Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function
Abstract
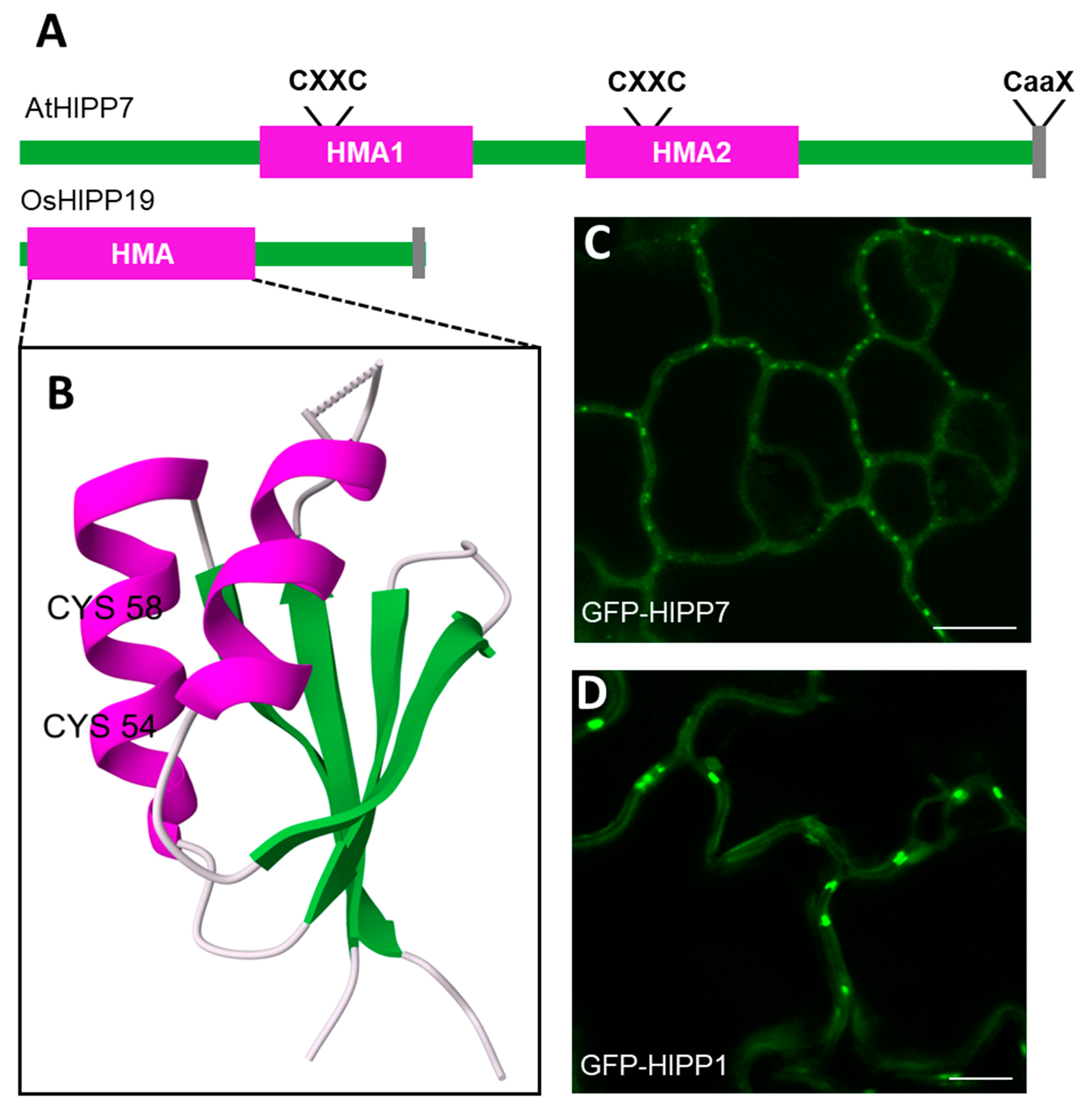
:1. Introduction
2. HIPP Proteins Identified in PD Proteomic Studies
| TAIR ID | Tehseen et al. [1]/de Abreu-Neto et al. [2] nomeclatures | Dykema et al. [34] nomenclature | Li et al. [12] Nomenclature | Number of HMA Domains | Clade [1] | Clade [2] | PD Proteomes/PD-Related Papers | PD-Localisation Confirmed | Known regulation, Functions and Interactions |
|---|---|---|---|---|---|---|---|---|---|
| AT1G01490 | HIPP39 | HMP01 | 1 | VI | V | N. benthamiana [27] | Fruit-specific [12]. | ||
| AT2G28090 | HIPP01 | HMP17 | 2 | I | I | Arabidopsis [33] | yes [33] | Low expression in all tissues [12], expressed during meiosis [35]. Alters cytokinin responses and development, regulates the CKX ERAD [33].Interacts with different cytokinin-degrading CKX proteins [33]. | |
| AT2G36950 | HIPP05 | FP2 | HMP20 | 2 | I | I | Arabidopsis [25,26] | Weakly Cu-binding [36]. ↑ Cu/Cd/Zn [12], ↑ cold [37], ↑ hypoxia [38],↓ brassinosteroids [39], ↓ NO [40], ↓ cytokinin [33]. Alters cytokinin responses, regulates the CKX ERAD and ER stress [33]. Interacts with different cytokinin-degrading CKX proteins [33]. | |
| AT3G05220 | HIPP32/HPP34 | HMP23 | 1 | III | III | Arabidopsis [24,25,26] | ↑Cu/Pb/Cd/Zn [12], ↑ cold [37], ↑ NO [40], ↓ Geminivirus infection [41] | ||
| AT3G06130 | HIPP34/HIPP32 | HMP25 | 1 | III | III | Arabidopsis [25], N. benthamiana [27] | ↑ Zn/Pb/Cu [12], ↓ NO [40], ↓ Geminivirus infection [41]. Interacts with cytokinin-degrading protein CKX1 [33]. | ||
| AT4G16380 | FP1 | HMP35 | 1 * | Arabidopsis [24,25,26], P. trichocarpa [28] | ↑ Cu/Cd/Zn/Pb [12] | ||||
| AT4G35060 | HIPP25 | HMP39 | 1 | IV | II | Arabidopsis [24,25] | Expressed in leaves [12], main roots, SAM, flower buds, trichomes [1], ↑ in trichomes [42]; expressed in endosperm but not embryo during germination [43]. ↓ auxin [39], ↓ NO [40], ↑ PtdInsP’s [44], initially ↑ then ↓ cold. No interaction with transcription factor ATHB29 [45]. | ||
