Different Ecological Niches of Poisonous Aristolochia clematitis in Central and Marginal Distribution Ranges—Another Contribution to a Better Understanding of Balkan Endemic Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetation Plots
2.2. Ecological Specialization Index and Niche Differentiation
2.3. Data for Niche Description
2.4. Statistical Analysis
3. Results
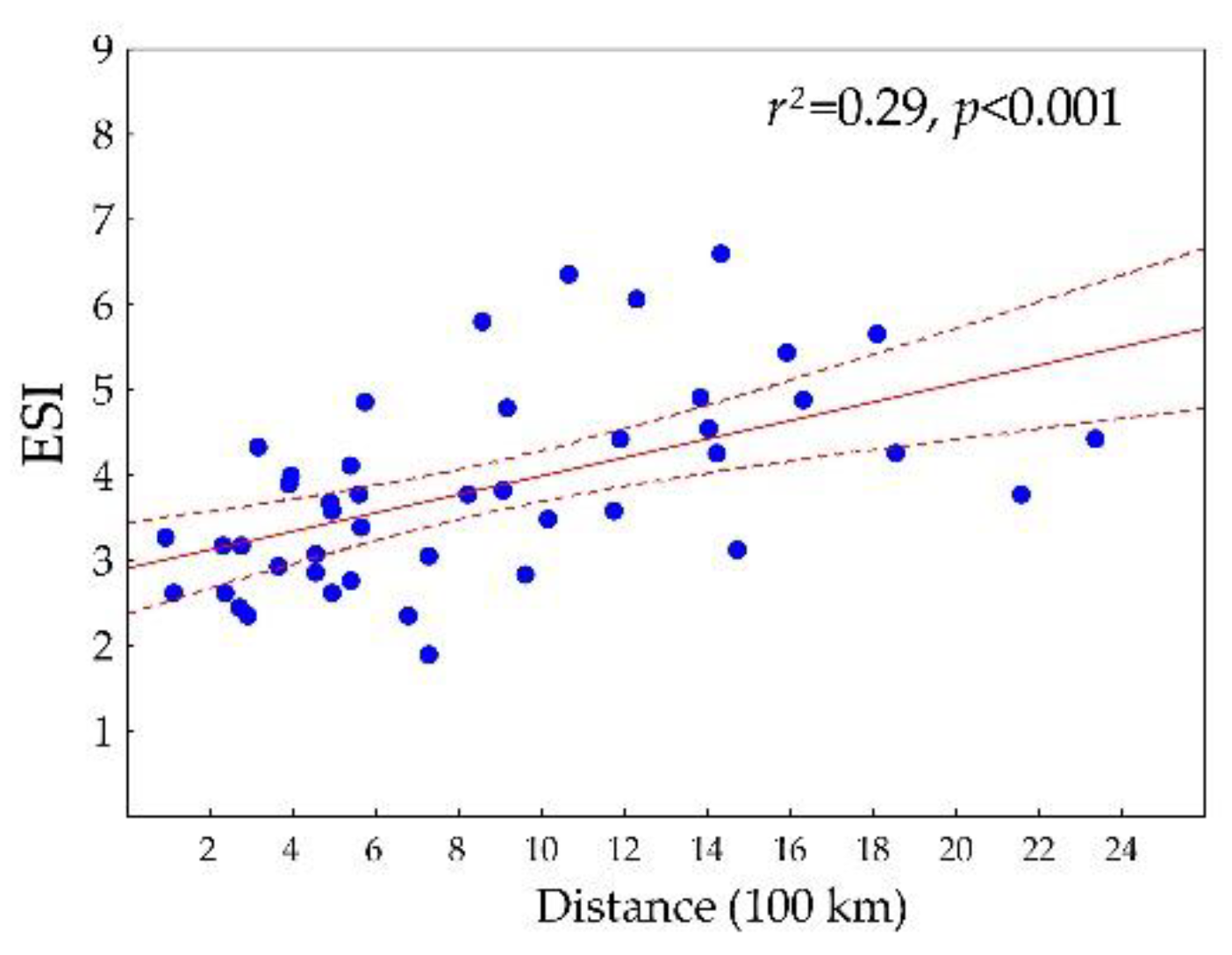
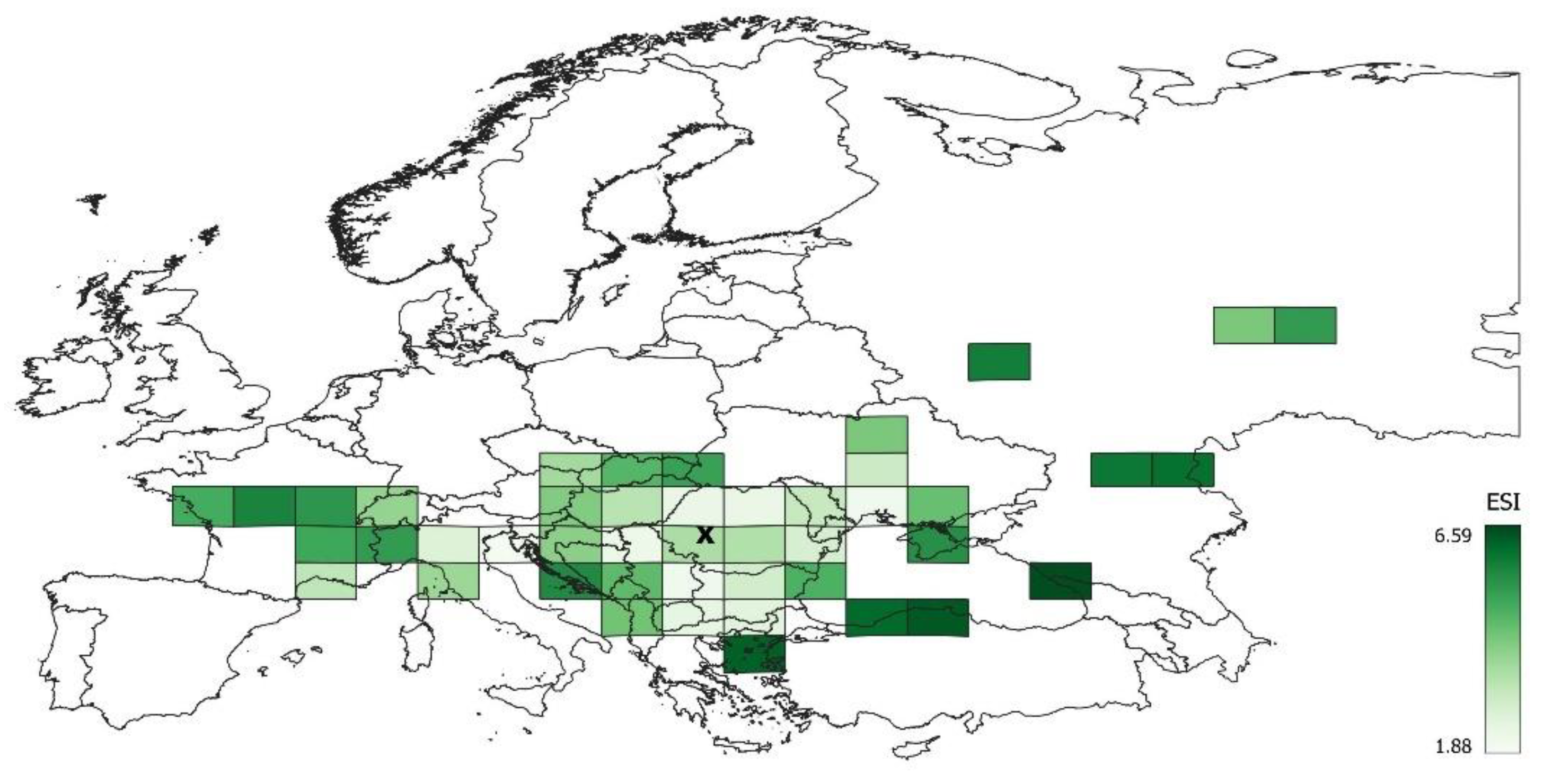
3.1. Characteristics of Ecological Niche in the Whole Studied Area
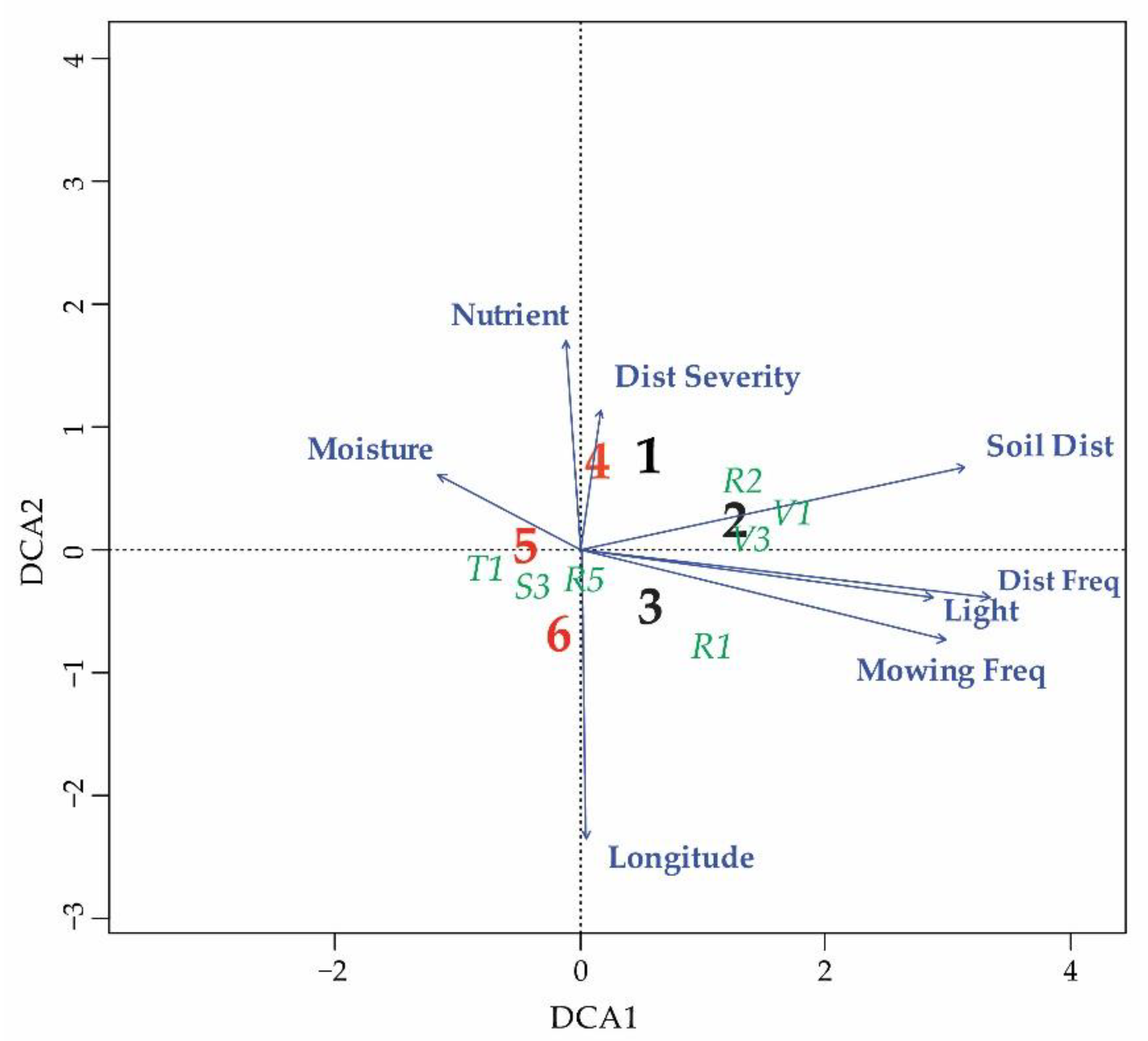
3.2. Differentiation of the Ecological Niche
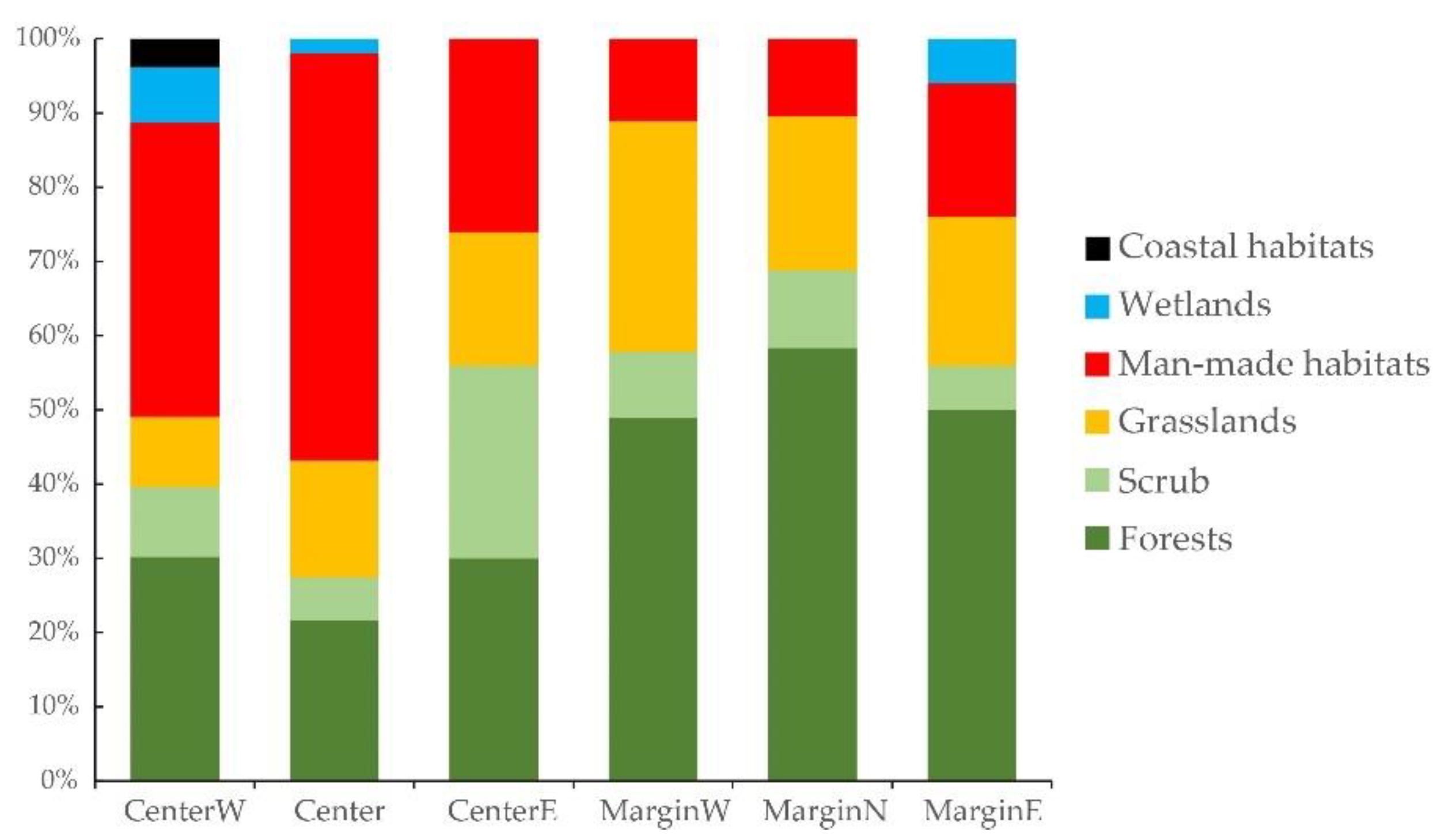
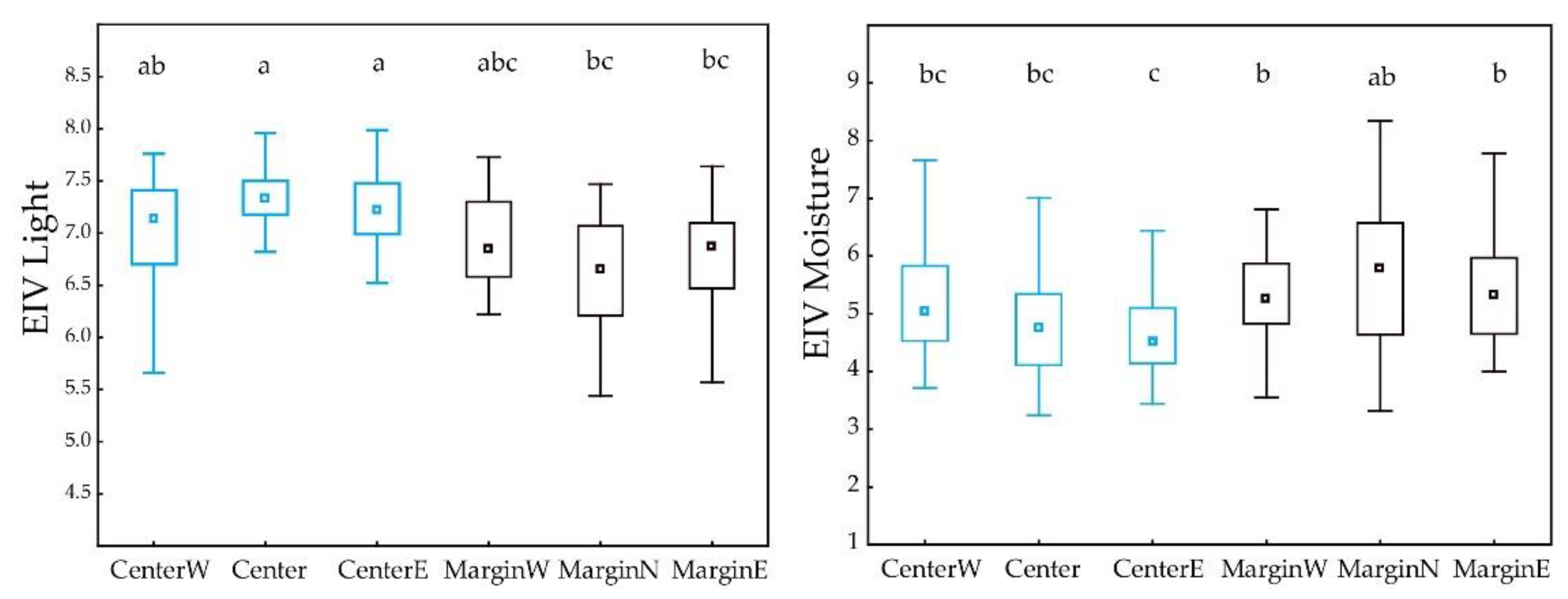
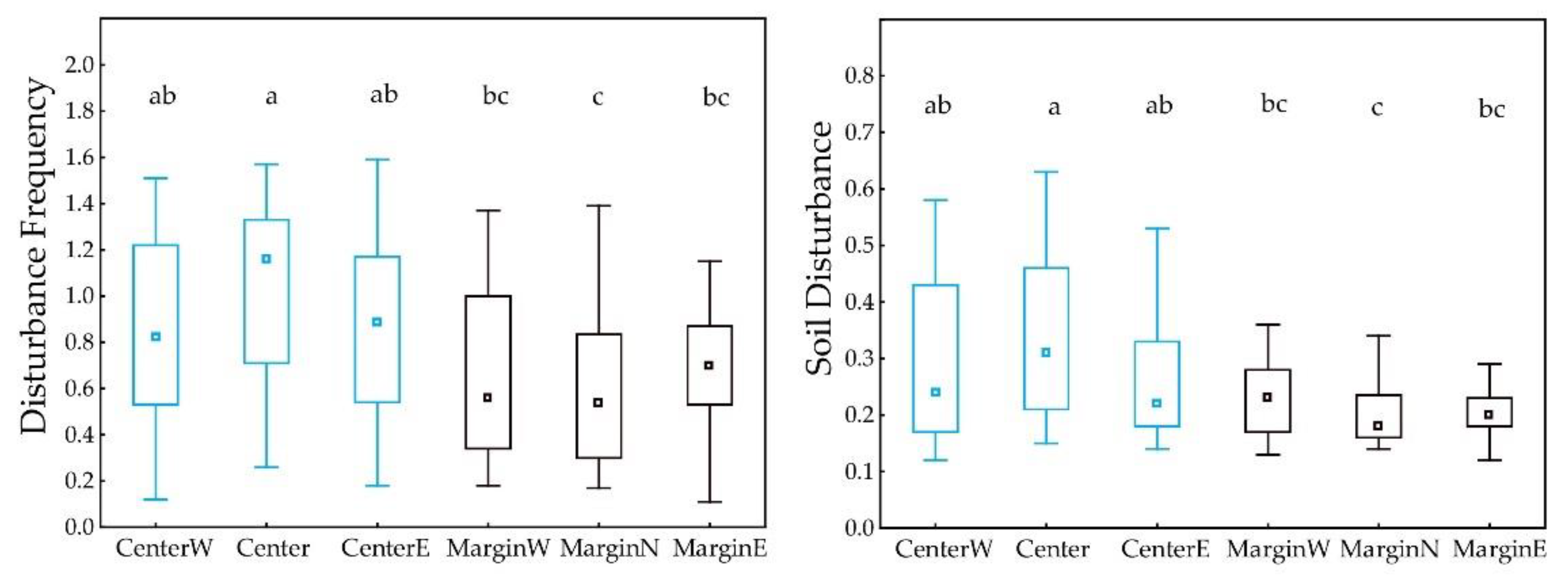
3.3. Differences between Margin and Centre of the Distribution Area
4. Discussion
4.1. Fundamental Niche
4.2. Availability of Suitable Habitats
4.3. Biotic Interactions
4.4. Methodology Strengths and Weaknesses
4.5. Balkan Endemic Nephropathy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoener, T.W. The ecological niche. In Ecological Concepts: The Contribution of Ecology to an Understanding of the Natural World; Cherret, M.J., Ed.; Blackwell Scientific Publication: Hoboken, NJ, USA, 1989; pp. 790–813. [Google Scholar]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harbor Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Ellenberg, H. Physiologisches und ökologisches Verhalten derselben Pflanzenarten. Ber. Dt. Bot. Ges. 1953, 65, 351–362. [Google Scholar]
- Walter, H.; Breckle, S.-W. Ökologie der Erde: Band 1: Ökologische Grundlagen in Globaler Sicht; Gustav Fischer Verlag: Jena, Germany, 1991; 238p. [Google Scholar]
