Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton
Abstract
1. Introduction
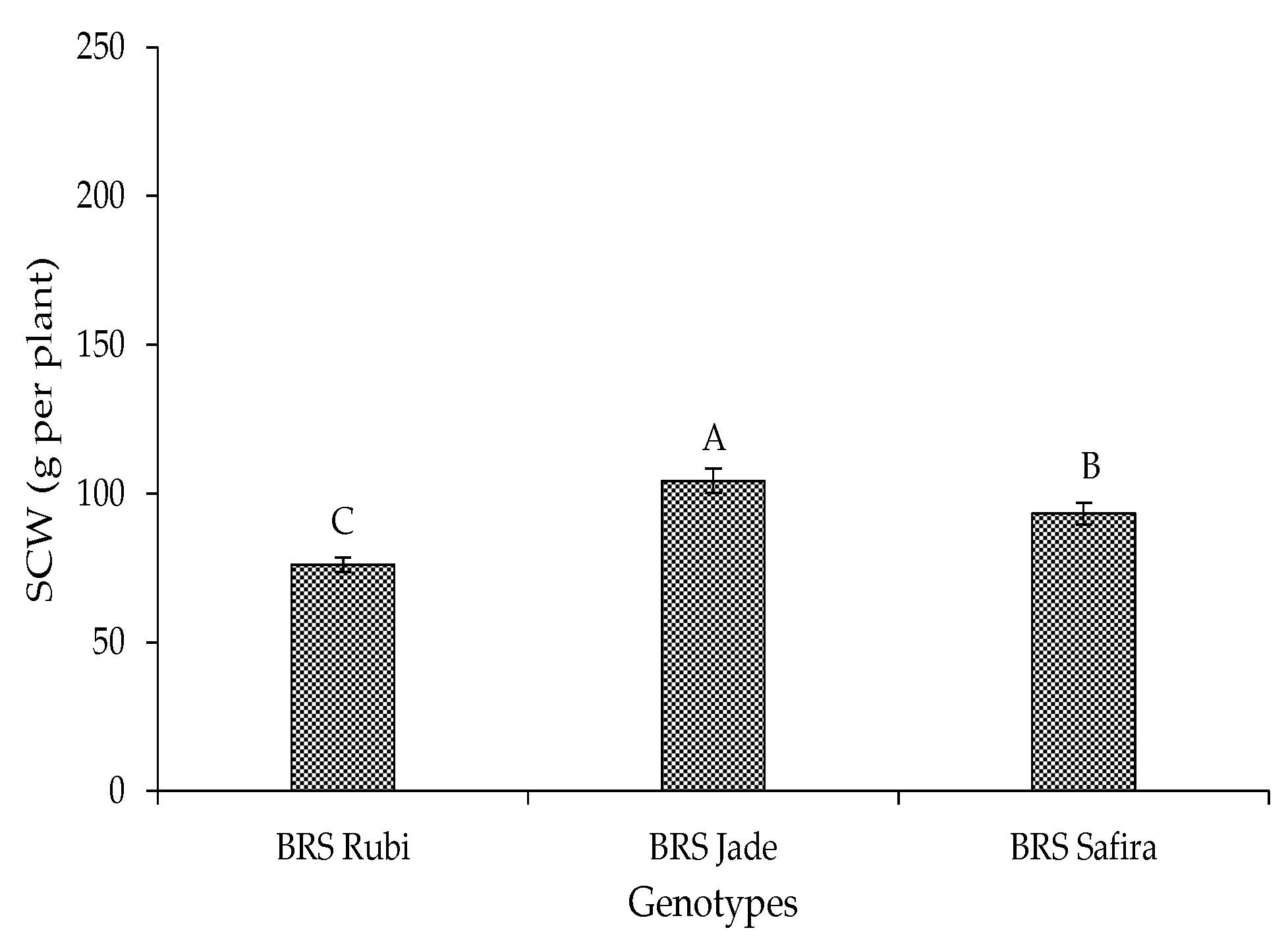
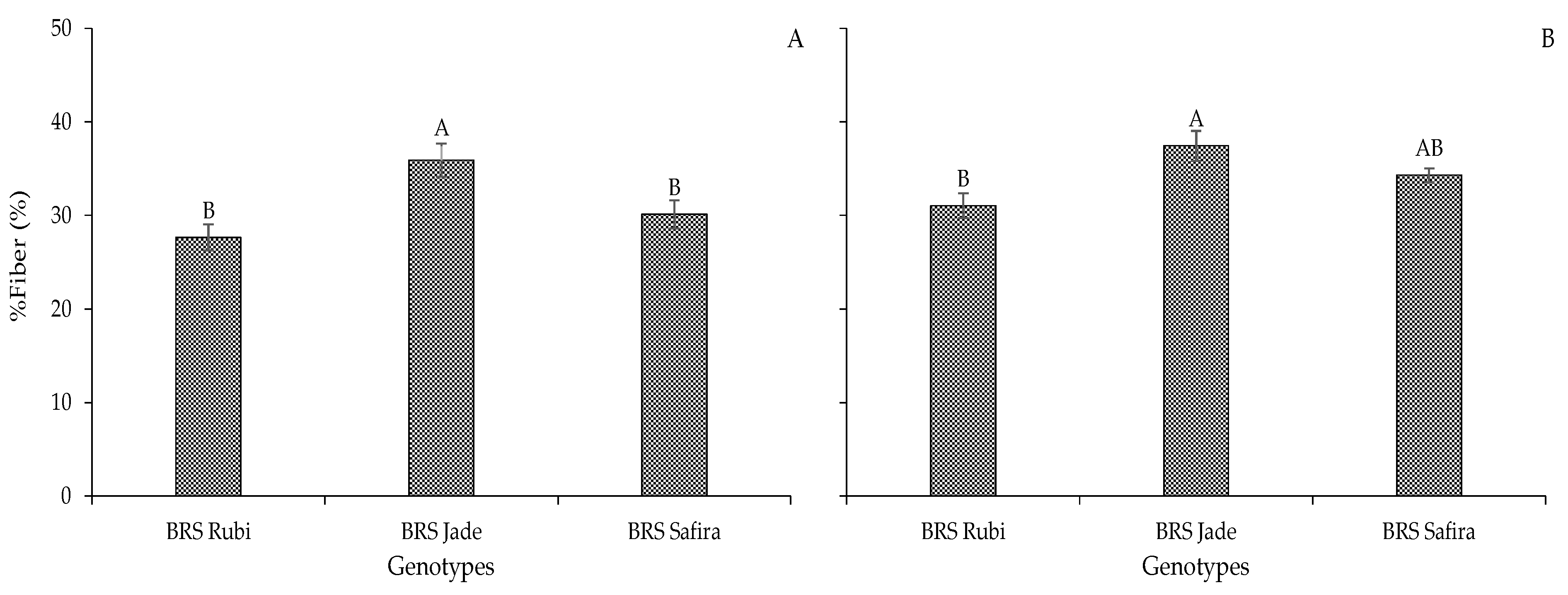
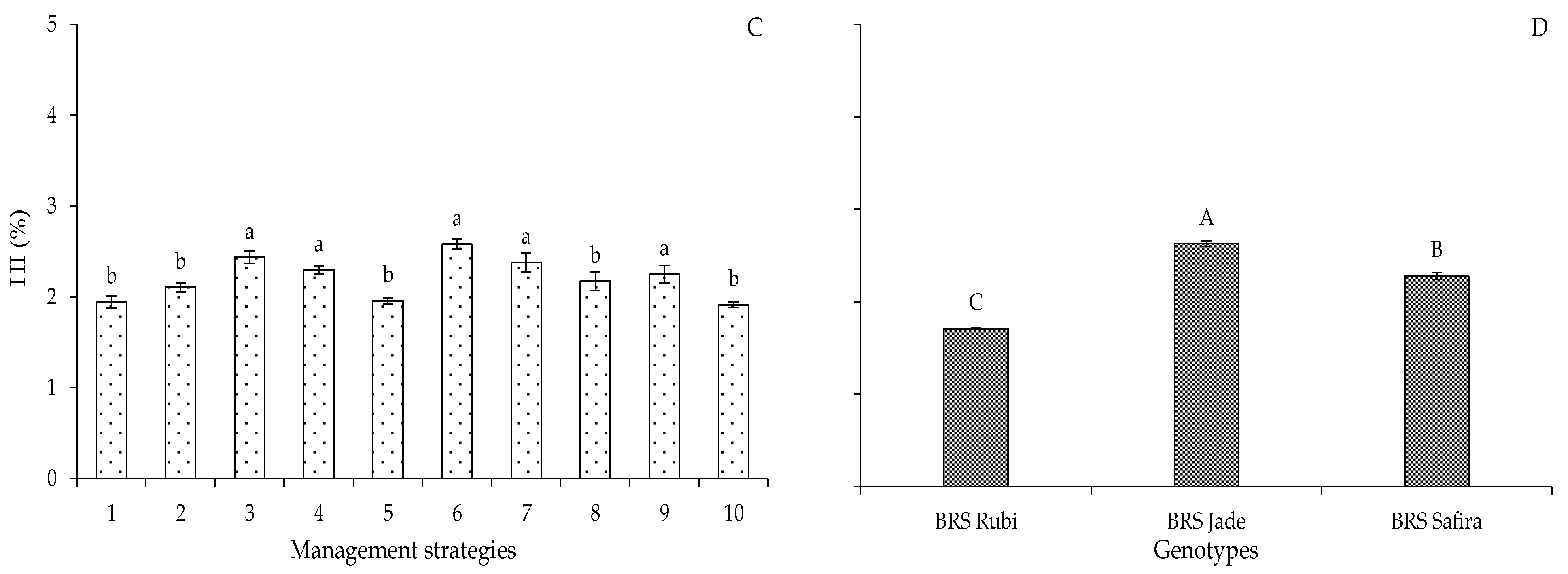
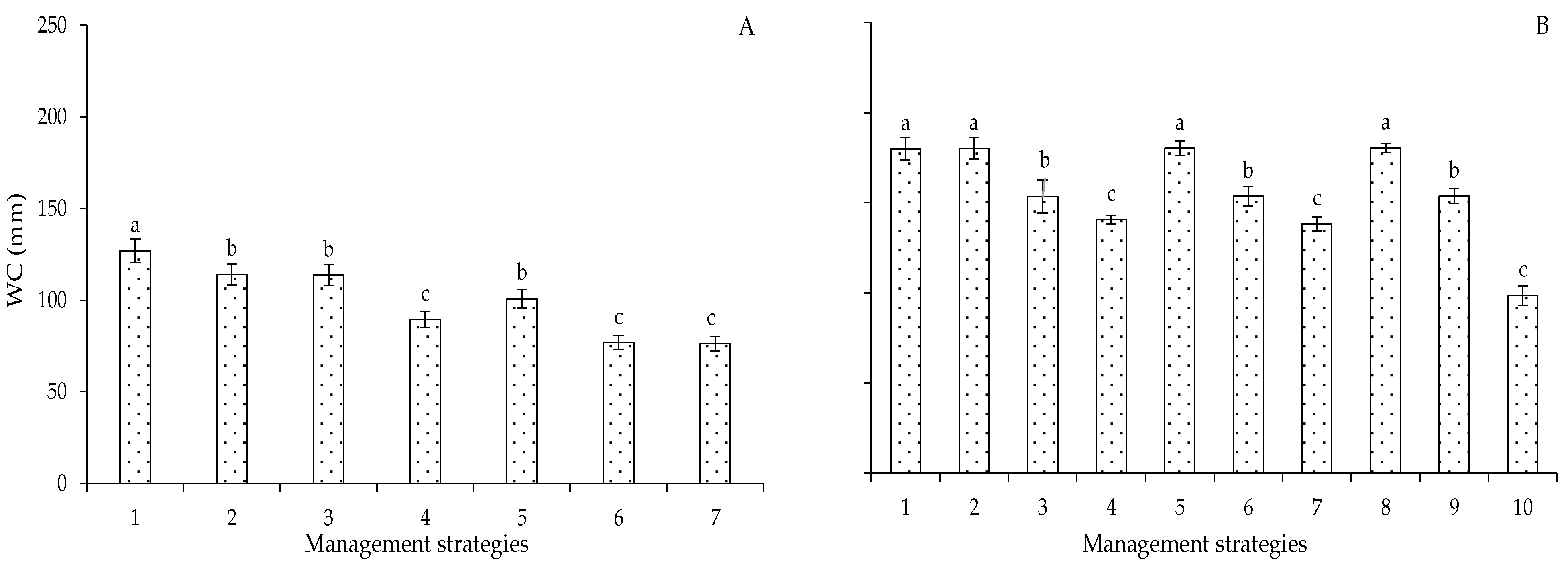
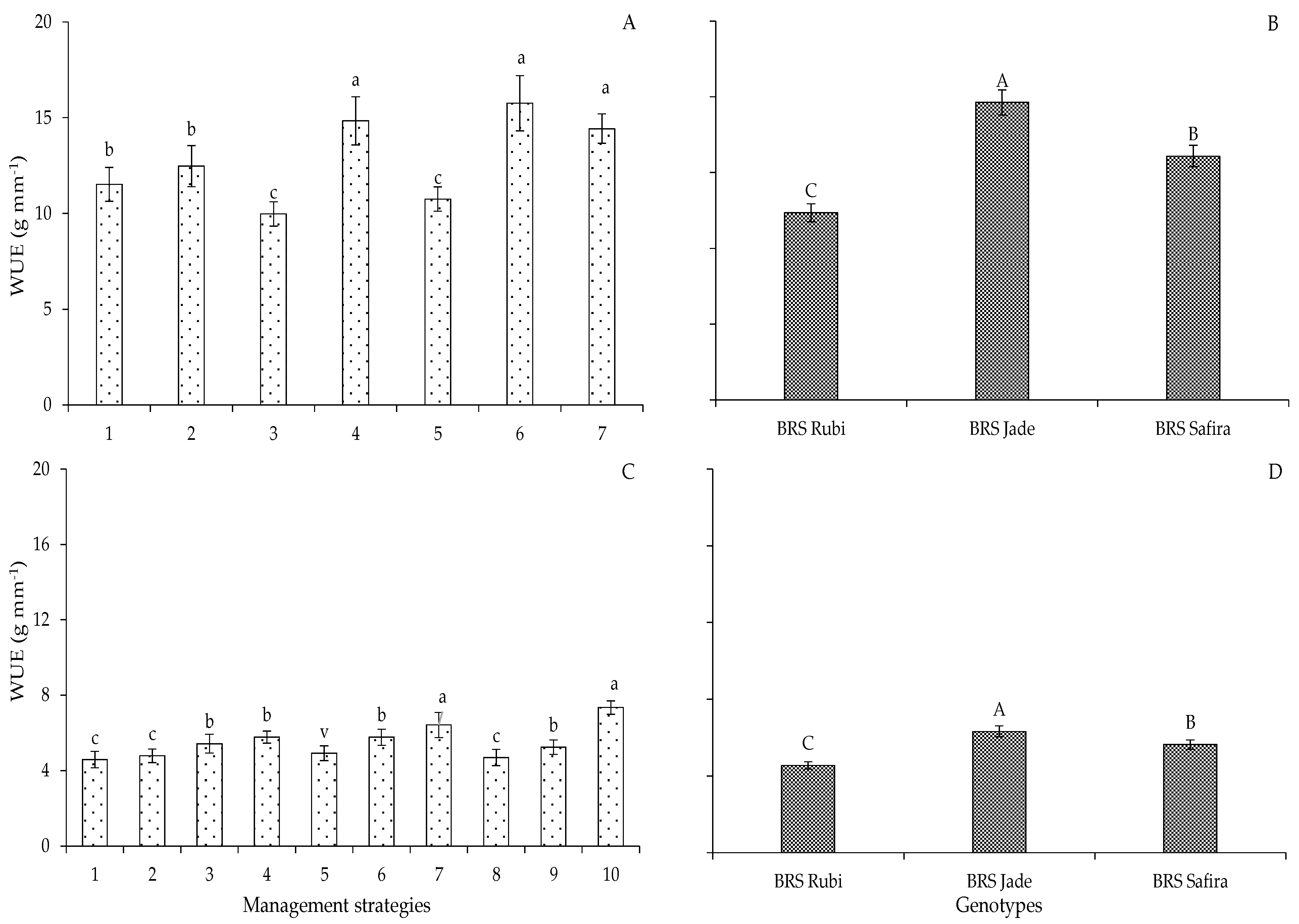
2. Results and Discussion
3. Materials and Methods
3.1. Location of the Experiment
3.2. Plant Material
3.3. Treatments and Experimental Design
3.4. Experiment Setup and Conduction
3.5. Variables Analyzed
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niu, Y.; Li, J.; Sun, F.; Song, T.; Han, B.; Liu, Z.; Su, P. Comparative transcriptome analysis reveals the key genes and pathways involved in drought stress response of two wheat (Triticum aestivum L.) varieties. Genomics 2023, 115, e110688. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Tang, H.; Fu, L.; Tan, J.; Guo, Y. A drought stress-sensing technique based on wavelet entropy of chlorophyll fluorescence excited with pseudo-random binary sequence. Comput. Electron. Agric. 