| AT4G38580 | HIPP26 | FP6 | HMP40 | 1 | IV | II | Arabidopsis [24,25], N. benthamiana [27,46], P. patens [29] | yes [46] ** | Binds Cd/Cu/Pb [47]. Expressed in lateral root tips, SAM, weakly in leaf vasculature [1], mostly vascular expression [45,46], ↑ in trichomes [42]. ↑ Cd [47], ↑ cold [45,48,49], ↑drought [45,46,49], ↑ salt [45], ↓ heat [50], ↑ Pomovirus infection [46]. Interacts with acyl-CoA binding protein ACBP2 [47], zinc finger homeobox domain transcription factor ATHB29 via HMA cysteines [45], metallocarboxipeptidase inhibitor TCMP-1 [51], viral movement protein [46]. Overexpression increases Cd tolerance [47], rescues Cd-sensitive yeast mutant [1]. S-acylated [46] |
| AT5G03380 | HIPP06 | HMP43 | 2 | I | I | Arabidopsis [25], P. trichocarpa [28] | Mainly root-expressed [12]. ↑ hypoxia [52], ↑ Geminivirus infection [41], ↑ wound responses [53], ↓ cytokinin [33]. Alters cytokinin responses and development, regulates the CKX ERAD and ER stress [33]. Interacts with different cytokinin-degrading CKX proteins [33]. | ||
| AT5G19090 | HIPP33 | HMP46 | 1 | III | III | Arabidopsis [24,25], N. benthamiana [27], P. trichocarpa [28] | ↑ Cu/Cd/Zn/Pb [12], ↓ NO [40], ↓ Geminivirus infection [41]. Non-canonical intron/likely alternatively spliced [54]. | ||
| AT5G50740 | HPP01 | HMP52 | 2 | I | N. benthamiana [27] | ↑ cytokinin [55], ↓ Geminivirus infection [41] | |||
| AT5G63530 | HIPP07 | FP3 | HMP54 | 2 | I | I | Arabidopsis [25,26,33] | yes [33] | High general expression except in roots [12]. ↓ Geminivirus infection (Ascencio-Ibanez), ↓ cytokinin [33]. Alters cytokinin responses and development, regulates the CKX ERAD and ER stress [33]. Interacts with different cytokinin-degrading CKX proteins [33]. |