- Diekmann, M.; Lawesson, J.E. Shifts in ecological behaviour of herbaceous forest species along a transect from northern Central to North Europe. Folia Geobot. 1999, 34, 127–141. [Google Scholar] [CrossRef]
- Wasof, S.; Lenoir, J.; Gallet-Moron, E.; Jamoneau, A.; Brunet, J.; Cousins, S.A.; De Frenne, P.; Diekmann, M.; Hermy, M.; Kolb, A.; et al. Ecological niche shifts of understorey plants along a latitudinal gradient of temperate forests in north-western Europe. Glob. Ecol. Biogeog. 2013, 22, 1130–1140. [Google Scholar] [CrossRef]
- Wasof, S.; Lenoir, J.; Aarrestad, P.A.; Alsos, I.G.; Armbruster, W.S.; Austrheim, G.; Bakkestuen, V.; Birks, H.J.B.; Bråthen, K.A.; Broennimann, O.; et al. Disjunct populations of European vascular plant species keep the same climatic niches. Glob. Ecol. Biogeog. 2015, 24, 1401–1412. [Google Scholar] [CrossRef]
- Prinzing, A.; Durka, W.; Klotz, S.; Brandl, R. Geographic variability of ecological niches of plant species: Are competition and stress relevant? Ecography 2002, 25, 721–729. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the great smoky mountains. Ecol. Monogr. 1956, 26, 1–80. [Google Scholar] [CrossRef]
- Pannek, A.; Manthey, M.; Diekmann, M. Comparing resource-based and co-occurrence-based methods for estimating species niche breadth. J. Veg. Sci. 2016, 27, 596–605. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M.; Lawton, J.H. Interspecific abundance range size relationships: An appraisal of mechanisms. J. Anim. Ecol. 1997, 66, 579–601. [Google Scholar] [CrossRef]
