Stability after Cryo-Storage. J. Anim. Plant Sci. 2023, 33, 345–356. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Kulus, D.; Vacaro De Souza, A.; Kaviani, B.; Vicente, E.F. Cryopreservation of Agronomic Plant Germplasm Using Vitrification-Based Methods: An Overview of Selected Case Studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Volk, G.M.; Walters, C. Plant Vitrification Solution 2 Lowers Water Content and Alters Freezing Behavior in Shoot Tips during Cryoprotection. Cryobiology 2006, 52, 48–61. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of Nucellar Cells of Navel Orange (Citrus sinensis Osb. Var. Brasiliensis tanaka) by Vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of Asparagus (Asparagus officinalis L.) Embryogenic Suspension Cells and Subsequent Plant Regeneration by Vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Volk, G.; Hummer, K.; Chen, K. Mint Shoot Tip Cryopreservation (Droplet Vitrification). In Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules; US Department of Agriculture: Fort Collins, CO, USA, 2020; Available online: https://colostate.pressbooks.pub/clonalcryopreservation/chapter/mint-cryopreservation/ (accessed on 15 November 2020).
- Senula, A.; Joachim Keller, E.R.; Sanduijav, T.; Yohannes, T. Cryopreservation of Cold-Acclimated Mint (Mentha Spp.) Shoot Tips Using a Simple Vitrification Protocol. Cryo Lett. 2007, 28, 1–12. [Google Scholar]
- Hirai, D.; Sakai, A. Cryopreservation of in Vitro-Grown Axillary Shoot-Tip Meristems of Mint (Mentha spicata L.) by Encapsulation Vitrification. Plant Cell Rep. 1999, 19, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Caspersen, A.M. Plasmolysis and Recovery of Different Cell Types in Cryoprotected Shoot Tips of Mentha × Piperita. Protoplasma 2007, 231, 215–226. [Google Scholar] [CrossRef]
- Ganino, T.; Silvanini, A.; Beghè, D.; Benelli, C.; Lambardi, M.; Fabbri, A. Anatomy and Osmotic Potential of the Vitis Rootstock Shoot Tips Recalcitrant to Cryopreservation. Biol. Plant. 2012, 56, 78–82. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Shepherd, A.; Yin, Z.; Lee, R.; Polek, M.; Krueger, R. Citrus Cryopreservation: Viability of Diverse Taxa and Histological Observations. Plant Cell Tissue Organ Cult. 2017, 128, 327–334. [Google Scholar] [CrossRef]
- Samuels, F.M.D.; Pearce, K.C.; Soderlund, S.; Stich, D.G.; Bonnart, R.; Volk, G.M.; Levinger, N.E. Direct Measurement of Rice (Oryza sativa) Callus Cell Responses to Common Molecular Cryoprotectants. Cell Rep. Phys. Sci. 2023. [Google Scholar] [CrossRef]
- Bi, W.-L.; Hao, X.-Y.; Cui, Z.-H.; Volk, G.M.; Wang, Q.-C. Droplet-Vitrification Cryopreservation of in Vitro-Grown Shoot Tips of Grapevine (Vitis spp.). Vitro Cell. Dev. Biol. Plant 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Mathew, L.; McLachlan, A.; Jibran, R.; Burritt, D.J.; Pathirana, R. Cold, Antioxidant and Osmotic Pre-Treatments Maintain the Structural Integrity of Meristematic Cells and Improve Plant Regeneration in Cryopreserved Kiwifruit Shoot Tips. Protoplasma 2018, 255, 1065–1077. [Google Scholar] [CrossRef]
- Faltus, M.; Bilavcik, A.; Zamecnik, J. Vitrification Ability of Combined and Single Cryoprotective Agents. Plants 2021, 10, 2392. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kinoshita, K.; Yamazaki, M. Low Concentration of DMSO Stabilizes the Bilayer Gel Phase Rather than the Interdigitated Gel Phase in Dihexadecylphosphatidylcholine Membrane. Biochim. Biophys. Acta BBA Biomembr. 2000, 1467, 395–405. [Google Scholar] [CrossRef]