3. HIPP Localization to PD and Targeting Mechanisms
4. HIPPs’ Role as Metallochaperones
5. Heavy Metal Effects on PD
6. HIPP Functions in Responses to Abiotic and Biotic Stresses
7. Functions of HIPPs in Regulating Plant Development
8. What Conclusions Are Currently Possible about PD-Associated HIPPs?
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tehseen, M.; Cairns, N.; Sherson, S.; Cobbett, C.S. Metallochaperone-like Genes in Arabidopsis thaliana. Metallomics 2010, 2, 556–564. [Google Scholar] [CrossRef] [PubMed]
- de Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.V.; Bodanese Zanettini, M.H.; Margis-Pinheiro, M. Heavy Metal-Associated Isoprenylated Plant Protein (HIPP): Characterization of a Family of Proteins Exclusive to Plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Rono, J.K.; Sun, D.; Yang, Z.M. Metallochaperones: A Critical Regulator of Metal Homeostasis and Beyond. Gene 2022, 822, 146352. [Google Scholar] [CrossRef]
- Maidment, J.H.R.; Franceschetti, M.; Maqbool, A.; Saitoh, H.; Jantasuriyarat, C.; Kamoun, S.; Terauchi, R.; Banfield, M.J. Multiple Variants of the Fungal Effector AVR-Pik Bind the HMA Domain of the Rice Protein OsHIPP19, Providing a Foundation to Engineer Plant Defense. J. Biol. Chem. 2021, 296, 100371. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Necci, M.; Piovesan, D.; Dosztányi, Z.; Tosatto, S.C.E. MobiDB-Lite: Fast and Highly Specific Consensus Prediction of Intrinsic Disorder in Proteins. Bioinformatics 2017, 33, 1402–1404. [Google Scholar] [CrossRef]
- Hála, M.; Žárský, V. Protein Prenylation in Plant Stress Responses. Molecules 2019, 24, 3906. [Google Scholar] [CrossRef]
- Magee, A.I.; Seabra, M.C. Are Prenyl Groups on Proteins Sticky Fingers or Greasy Handles? Biochem. J. 2003, 376, e3–e4. [Google Scholar] [CrossRef]
- Wang, M.; Casey, P.J. Protein Prenylation: Unique Fats Make Their Mark on Biology. Nat. Rev. Mol. Cell Biol. 2016, 17, 110–122. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Chen, X.; Aramsangtienchai, P.; Tong, Z.; Lin, H. Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem. Rev. 2018, 118, 919–988. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Sun, J.; Mao, X.; Wang, J.; Liu, H.; Zheng, H.; Li, X.; Zhao, H.; Zou, D. Heavy Metal Stress-Associated Proteins in Rice and Arabidopsis: Genome-Wide Identification, Phylogenetics, Duplication, and Expression Profiles Analysis. Front. Genet. 2020, 11, 477. [Google Scholar] [CrossRef]
- Roberts, A.G.; Oparka, K.J. Plasmodesmata and the Control of Symplastic Transport. Plant Cell Environ. 2003, 26, 103–124. [Google Scholar] [CrossRef]
- Tilsner, J.; Nicolas, W.; Rosado, A.; Bayer, E.M. Staying Tight: Plasmodesmal Membrane Contact Sites and the Control of Cell-to-Cell Connectivity in Plants. Annu. Rev. Plant Biol. 2016, 67, 337–364. [Google Scholar] [CrossRef]
- Sager, R.E.; Lee, J.-Y. Plasmodesmata at a Glance. J. Cell Sci. 2018, 131, jcs209346. [Google Scholar] [CrossRef]
- Peters, W.S.; Jensen, K.H.; Stone, H.A.; Knoblauch, M. Plasmodesmata and the Problems with Size: Interpreting the Confusion. J. Plant Physiol. 2021, 257, 153341. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lu, H. Plasmodesmata: The Battleground against Intruders. Trends Plant Sci. 2011, 16, 201–210. [Google Scholar] [CrossRef]
- Cheval, C.; Faulkner, C. Plasmodesmal Regulation during Plant–Pathogen Interactions. New Phytol. 2018, 217, 62–67. [Google Scholar] [CrossRef]
- Reagan, B.C.; Burch-Smith, T.M. Viruses Reveal the Secrets of Plasmodesmal Cell Biology. Mol. Plant-Microbe Interact. 2020, 33, 26–39. [Google Scholar] [CrossRef]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of Callose (β-1,3-Glucan) Turnover at Plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef]
- German, L.; Yeshvekar, R.; Benitez-Alfonso, Y. Callose Metabolism and the Regulation of Cell Walls and Plasmodesmata during Plant Mutualistic and Pathogenic Interactions. Plant Cell Environ. 2023, 46, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y. Plasmodesmata: A Signaling Hub at the Cellular Boundary. Curr. Opin. Plant Biol. 2015, 27, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Faulkner, C. Receptor Complex Mediated Regulation of Symplastic Traffic. Trends Plant Sci. 2016, 21, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Calvino, L.; Faulkner, C.; Walshaw, J.; Saalbach, G.; Bayer, E.; Benitez-Alfonso, Y.; Maule, A. Arabidopsis Plasmodesmal Proteome. PLoS ONE 2011, 6, e18880. [Google Scholar] [CrossRef]