- Fridley, J.; Vandermast, D.; Kuppinger, D.; Manthey, M.; Peet, R. Co-occurrence based assessment of habitat generalists and specialists: A new approach for the measurement of niche width. J. Ecol. 2007, 95, 707–722. [Google Scholar] [CrossRef]
- Manthey, M.; Fridley, J. Beta diversity metrics and the estimation of niche width via species co-occurrence data: Reply to Zeleny. J. Ecol. 2009, 97, 18–22. [Google Scholar] [CrossRef]
- Zelený, D. Co-occurrence based assessment of species habitat specialization is affected by the size of species pool: Reply to Fridley et al. J. Ecol. 2009, 97, 10–17. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Co-occurrence-based measure of species’ habitat specialization: Robust, unbiased estimation in saturated communities. J. Veg. Sci. 2012, 23, 201–207. [Google Scholar] [CrossRef]
- Šilc, U.; Lososová, Z.; Vrbničanin, S. Weeds shift from generalist to specialist: Narrowing of ecological niches along a north-south gradient. Preslia 2014, 86, 35–46. [Google Scholar]
- Marinšek, A.; Čarni, A.; Šilc, U.; Manthey, M. What makes a plant species specialist in mixed broadleaved deciduous forests? Plant Ecol. 2015, 216, 1469–1479. [Google Scholar] [CrossRef]
- Zelený, D.; Chytrý, M. Ecological specialization indices for species of the Czech flora. Preslia 2019, 91, 93–116. [Google Scholar] [CrossRef]
- Hegi, G. Illustrierte Flora von Mitteleuropa; Wagenitz, G., Melzheimer, V., Straka, H., Eds.; Paul Parey Verlag: Singhofen, Germany, 1981; Volume 3. [Google Scholar]
- Sekerka, P. Podražce v Evropě původní a pěstované. Živa 2007, 6, 254–256. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19 (Suppl. 1), 3–264. [Google Scholar] [CrossRef]