2023, 210, e107933. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, R.; Wang, L.; Li, J.; Ma, B.; Ma, F.; Li, M. Overexpression of MdFRK2 enhances apple drought resistance by promoting carbohydrate metabolism and root growth under drought stress. Hortic. Plant J. 2023, 475, 1–32. [Google Scholar] [CrossRef]
- Chakhchar, A.; Lamaoui, M.; Aissam, S.; Ferradous, A.; Wahbi, S.; El Mousadik, A.; El Modafar, C. Using chlorophyll fluorescence, photosynthetic enzymes and pigment composition to discriminate drought-tolerant ecotypes of Argania spinosa. Plant Biosyst. 2018, 152, 356–367. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021, 10, 259. [Google Scholar] [CrossRef]
- Soufiani, M.; Chakhchar, A.; Aissam, S.; Ferradous, A.; Douira, A.; Meddich, A.; El Modafar, C. Can an indigenous consortium of arbuscular mycorrhizae effectively mitigate drought stress in argan trees (Argania spinosa L. Skeels) by modulating mineral nutrition and phosphatase activities. S. Afr. J. Bot. 2023, 159, 439–446. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Fan, R.; Sun, F.; Chen, Q.; Shi, J.; Yan, G. Regulating drought tolerance in cotton by the expression of a specific allele of heat shock protein 70. Ind. Crops Prod. 2023, 202, e116820. [Google Scholar] [CrossRef]
- Wu, F.; Guo, S.; Huang, W.; Han, Y.; Wang, Z.; Feng, L.; Li, Y. Adaptation of cotton production to climate change by sowing date optimization and precision resource management. Ind. Crops Prod. 2023, 203, e117167. [Google Scholar] [CrossRef]
- ABRAPA. Associação Brasileira dos Produtores de Algodão. Algodão no Brasil. Available online: https://www.abrapa.com.br/Paginas/dados/algodao-no-brasil.aspx (accessed on 29 March 2022).