- Brault, M.L.; Petit, J.D.; Immel, F.; Nicolas, W.J.; Glavier, M.; Brocard, L.; Gaston, A.; Fouché, M.; Hawkins, T.J.; Crowet, J.; et al. Multiple C2 Domains and Transmembrane Region Proteins (MCTPs) Tether Membranes at Plasmodesmata. EMBO Rep. 2019, 20, e47182. [Google Scholar] [CrossRef]
- Kraner, M.E.; Müller, C.; Sonnewald, U. Comparative Proteomic Profiling of the Choline Transporter-Like1 (CHER1) Mutant Provides Insights into Plasmodesmata Composition of Fully Developed Arabidopsis thaliana Leaves. Plant J. 2017, 92, 696–709. [Google Scholar] [CrossRef]
- Park, S.-H.; Li, F.; Renaud, J.; Shen, W.; Li, Y.; Guo, L.; Cui, H.; Sumarah, M.; Wang, A. NbEXPA1, an α-Expansin, Is Plasmodesmata-Specific and a Novel Host Factor for Potyviral Infection. Plant J. 2017, 92, 846–861. [Google Scholar] [CrossRef]
- Leijon, F.; Melzer, M.; Zhou, Q.; Srivastava, V.; Bulone, V. Proteomic Analysis of Plasmodesmata from Populus Cell Suspension Cultures in Relation With Callose Biosynthesis. Front. Plant Sci. 2018, 9, 1681. [Google Scholar] [CrossRef]
- Gombos, S.; Miras, M.; Howe, V.; Xi, L.; Pottier, M.; Kazemein Jasemi, N.S.; Schladt, M.; Ejike, J.O.; Neumann, U.; Hänsch, S.; et al. A High-Confidence Physcomitrium Patens Plasmodesmata Proteome by Iterative Scoring and Validation Reveals Diversification of Cell Wall Proteins during Evolution. New Phytol. 2023, 238, 637–653. [Google Scholar] [CrossRef]
- Johnston, M.G.; Breakspear, A.; Samwald, S.; Zhang, D.; Papp, D.; Faulkner, C.; de Keijzer, J. Comparative Phyloproteomics Identifies Conserved Plasmodesmal Proteins. J. Exp. Bot. 2023, 74, 1821–1835. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the Worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Weber, H.; Niemann, M.C.E.; Theisl, L.; Leonte, G.; Novák, O.; Werner, T. Arabidopsis HIPP Proteins Regulate Endoplasmic Reticulum-Associated Degradation of CKX Proteins and Cytokinin Responses. Mol. Plant 2021, 14, 1918–1934. [Google Scholar] [CrossRef] [PubMed]
- Dykema, P.E.; Sipes, P.R.; Marie, A.; Biermann, B.J.; Crowell, D.N.; Randall, S.K. A New Class of Proteins Capable of Binding Transition Metals. Plant Mol. Biol. 1999, 41, 139–150. [Google Scholar] [CrossRef]
- Li, J.; Farmer, A.D.; Lindquist, I.E.; Dukowic-Schulze, S.; Mudge, J.; Li, T.; Retzel, E.F.; Chen, C. Characterization of a Set of Novel Meiotically-Active Promoters in Arabidopsis. BMC Plant Biol. 2012, 12, 104. [Google Scholar] [CrossRef]
- Tan, Y.-F.; O’Toole, N.; Taylor, N.L.; Millar, A.H. Divalent Metal Ions in Plant Mitochondria and Their Role in Interactions with Proteins and Oxidative Stress-Induced Damage to Respiratory Function. Plant Physiol. 2010, 152, 747–761. [Google Scholar] [CrossRef]
- Vergnolle, C.; Vaultier, M.-N.; Taconnat, L.; Renou, J.-P.; Kader, J.-C.; Zachowski, A.; Ruelland, E. The Cold-Induced Early Activation of Phospholipase C and D Pathways Determines the Response of Two Distinct Clusters of Genes in Arabidopsis Cell Suspensions. Plant Physiol. 2005, 139, 1217–1233. [Google Scholar] [CrossRef]
- Branco-Price, C.; Kawaguchi, R.; Ferreira, R.B.; Bailey-Serres, J. Genome-Wide Analysis of Transcript Abundance and Translation in Arabidopsis Seedlings Subjected to Oxygen Deprivation. Ann. Bot. 2005, 96, 647–660. [Google Scholar] [CrossRef]
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive Comparison of Auxin-Regulated and Brassinosteroid-Regulated Genes in Arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.-G.; Sharma, A.; Lee, S.-U.; Kim, K.-M.; Yun, B.-W. Nitric Oxide Responsive Heavy Metal-Associated Gene AtHMAD1 Contributes to Development and Disease Resistance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1712. [Google Scholar] [CrossRef]