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene: Aristolochia species and aristolochic acids. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 69–128. [Google Scholar]
- Čeović, S.; Miletić-Medved, M. Epidemiological features of endemic nephropathy in focal area of Brodska Posavina, Croatia. In Endemic Nephropathy in Croatia; Cvorisćec, D., Ceović, S., Borso, G., Rukavina, A.S., Eds.; Academia Croatica Scientiarum Medicarum: Zagreb, Croatia, 1996; pp. 7–21. [Google Scholar]
- Stefanović, V.; Radovanović, Z. Balkan endemic nephropathy and associated urothelial cancer. Nat. Clin. Pract. Urol. 2008, 5, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Grollman, A.; Jelaković, B. Role of environmental toxins in endemic (Balkan) nephropathy. J. Am. Soc. Nephrol. 2007, 18, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Ivić, M. The problem of etiology of endemic nephropathy. Lije. Vjesć. 1969, 91, 1273–1281. [Google Scholar]
- Jelaković, B.; Karanović, S.; Vuković-Lela, I.; Miller, F.; Edwards, K.; Nikolić, J.; Tomić, K.; Slade, N.; Brdar, B.; Turesky, R.; et al. Aristolactam-DNA Adducts in the Renal Cortex: Biomarker of Environmental Exposure to Aristolochic Acid. Kidney Int. 2012, 81, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, T.; Kovač, A.; Kos, J.; Mao, W.; Chen, J.J.; Grollman, A.P.; Jelaković, B. Endemic nephropathy: The case for chronic poisoning by Aristolochia. Croat. Med. J. 2005, 46, 116–125. [Google Scholar]
- Jelaković, B.; Vuković Lela, I.; Karanović, S.; Dika, Ž.; Kos, J.; Dickman, K.; Šekoranja, M.; Poljičanin, T.; Mišić, M.; Premužić, V.; et al. Chronic dietary exposure to aristolochic acid and kidney function in native farmers from a Croatian endemic area and Bosnian immigrants. Clin. J. Am. Soc. Nephrol. 2015, 6, 215–223. [Google Scholar] [CrossRef]