- Gordeliy, V.I.; Kiselev, M.A.; Lesieur, P.; Pole, A.V.; Teixeira, J. Lipid Membrane Structure and Interactions in Dimethyl Sulfoxide/Water Mixtures. Biophys. J. 1998, 75, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Rudolph, A.S.; Wistrom, C.A.; Spargo, B.J.; Anchordoguy, T.J. Interactions of Sugars with Membranes. Biochim. Biophys. Acta BBA Rev. Biomembr. 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Kent, B.; Hauß, T.; Demé, B.; Cristiglio, V.; Darwish, T.; Hunt, T.; Bryant, G.; Garvey, C.J. Direct Comparison of Disaccharide Interaction with Lipid Membranes at Reduced Hydrations. Langmuir 2015, 31, 9134–9141. [Google Scholar] [CrossRef] [PubMed]
- Konov, K.B.; Isaev, N.P.; Dzuba, S.A. Glycerol Penetration Profile in Phospholipid Bilayers Measured by ESEEM of Spin-Labelled Lipids. Mol. Phys. 2013, 111, 2882–2886. [Google Scholar] [CrossRef]
- Arakawa, T.; Carpenter, J.F.; Kita, Y.A.; Crowe, J.H. The Basis for Toxicity of Certain Cryoprotectants: A Hypothesis. Cryobiology 1990, 27, 401–415. [Google Scholar] [CrossRef]
- Arakawa, T.; Kita, Y.; Timasheff, S.N. Protein Precipitation and Denaturation by Dimethyl Sulfoxide. Biophys. Chem. 2007, 131, 62–70. [Google Scholar] [CrossRef]
- Hills, B.P.; Favret, F.A. A Comparative Multinuclear Relaxation Study of Protein-DMSO and Protein-Water Interactions. J. Magn. Reson. B 1994, 103, 142–151. [Google Scholar] [CrossRef]
- Towill, L.E. Survival of Shoot Tips from Mint Species after Short-Term Exposure to Cryogenic Conditions. HortScience 1988, 23, 839–841. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Reed, B.M. A Comparative Study of Three Cryopreservation Protocols for Effective Storage of in Vitro-Grown Mint (Mentha spp.). CryoLetters 2008, 29, 181–188. [Google Scholar]
- Martín, C.; Kremer, C.; González, I.; González-Benito, M.E. Influence of the Cryopreservation Technique, Recovery Medium and Genotype on Genetic Stability of Mint Cryopreserved Shoot Tips. Plant Cell Tissue Organ Cult. 2015, 122, 185–195. [Google Scholar] [CrossRef]
- Senula, A.; Büchner, D.; Keller, E.R.J.; Nagel, M. An Improved Cryopreservation Protocol for Mentha spp. Based on PVS3 as the Cryoprotectant. Cryoletters 2018, 39, 345–353. [Google Scholar]
- Cheng, J.-X.; Xie, X.S. Coherent Anti-Stokes Raman Scattering Microscopy: Instrumentation, Theory, and Applications. J. Phys. Chem. B 2004, 108, 827–840. [Google Scholar] [CrossRef]
- Samuels, F.M.D.; Stich, D.G.; Bonnart, R.; Volk, G.M.; Levinger, N.E. Non-Uniform Distribution of Cryoprotecting Agents in Rice Culture Cells Measured by CARS Microscopy. Plants 2021, 10, 589. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and Quantification of Valuable Plant Substances by IR and Raman Spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Nakayama, N.; Routier-Kierzkowska, A.-L.; Weber, A.; Bayer, E.; Schorderet, M.; Reinhardt, D.; Kuhlemeier, C.; Smith, R.S. Elastic Domains Regulate Growth and Organogenesis in the Plant Shoot Apical Meristem. Science 2012, 335, 1096–1099. [Google Scholar] [CrossRef]
- Milani, P.; Braybrook, S.A.; Boudaoud, A. Shrinking the Hammer: Micromechanical Approaches to Morphogenesis. J. Exp. Bot. 2013, 64, 4651–4662. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J. Plasmolysis Shape in Relation to Freeze-Hardening of Cabbage Plants and to the Effect of Penetrating Solutes. Plant Cell Environ. 1983, 6, 465–470. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Cuellar-Bermudez, S.P.; Cuaresma, M.; Bosma, R.; Muylaert, K.; Ritmann, B.E.; Parra, R. How to Use Nile Red, a Selective Fluorescent Stain for Microalgal Neutral Lipids. J. Microbiol. Methods 2016, 128, 74–79. [Google Scholar] [CrossRef]