- CONAB. Companhia Nacional de Abastecimento. Conab. Available online: https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras/itemlist/category/898-algodao (accessed on 10 February 2022).
- Zonta, J.H.; Bezerra, J.R.C.; Sofiatti, V.; Farias, F.J.C.; Carvalho, L.P.D. Efeito da irrigação no rendimento e qualidade de fibras em cultivares de algodoeiro herbáceo. Rev. Caatinga 2015, 28, 43–52. [Google Scholar] [CrossRef][Green Version]
- Soares, L.A.D.A.; Fernandes, P.D.; de Lima, G.S.; Gheyi, H.R.; Nobre, R.G.; Sá, F.V.D.S.; Moreira, R.C.L. Saline water irrigation strategies in two production cycles of naturally colored cotton. Irrig. Sci. 2020, 38, 401–413. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Silva, C.R.C.; Pereira, R.F.; Ramos, J.P.C.; Melo Filho, P.A.; Cavalcanti, J.J.V.; Santos, R.C. Characterization of water-stress tolerant cotton cultivars based on plant growth and in activity of antioxidant enzymes. Afr. J. Agric. Res. 2016, 11, 3763–3770. [Google Scholar] [CrossRef]
- Sun, C.; Ali, K.; Yan, K.; Fiaz, S.; Dormatey, R.; Bi, Z.; Bai, J. Exploration of epigenetics for improvement of drought and other stress resistance in crops: A review. Plants 2021, 10, 1226. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Paixão, J.F.R. Melhoramento da Tolerância de Plantas a Estresses Biótico e Abiótico: Uso de Regulação Transcricional por CRISPR/dCas9 e Algodão Transgênico. 2018. 119f. Ph.D. Thesis, Tese de Doutorado (Doutorado em Biotecnologia e Biodiversidade)—Programa de Biotecnologia e Biodiversidade da Universidade de Brasília, Brasília, DF, Brazil, 2018. [Google Scholar]