- Leuschner, C.; Köckemann, B.; Buschmann, H. Abundance, niche breadth, and niche occupation of Central European tree species in the centre and at the margin of their distribution range. For. Ecol. Manag. 2009, 258, 1248–1259. [Google Scholar] [CrossRef]
- Morin, X.; Thuiller, W. Comparing niche-and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 2009, 90, 1301–1313. [Google Scholar] [CrossRef]
- Papuga, G.; Gauthier, P.; Ramos, J.; Pons, V.; Pironon, S.; Farris, E.; Thompson, J.D. Range-wide variation in the ecological niche and floral polymorphism of the western Mediterranean geophyte Narcissus dubius Gouan. Int. J. Plant Sci. 2015, 176, 724–738. [Google Scholar] [CrossRef]
- Chytrý, M.; Hennekens, S.M.; Jiménez-Alfaro, B.; Knollová, I.; Dengler, J.; Jansen, F.; Landucci, F.; Schaminée, J.H.J.; Aćić, S.; Agrillo, E.; et al. European Vegetation Archive (EVA): An integrated database of European vegetation plots. Appl. Veg. Sci. 2016, 19, 173–180. [Google Scholar] [CrossRef]
- Knollová, I.; Chytrý, M.; Tichý, L.; Hájek, O. Stratified resampling of phytosociological databases: Some strategies for obtaining more representative data sets for classification studies. J. Veg. Sci. 2006, 16, 479–486. [Google Scholar] [CrossRef]
- Lengyel, A.; Chytrý, M.; Tichý, L. Heterogeneity-constrained random resampling of phytosociological databases. J. Veg. Sci. 2011, 22, 175–183. [Google Scholar] [CrossRef]