- Van Der Kolk, J.; Lesina, A.C.; Ramunno, L. Effects of Refractive Index Mismatch on SRS and CARS Microscopy. Opt. Express 2016, 24, 25752. [Google Scholar] [CrossRef]
- Djaker, N.; Gachet, D.; Sandeau, N.; Lenne, P.-F.; Rigneault, H. Refractive Effects in Coherent Anti-Stokes Raman Scattering Microscopy. Appl. Opt. 2006, 45, 7005. [Google Scholar] [CrossRef]
- Petrov, G.I.; Arora, R.; Yakovlev, V.V.; Wang, X.; Sokolov, A.V.; Scully, M.O. Comparison of Coherent and Spontaneous Raman Microspectroscopies for Noninvasive Detection of Single Bacterial Endospores. Proc. Natl. Acad. Sci. USA 2007, 104, 7776–7779. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J.E.; Bishop, M.W.H. Prevention of Freezing Damage to Living Cells by Dimethyl Sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Quatrano, R.S. Freeze-Preservation of Cultured Flax Cells Utilizing Dimethyl Sulfoxide. Plant Physiol. 1968, 43, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, D.H.; Mackenzie, A.P. Phase Diagram for the System Water–Dimethylsulphoxide. Nature 1968, 220, 1315–1317. [Google Scholar] [CrossRef]
- Strauss, J.H.; Kelly, R.B.; Sinsheimer, R.L. Denaturation of RNA with Dimethyl Sulfoxide. Biopolymers 1968, 6, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dai, T.; Wang, Y.; Yang, G. The Incipient Denaturation Mechanism of DNA. RSC Adv. 2022, 12, 23356–23365. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Harris, J.L.; Rotindo, K.E. Survival of Mint Shoot Tips after Exposure to Cryoprotectant Solution Components. Cryobiology 2006, 52, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Ortiz, A.; Hernandez-Walls, R. Levenetest. 2003. Available online: https://www.mathworks.com/matlabcentral/fileexchange/3375-levenetest (accessed on 17 February 2023).

| Shoot Tip | Region | Range (Pixel Intensity) | IQR (Pixel Intensity) |
|---|---|---|---|
| ST1 | Bkgd1 | 584 | 124 |
| ST2 | 679 | 132 | |
| ST3 | 708 | 127 | |
| ST1 | Bkgd2 | 661 | 113 |
| ST2 | 651 | 125 | |
| ST3 | 696 | 119 | |
| ST1 | LP1 | 2686 | 639 |
| ST2 | 2646 | 596 | |
| ST3 | 1525 | 245 | |
| ST1 | LP2 | 2677 | 524 |
| ST2 | 2625 | 455 | |
| ST3 | 1244 | 201 | |
| ST1 | Meristem | 2241 | 535 |
| ST2 | 2453 | 450 | |
| ST3 | 1849 | 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreckel, H.D.; Samuels, F.M.D.; Bonnart, R.; Volk, G.M.; Stich, D.G.; Levinger, N.E. Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy. Plants 2023, 12, 2247. https://doi.org/10.3390/plants12122247
Kreckel HD, Samuels FMD, Bonnart R, Volk GM, Stich DG, Levinger NE. Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy. Plants. 2023; 12(12):2247. https://doi.org/10.3390/plants12122247
Chicago/Turabian StyleKreckel, Heidi D., Fionna M. D. Samuels, Remi Bonnart, Gayle M. Volk, Dominik G. Stich, and Nancy E. Levinger. 2023. "Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy" Plants 12, no. 12: 2247. https://doi.org/10.3390/plants12122247
APA StyleKreckel, H. D., Samuels, F. M. D., Bonnart, R., Volk, G. M., Stich, D. G., & Levinger, N. E. (2023). Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy. Plants, 12(12), 2247. https://doi.org/10.3390/plants12122247