- Ascencio-Ibáñez, J.T.; Sozzani, R.; Lee, T.-J.; Chu, T.-M.; Wolfinger, R.D.; Cella, R.; Hanley-Bowdoin, L. Global Analysis of Arabidopsis Gene Expression Uncovers a Complex Array of Changes Impacting Pathogen Response and Cell Cycle during Geminivirus Infection. Plant Physiol. 2008, 148, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.J.; Falkenhan, D.; Mader, M.T.; Brininstool, G.; Wischnitzki, E.; Platz, N.; Hudson, A.; Hülskamp, M.; Larkin, J.; Schnittger, A. Transcriptional Profiling of Mature Arabidopsis Trichomes Reveals That NOECK Encodes the MIXTA-Like Transcriptional Regulator MYB106. Plant Physiol. 2008, 148, 1583–1602. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.J.W.; Pearce, S.; van Bolderen-Veldkamp, R.P.; Marshall, A.; Widera, P.; Gilbert, J.; Drost, H.-G.; Bassel, G.W.; Müller, K.; King, J.R.; et al. Transcriptional Dynamics of Two Seed Compartments with Opposing Roles in Arabidopsis Seed Germination. Plant Physiol. 2013, 163, 205–215. [Google Scholar] [CrossRef]
- Chen, X.; Lin, W.-H.; Wang, Y.; Luan, S.; Xue, H.-W. An Inositol Polyphosphate 5-Phosphatase Functions in PHOTOTROPIN1 Signaling in Arabidopis by Altering Cytosolic Ca2+. Plant Cell 2008, 20, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress Induced and Nuclear Localized HIPP26 from Arabidopsis thaliana Interacts via Its Heavy Metal Associated Domain with the Drought Stress Related Zinc Finger Transcription Factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Cowan, G.H.; Roberts, A.G.; Jones, S.; Kumar, P.; Kalyandurg, P.B.; Gil, J.F.; Savenkov, E.I.; Hemsley, P.A.; Torrance, L. Potato Mop-Top Virus Co-Opts the Stress Sensor HIPP26 for Long-Distance Movement. Plant Physiol. 2018, 176, 2052–2070. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, S.; Li, H.-Y.; Tsao, S.-W.; Chye, M.-L. Arabidopsis thaliana Acyl-CoA-Binding Protein ACBP2 Interacts with Heavy-Metal-Binding Farnesylated Protein AtFP6. New Phytol. 2009, 181, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Sakuma, Y.; Kasuga, M.; Ito, Y.; Seki, M.; Goda, H.; Shimada, Y.; Yoshida, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Identification of Cold-Inducible Downstream Genes of the Arabidopsis DREB1A/CBF3 Transcriptional Factor Using Two Microarray Systems. Plant J. 2004, 38, 982–993. [Google Scholar] [CrossRef]
- Oono, Y.; Seki, M.; Satou, M.; Iida, K.; Akiyama, K.; Sakurai, T.; Fujita, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Monitoring Expression Profiles of Arabidopsis Genes during Cold Acclimation and Deacclimation Using DNA Microarrays. Funct. Integr. Genom. 2006, 6, 212–234. [Google Scholar] [CrossRef]
- Lim, C.J.; Yang, K.A.; Hong, J.K.; Choi, J.S.; Yun, D.-J.; Hong, J.C.; Chung, W.S.; Lee, S.Y.; Cho, M.J.; Lim, C.O. Gene Expression Profiles during Heat Acclimation in Arabidopsis thaliana Suspension-Culture Cells. J. Plant Res. 2006, 119, 373–383. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Molesini, B.; DalCorso, G.; Pennisi, F.; Pandolfini, T.; Furini, A. The Tomato Metallocarboxypeptidase Inhibitor I, Which Interacts with a Heavy Metal-Associated Isoprenylated Protein, Is Implicated in Plant Response to Cadmium. Molecules 2020, 25, 700. [Google Scholar] [CrossRef]
- Lee, T.A.; Bailey-Serres, J. Integrative Analysis from the Epigenome to Translatome Uncovers Patterns of Dominant Nuclear Regulation during Transient Stress. Plant Cell 2019, 31, 2573–2595. [Google Scholar] [CrossRef]
- Guan, Y.; Nothnagel, E.A. Binding of Arabinogalactan Proteins by Yariv Phenylglycoside Triggers Wound-Like Responses in Arabidopsis Cell Cultures. Plant Physiol. 2004, 135, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Guo, L.; Lu, F.; Feng, X.; He, K.; Wei, L.; Chen, Z.; Qu, L.-J.; Gu, H. A Subgroup of MYB Transcription Factor Genes Undergoes Highly Conserved Alternative Splicing in Arabidopsis and Rice. J. Exp. Bot. 2006, 57, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Naitou, T.; Koizumi, N.; Yamashino, T.; Sakakibara, H.; Mizuno, T. Combinatorial Microarray Analysis Revealing Arabidopsis Genes Implicated in Cytokinin Responses through the His→Asp Phosphorelay Circuitry. Plant Cell Physiol. 2005, 46, 339–355. [Google Scholar] [CrossRef]