- Wiser, S.; De Cáceres, M. Updating vegetation classifications: And example with New Zealand’s woody vegetation. J. Veg. Sci. 2013, 24, 80–93. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Hennekens, S.M.; Knollová, I.; Janssen, J.A.M.; Rodwell, J.S.; Peterka, T.; Marcenò, C.; Landucci, F.; Danihelka, J.; et al. EUNIS Habitat Classification: Expert system, characteristic species combinations and distribution maps of European habitats. Appl. Veg. Sci. 2020, 23, 648–675. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, P.; Kessler, M. Climatologies at high resolution for the Earth land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-type indicator values for European vascular plant species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Midolo, G.; Herben, T.; Axmanová, I.; Marcenò, C.; Pätsch, R.; Bruelheide, H.; Karger, D.N.; Aćić, S.; Bergamini, A.; Bergmeier, E.; et al. Disturbance indicator values for European plants. Glob. Ecol. Biogeog. 2023, 32, 24–34. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Chardon, N.I.; Cornwell, W.K.; Flint, L.E.; Flint, A.L.; Ackerly, D.D. Topographic, latitudinal and climatic distribution of Pinus coulteri: Geographic range limits are not at the edge of the climate envelope. Ecography 2014, 38, 590–601. [Google Scholar] [CrossRef]
- Pironon, S.; Villellas, J.; Morris, W.F.; Doak, D.F.; García, M.B. Do geographic, climatic or historical ranges differentiate the performance of central versus peripheral populations? Glob. Ecol. Biogeogr. 2015, 24, 611–620. [Google Scholar] [CrossRef]
- Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–390. [Google Scholar] [CrossRef]