- Nazim, M.; ALI, M.; Shahzad, K.; Ahmad, F.; Nawaz, F.; Amin, M.; Datta, R. Kaolin and Jasmonic acid improved cotton productivity under water stress conditions. Saudi J. Biol. Sci. 2021, 28, 6606–6614. [Google Scholar] [CrossRef]
- Kholliyev, A.E.; Norboyeva, U.T.; Kholov, Y.D.; Boltayeva, Z.A. Productivity of cotton varieties in soil salinity and water deficiency. Am. J. Appl. Sci. 2020, 2, 7–13. [Google Scholar] [CrossRef]
- Makamov, A.; Shavkiev, J.; Kholmuradova, M.; Boyqobilov, U.; Normamatov, I.; Norbekov, J.; Buriev, Z. Cotton genotypes appraisal for morpho-physiological and Yield contributing traits under optimal and deficit irrigated conditions. SABRAO J. Breed. Genet. 2023, 55, 74–89. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Marc, R.A. Plants physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, T.T.; He, L.; Wang, K.; Chen, Y. Qualitative analysis of cotton fiber pigment composition. Text. Res. J. 2021, 91, 456–463. [Google Scholar] [CrossRef]
- Veloso, L.L.D.S.A.; Azevedo, C.A.V.D.; Nobre, R.G.; Lima, G.S.D.; Bezerra, J.R.C.; Silva, A.A.R.D.; Fátima, R.T.D.; Gheyi, H.R.; Soares, L.A.D.A.; Fernandes, P.D.; et al. Production and fiber characteristics of colored cotton cultivares under salt stress and H2O2. Plants 2023, 12, 2090. [Google Scholar] [CrossRef]
- Veesar, N.F.; Baloch, M.J.; Kumbher, M.B.; Chachar, Q.D. Field screening of cotton genotypes for drought tolerance on the basis of yield and fibre traits. Sind. Univ Res. J. 2018, 50, 45–52. [Google Scholar] [CrossRef]
- Bakhsh, A.; Rehman, M.; Salman, S.; Ullah, R. Evaluation of cotton genotypes for seed cotton yield and fiber quality traits under water stress and non-stress conditions. Sarhad J. Agric. 2019, 35, 161–170. [Google Scholar] [CrossRef]