- Kirk, P.; Benitez-Alfonso, Y. Plasmodesmata Structural Components and Their Role in Signaling and Plant Development. In Plasmodesmata: Methods and Protocols; Benitez-Alfonso, Y., Heinlein, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; pp. 3–22. ISBN 978-1-07-162132-5. [Google Scholar]
- Hurst, C.H.; Hemsley, P.A. Current Perspective on Protein S-Acylation in Plants: More than Just a Fatty Anchor? J. Exp. Bot. 2015, 66, 1599–1606. [Google Scholar] [CrossRef]
- Hemsley, P.A.; Weimar, T.; Lilley, K.S.; Dupree, P.; Grierson, C.S. A Proteomic Approach Identifies Many Novel Palmitoylated Proteins in Arabidopsis. New Phytol. 2013, 197, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.R.; Li, J.; Wang, X.; Liao, L.; Lee, J.-Y. Targeting of Plasmodesmal Proteins Requires Unconventional Signals. Plant Cell 2023, 35, 3035–3052. [Google Scholar] [CrossRef]
- Thomas, C.L.; Bayer, E.M.; Ritzenthaler, C.; Fernandez-Calvino, L.; Maule, A.J. Specific Targeting of a Plasmodesmal Protein Affecting Cell-to-Cell Communication. PLoS Biol. 2008, 6, e7. [Google Scholar] [CrossRef]
- Zavaliev, R.; Dong, X.; Epel, B.L. Glycosylphosphatidylinositol (GPI) Modification Serves as a Primary Plasmodesmal Sorting Signal. Plant Physiol. 2016, 172, 1061–1073. [Google Scholar] [CrossRef]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. Identification of a Functional Plasmodesmal Localization Signal in a Plant Viral Cell-to-Cell-Movement Protein. mBio 2016, 7, e02052-15. [Google Scholar] [CrossRef]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. The Plasmodesmal Localization Signal of TMV MP Is Recognized by Plant Synaptotagmin SYTA. mBio 2018, 9, e01314-18. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Zeng, J.; Yu, H.; Li, Y.; Yuan, C. Identification of Two Additional Plasmodesmata Localization Domains in the Tobacco Mosaic Virus Cell-to-Cell-Movement Protein. Biochem. Biophys. Res. Commun. 2020, 521, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Grison, M.S.; Kirk, P.; Brault, M.L.; Wu, X.N.; Schulze, W.X.; Benitez-Alfonso, Y.; Immel, F.; Bayer, E.M. Plasma Membrane-Associated Receptor-like Kinases Relocalize to Plasmodesmata in Response to Osmotic Stress. Plant Physiol. 2019, 181, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Functional Characterization of a Heavy Metal Binding Protein CdI19 from Arabidopsis. Plant J. 2002, 32, 165–173. [Google Scholar] [CrossRef]
- Zschiesche, W.; Barth, O.; Daniel, K.; Böhme, S.; Rausche, J.; Humbeck, K. The Zinc-Binding Nuclear Protein HIPP3 Acts as an Upstream Regulator of the Salicylate-Dependent Plant Immunity Pathway and of Flowering Time in Arabidopsis thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Winge, D.R. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and Metal Sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.E.J.; Bridwell-Rabb, J.; Drennan, C.L. Metalloprotein Crystallography: More than a Structure. Acc. Chem. Res. 2016, 49, 695–702. [Google Scholar] [CrossRef]
- Kami, K.; Takeya, R.; Sumimoto, H.; Kohda, D. Diverse Recognition of Non-PxxP Peptide Ligands by the SH3 Domains from P67phox, Grb2 and Pex13p. EMBO J. 2002, 21, 4268–4276. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. In Planta Mutagenesis of Src Homology 3 Domain-like Fold of NdhS, a Ferredoxin-Binding Subunit of the Chloroplast NADH Dehydrogenase-like Complex in Arabidopsis: A Conserved ARG-193 Plays a Critical Role in Ferredoxin Binding. J. Biol. Chem. 2013, 288, 36328–36337. [Google Scholar] [CrossRef]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Hayashi, N.; Takahashi, A.; Hirochika, H.; Okuno, K.; et al. Loss of Function of a Proline-Containing Protein Confers Durable Disease Resistance in Rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Li, Y.; Chen, Y.; Tang, X.; Zhao, J.; Yu, F.; Wang, H.; Xiao, J.; Liu, J.; et al. Isoprenylation Modification Is Required for HIPP1-Mediated Powdery Mildew Resistance in Wheat. Plant Cell Environ. 2023, 46, 288–305. [Google Scholar] [CrossRef] [PubMed]