- Preislerová, Z.; Jiménez-Alfaro, B.; Mucina, L.; Berg, C.; Bonari, G.; Kuzemko, A.; Landucci, F.; Marcenò, C.; Monteiro-Henriques, T.; Novák, P.; et al. Distribution maps of vegetation alliances in Europe. Appl. Veg. Sci. 2022, 25, e12642. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Env. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Küzmič, F. Classification and Gradient Analysis of Weed Vegetation in Europe. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2020. [Google Scholar]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Manthey, M.; Fridley, J.D.; Peet, R.K. Niche expansion after competitor extinction? A comparative assessment of habitat generalists and specialists in the tree floras of southeastern North America and south-eastern Europe. J. Biogeogr. 2011, 38, 840–853. [Google Scholar] [CrossRef]
- Fogazzi, G.B.; Bellincioni, C. Aristolochia clematitis, the herb responsible for aristolochic acid nephropathy, in an uncultivated piece of land of an Italian nephrologist. Nephrol. Dial. Transplant. 2015, 30, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Cvitković, A.; Vuković-Lela, I.; Edwards, K.L.; Karanović, S.; Jurić, D.; Čvorišćec, D.; Fuček, M.; Jelaković, B. Could disappearance of endemic (Balkan) nephropathy be expected in forthcoming decades? Kidney Blood Press. Res. 2012, 35, 147–152. [Google Scholar] [CrossRef]

| Variable | Spearman R Between | |
|---|---|---|
| ESI and Median | ESI and Quartile Range | |
| Light | −0.35 | - |
| Moisture | - | −0.48 |
| Reaction | −0.33 | - |
| Disturbance Severity | - | −0.49 |
| Disturbance Frequency | −0.46 | −0.50 |
| Mowing Frequency | - | −0.45 |
| Soil Disturbance | −0.38 | −0.43 |
| Perecentage of forest habitats | 0.45 | NA |