- Cordão, M.A.; Araújo, W.P.; Pereira, J.R.; Zonta, J.H.; Lima, R.F.; Ferreira, F.N. Cultivares de algodoeiro herbáceo sob déficit hídrico aplicado em fases fenológicas. Rev. Verde Agroec. Desenv. Sustent. 2018, 13, 313–321. [Google Scholar] [CrossRef]
- Zonta, J.H.; Brandao, Z.N.; Rodrigues, J.I.D.S.; Sofiatti, V. Cotton response to water deficits at different growth stages. Rev. Caatinga 2017, 30, 980–990. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Ödemiş, B.; Candemir, D.K. The effects of water stress on cotton leaf area and leaf morphology. Kahramanmaraş Sütçü İmam Üniv. Tarım Ve Doğa Derg. 2023, 26, 140–149. [Google Scholar] [CrossRef]
- Pereira Filho, J.V.; Lima Bezerra, F.M.; Cavalcante da Silva, T.; Mareco de Sousa Pereira, C.C. Crescimento vegetativo do feijão caupi cultivado sob salinidade e déficit hídrico. Rev. Bras. Agric. Irr. 2017, 11, 2217–2228. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, RS, Brazil, 2017; 858p. [Google Scholar]
- Vidal, V.M.; Soares, F.A.L.; Teixeira, M.B.; Cunha, F.N.; Santos, L.N.S.; Costa, C.T.S.; Pereira, L.S. Cotton growth in response to water supply in Red Latosol Cerrado. Afr. J. Agric. Res. 2018, 13, 452–459. [Google Scholar] [CrossRef]
- Akhter, Z.; Bi, Z.; Ali, K.; Sun, C.; Fiaz, S.; Haider, F.U.; Bai, J. In response to abiotic stress, DNA methylation confers epigenetic changes in plants. Plants 2021, 10, 1096. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Sá, F.V.S. ; Ferreira Neto, M.; Lima, Y.B.; Paiva, E.P.; Prata, R.C.; Lacerda, C.F.; Brito, M.B. Growth, gas exchange and photochemical efficiency of the cowpea bean under salt stress and phosphorus fertilization. Com. Sci. 2018, 9, 668–679. [Google Scholar] [CrossRef]