- O’Lexy, R.; Kasai, K.; Clark, N.; Fujiwara, T.; Sozzani, R.; Gallagher, K.L. Exposure to Heavy Metal Stress Triggers Changes in Plasmodesmatal Permeability via Deposition and Breakdown of Callose. J. Exp. Bot. 2018, 69, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Rono, J.K.; Zhang, B.Q.; Liu, X.S.; Wang, M.Q.; Wang, L.L.; Wu, X.C.; Chen, X.; Cao, H.W.; Yang, Z.M. Identification of Novel Rice (Oryza sativa) HPP and HIPP Genes Tolerant to Heavy Metal Toxicity. Ecotoxicol. Environ. Saf. 2019, 175, 8–18. [Google Scholar] [CrossRef]
- Cao, M.; Morozov, S.; Komatsu, K.; Wei, Z.; Sun, Z.; Jiang, C.; Shan, S.; Huang, Y.; Mao, C.; Zhang, H.; et al. The C-Terminal Transmembrane Domain of Cowpea Mild Mottle Virus TGBp2 Is Critical for Plasmodesmata Localization and for Its Interaction with TGBp1 and TGBp3. Front. Microbiol. 2022, 13, 860695. [Google Scholar] [CrossRef]
- Xiong, S.; Kong, X.; Chen, G.; Tian, L.; Qian, D.; Zhu, Z.; Qu, L.Q. Metallochaperone OsHIPP9 Is Involved in the Retention of Cadmium and Copper in Rice. Plant Cell Environ. 2023, 46, 1946–1961. [Google Scholar] [CrossRef]
- Ma, L.; An, R.; Jiang, L.; Zhang, C.; Li, Z.; Zou, C.; Yang, C.; Pan, G.; Lübberstedt, T.; Shen, Y. Effects of ZmHIPP on Lead Tolerance in Maize Seedlings: Novel Ideas for Soil Bioremediation. J. Hazard. Mater. 2022, 430, 128457. [Google Scholar] [CrossRef]
- Kabir, A.H.; Das, U.; Rahman, M.A.; Lee, K.-W. Silicon Induces Metallochaperone-Driven Cadmium Binding to the Cell Wall and Restores Redox Status through Elevated Glutathione in Cd-Stressed Sugar Beet. Physiol. Plant. 2021, 173, 352–368. [Google Scholar] [CrossRef]
- Ye, X.; Liu, C.; Yan, H.; Wan, Y.; Wu, Q.; Wu, X.; Zhao, G.; Zou, L.; Xiang, D. Genome-Wide Identification and Transcriptome Analysis of the Heavy Metal-Associated (HMA) Gene Family in Tartary Buckwheat and Their Regulatory Roles under Cadmium Stress. Gene 2022, 847, 146884. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Z.; Li, J.; Yao, Q.; Tan, W.; Xing, W.; Lu, Z. Effects of Cadmium Stress on the Morphology, Physiology, Cellular Ultrastructure, and BvHIPP24 Gene Expression of Sugar Beet (Beta vulgaris L.). Int. J. Phytoremediat. 2022, 25, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, Y.; Mu, S.; Yan, D.; Xu, X.; Zhang, L.; Xu, B. Changes in Phenotype and Gene Expression under Lead Stress Revealed Key Genetic Responses to Lead Tolerance in Medicago sativa L. Gene 2021, 791, 145714. [Google Scholar] [CrossRef]
- Lu, Y.; Peng, F.; Wang, Y.; Yang, Z.; Li, H. Transcriptomic Analysis Reveals the Molecular Mechanisms of Boehmeria nivea L. in Response to Antimonite and Antimonate Stresses. J. Environ. Manag. 2023, 343, 118195. [Google Scholar] [CrossRef]
- Parasyri, A.; Barth, O.; Zschiesche, W.; Humbeck, K. The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses. Plants 2022, 11, 2851. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yamamoto, N.; Yang, G.; Lin, H.; Jiang, L.; Liu, Y.; Zheng, A. A Small Secreted Protein, RsMf8HN, in Rhizoctonia Solani Triggers Plant Immune Response, Which Interacts with Rice OsHIPP28. Microbiol. Res. 2023, 266, 127219. [Google Scholar] [CrossRef]
- Brunkard, J.O.; Zambryski, P.C. Plasmodesmata Enable Multicellularity: New Insights into Their Evolution, Biogenesis, and Functions in Development and Immunity. Curr. Opin. Plant Biol. 2017, 35, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Jackson, D. Plasmodesmata-Mediated Cell-to-Cell Communication in the Shoot Apical Meristem: How Stem Cells Talk. Plants 2017, 6, 12. [Google Scholar] [CrossRef]
- Band, L.R. Auxin Fluxes through Plasmodesmata. New Phytol. 2021, 231, 1686–1692. [Google Scholar] [CrossRef]
- Werner, T.; Köllmer, I.; Bartrina, I.; Holst, K.; Schmülling, T. New Insights into the Biology of Cytokinin Degradation. Plant Biol. 2006, 8, 371–381. [Google Scholar] [CrossRef]
- Ormenese, S.; Bernier, G.; Périlleux, C. Cytokinin Application to the Shoot Apical Meristem of Sinapis alba Enhances Secondary Plasmodesmata Formation. Planta 2006, 224, 1481–1484. [Google Scholar] [CrossRef]
- Horner, W.; Brunkard, J.O. Cytokinins Stimulate Plasmodesmatal Transport in Leaves. Front. Plant Sci. 2021, 12, 674128. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Šamaj, J.; Napier, R.; Volkmann, D. Maize Calreticulin Localizes Preferentially to Plasmodesmata in Root Apex. Plant J. 1999, 19, 481–488. [Google Scholar] [CrossRef]