| Perecentage of man-made habitats | −0.41 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzić, I.; Brener, M.; Čarni, A.; Ćušterevska, R.; Čulig, B.; Dziuba, T.; Golub, V.; Irimia, I.; Jelaković, B.; Kavgacı, A.; et al. Different Ecological Niches of Poisonous Aristolochia clematitis in Central and Marginal Distribution Ranges—Another Contribution to a Better Understanding of Balkan Endemic Nephropathy. Plants 2023, 12, 3022. https://doi.org/10.3390/plants12173022
Brzić I, Brener M, Čarni A, Ćušterevska R, Čulig B, Dziuba T, Golub V, Irimia I, Jelaković B, Kavgacı A, et al. Different Ecological Niches of Poisonous Aristolochia clematitis in Central and Marginal Distribution Ranges—Another Contribution to a Better Understanding of Balkan Endemic Nephropathy. Plants. 2023; 12(17):3022. https://doi.org/10.3390/plants12173022
Chicago/Turabian StyleBrzić, Ivan, Magdalena Brener, Andraž Čarni, Renata Ćušterevska, Borna Čulig, Tetiana Dziuba, Valentin Golub, Irina Irimia, Bojan Jelaković, Ali Kavgacı, and et al. 2023. "Different Ecological Niches of Poisonous Aristolochia clematitis in Central and Marginal Distribution Ranges—Another Contribution to a Better Understanding of Balkan Endemic Nephropathy" Plants 12, no. 17: 3022. https://doi.org/10.3390/plants12173022
APA StyleBrzić, I., Brener, M., Čarni, A., Ćušterevska, R., Čulig, B., Dziuba, T., Golub, V., Irimia, I., Jelaković, B., Kavgacı, A., Krstivojević Ćuk, M., Krstonošić, D., Stupar, V., Trobonjača, Z., & Škvorc, Ž. (2023). Different Ecological Niches of Poisonous Aristolochia clematitis in Central and Marginal Distribution Ranges—Another Contribution to a Better Understanding of Balkan Endemic Nephropathy. Plants, 12(17), 3022. https://doi.org/10.3390/plants12173022