- Gomes, I.H.; Cavalcanti, J.J.; Farias, F.J.; Paixão, F.J.; Silva Filho, J.L.D.; Suassuna, N.D. Selection of cotton genotypes for yield and fiber quality under water stress. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 610–617. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Tapia, G.; Jimenéz-Aspee, F.; Márquez, K.; Schmeda-Hirschmann, G. Changes in polyphenol composition, antioxidant capacity and enzyme inhibition in Phaseolus vulgaris L. submitted to hydric stress. Sci. Hortic. 2023, 317, e112070. [Google Scholar] [CrossRef]
- Himanshu, S.K.; Fan, Y.; Ale, S.; Bordovsky, J. Simulated efficient growth-stage-based deficit irrigation strategies for maximizing cotton yield, crop water productivity and net returns. Agric. Water Manag. 2021, 250, 106840. [Google Scholar] [CrossRef]
- EMBRAPA. Empresa Brasileira de Pesquisa Agropecuária. Cultivo de Algodão Irrigado. 2015. Available online: https://www.embrapa.br/busca-de-solucoes-tecnologicas/-/produto-servico/2810/algodao-colorido---brs-jade (accessed on 29 June 2023).
- Basal, H.; Dagdelen, N.; Unay, A.; Yilmaz, E. Effects of deficit drip irrigation ratios on cotton (Gossypium hirsutum L.) yield and fiber quality. J. Agron. Crop Sci. 2009, 195, 19–29. [Google Scholar] [CrossRef]
- Hussein, F.; Janat, M.; Yakoub, A. Assessment of yield and water use efficiency of drip-irrigated cotton (Gossypium hirsutum L.) as affected by deficit irrigation. Turk. J. Agric. For. 2011, 35, 611–621. [Google Scholar] [CrossRef]