- Laporte, C.; Vetter, G.; Loudes, A.-M.; Robinson, D.G.; Hillmer, S.; Stussi-Garaud, C.; Ritzenthaler, C. Involvement of the Secretory Pathway and the Cytoskeleton in Intracellular Targeting and Tubule Assembly of Grapevine fanleaf Virus Movement Protein in Tobacco BY-2 Cells. Plant Cell 2003, 15, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Thomas, C.L.; Maule, A.J. Plasmodesmata in Arabidopsis thaliana Suspension Cells. Protoplasma 2004, 223, 93–102. [Google Scholar] [CrossRef]
- Liu, D.Y.T.; Smith, P.M.C.; Barton, D.A.; Day, D.A.; Overall, R.L. Characterisation of Arabidopsis Calnexin 1 and Calnexin 2 in the Endoplasmic Reticulum and at Plasmodesmata. Protoplasma 2017, 254, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.-K.; Wang, X.; Toscano-Morales, R.; Lin, J.; Lucas, W.J. Plasmodesmal Endoplasmic Reticulum Proteins Regulate Intercellular Trafficking of Cucumber Mosaic Virus in Arabidopsis. J. Exp. Bot. 2023, erad190. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, M.; Yang, Y.; Wang, K.; Che, Y.; Yang, S.; Wang, J.; Yu, X.; Li, L.; Wu, S.; et al. CDC48B Facilitates the Intercellular Trafficking of SHORT-ROOT during Radial Patterning in Roots. J. Integr. Plant Biol. 2022, 64, 843–858. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Li, L.; Xi, Y.; Wang, J.; Mao, X.; Jing, R. RING Finger E3 Ubiquitin Ligase Gene TaAIRP2-1B Controls Spike Length in Wheat. J. Exp. Bot. 2023, erad226. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, L.; Shen, L.; Yu, H. NaKR1 Regulates Long-Distance Movement of FLOWERING LOCUS T in Arabidopsis. Nat. Plants 2016, 2, 16075. [Google Scholar] [CrossRef]
- Holdaway-Clarke, T.L.; Walker, N.A.; Hepler, P.K.; Overall, R.L. Physiological Elevations in Cytoplasmic Free Calcium by Cold or Ion Injection Result in Transient Closure of Higher Plant Plasmodesmata. Planta 2000, 210, 329–335. [Google Scholar] [CrossRef]
- Rinne, P.L.H.; Kaikuranta, P.M.; Van Der Schoot, C. The Shoot Apical Meristem Restores Its Symplasmic Organization during Chilling-Induced Release from Dormancy. Plant J. 2001, 26, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Sowiński, P. Closure of Plasmodesmata in Maize (Zea mays) at Low Temperature: A New Mechanism for Inhibition of Photosynthesis. Ann. Bot. 2010, 106, 675–686. [Google Scholar] [CrossRef]
- Rinne, P.L.H.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjärvi, J.; van der Schoot, C. Chilling of Dormant Buds Hyperinduces FLOWERING LOCUS T and Recruits GA-Inducible 1,3-β-Glucanases to Reopen Signal Conduits and Release Dormancy in Populus. Plant Cell 2011, 23, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, W.; Sun, C.; Xu, C.; Li, S.; Li, P.; Xu, H.; Zhu, D.; Li, M.; Yang, L.; et al. Cold Stress Induces Malformed Tomato Fruits by Breaking the Feedback Loops of Stem Cell Regulation in Floral Meristem. New Phytol. 2023, 237, 2268–2283. [Google Scholar] [CrossRef] [PubMed]
- Ganusova, E.E.; Burch-Smith, T.M. Review: Plant-Pathogen Interactions through the Plasmodesma Prism. Plant Sci. 2019, 279, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Iswanto, A.B.B.; Vu, M.H.; Pike, S.; Lee, J.; Kang, H.; Son, G.H.; Kim, J.-Y.; Kim, S.H. Pathogen Effectors: What Do They Do at Plasmodesmata? Mol. Plant Pathol. 2022, 23, 795–804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barr, Z.K.; Werner, T.; Tilsner, J. Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function. Plants 2023, 12, 3015. https://doi.org/10.3390/plants12163015
Barr ZK, Werner T, Tilsner J. Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function. Plants. 2023; 12(16):3015. https://doi.org/10.3390/plants12163015
Chicago/Turabian StyleBarr, Zoe Kathleen, Tomáš Werner, and Jens Tilsner. 2023. "Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function" Plants 12, no. 16: 3015. https://doi.org/10.3390/plants12163015
APA StyleBarr, Z. K., Werner, T., & Tilsner, J. (2023). Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function. Plants, 12(16), 3015. https://doi.org/10.3390/plants12163015