- Nguyen, D.; Rieu, I.; Mariani, C.; Van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Soares, L.A.D.A.; Felix, C.M.; Lima, G.S.D.; Gheyi, H.R.; Silva, L.D.A.; Fernandes, P.D. Gas exchange, growth, and production of cotton genotypes under water deficit in phenological stages. Rev. Caatinga 2023, 36, 145–157. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Andrade, F.P.; Silva Filho, J.L. Cultivares de algodão colorido no Brasil. Rev. bras. Ol. Fibros. 2011, 15, 37–44. [Google Scholar]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Algodão Colorido: “Tecnologia Embrapa para a Geração de Emprego e Renda na Agricultura Familiar do Brasil”; BRS Jade: Campina Grande, PB, Brazil, 2009; 2p. [Google Scholar]
- Texeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Revista e ampliada; Embrapa: Brasília, DF, Brazil, 2017. [Google Scholar]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio em ambiente controlado. In Métodos de Pesquisa em Fertilidade do Solo; Oliveira, A.J., Garrido, W.E., Araújo, J.D., Lourenço, S., Eds.; Embrapa-SEA: Brasília, DF, Brazil, 1991; pp. 189–254. [Google Scholar]
- Bernardo, S.S.; Soares, A.A.; Mantovani, E.C. Manual de Irrigação, 9th ed.; UFV: Viçosa, MG, Brazil, 2019; 545p. [Google Scholar]
- Grimes, D.W.; Carter, L.M. A linear rule for direct nondestructive leaf area measurements. J. Agron. 1969, 3, 477–479. [Google Scholar] [CrossRef]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. BRS 336 Cultivar de Alta Qualidade de Fibra para Cultivo no Cerrado e no Semiárido do Brasil; BRS Jade: Campina Grande, PB, Brazil, 2011. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
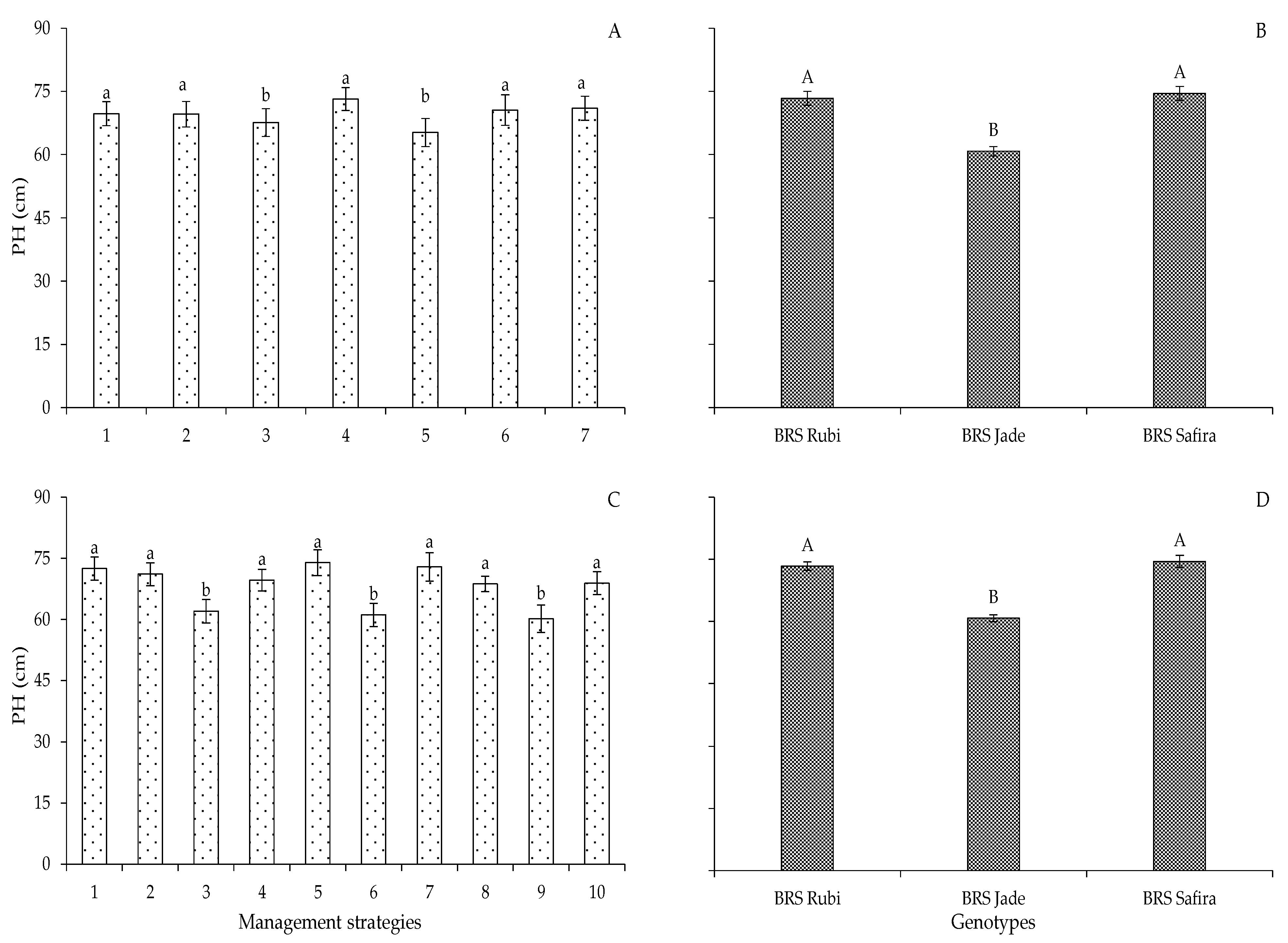
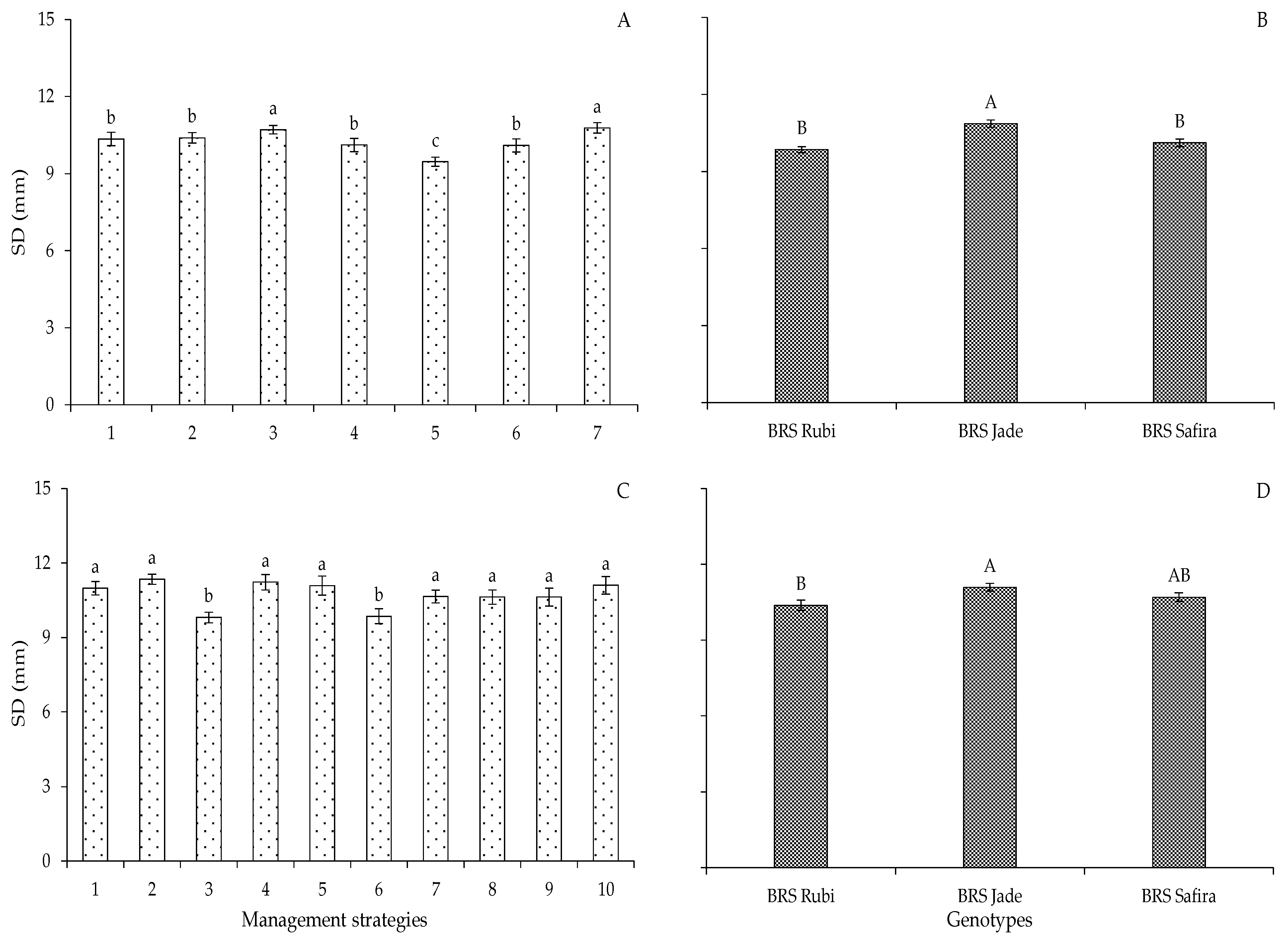
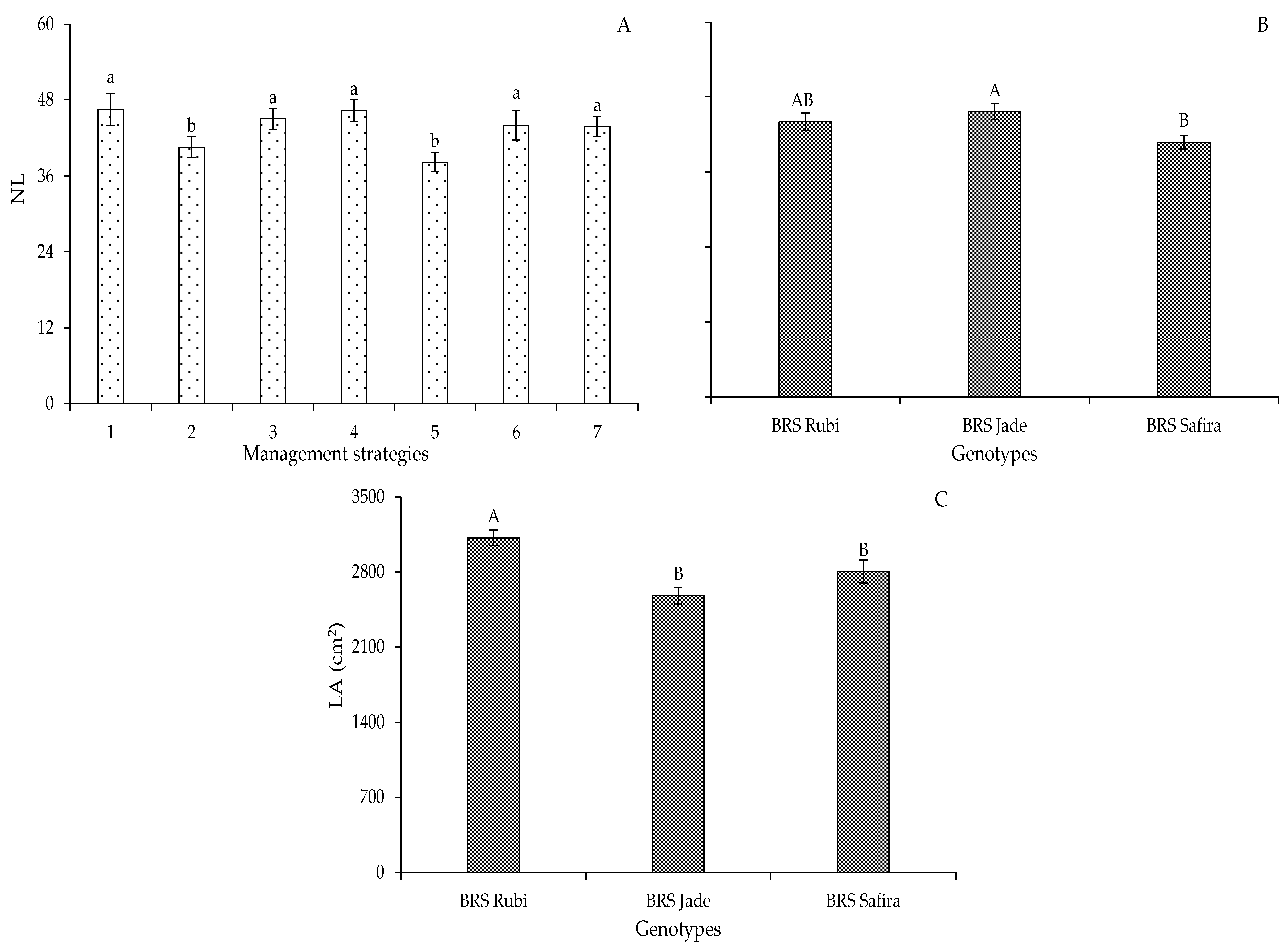
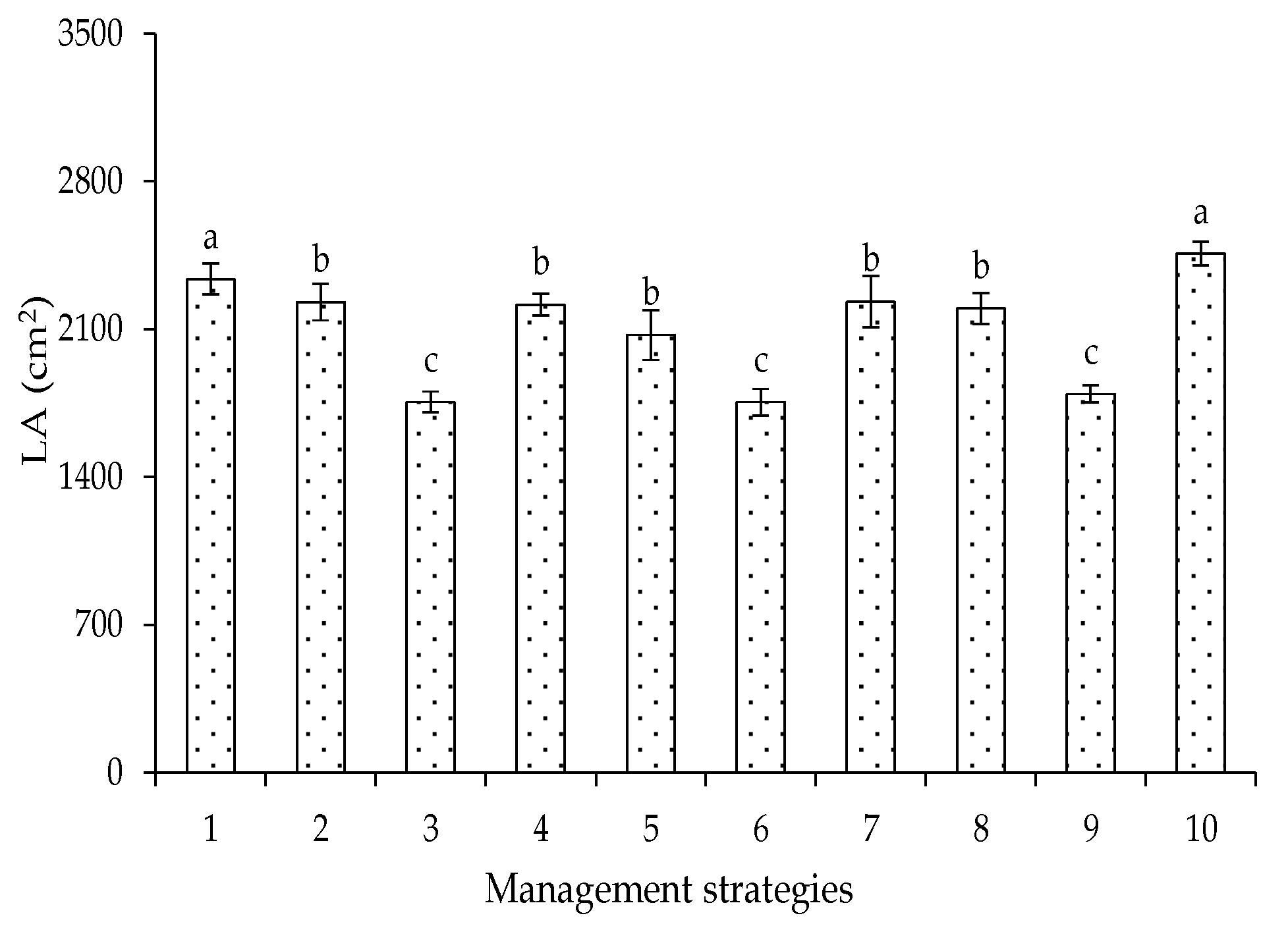

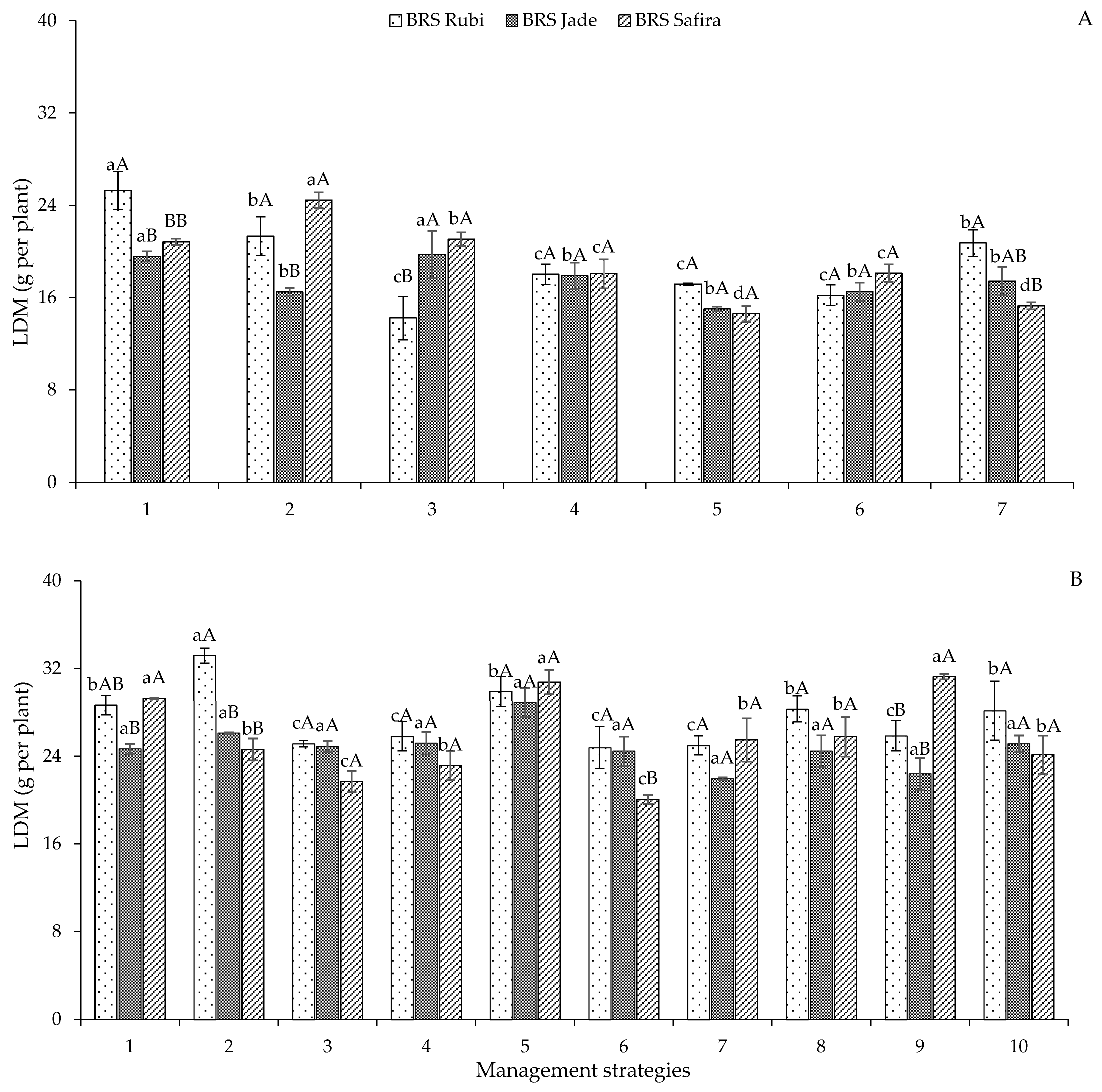
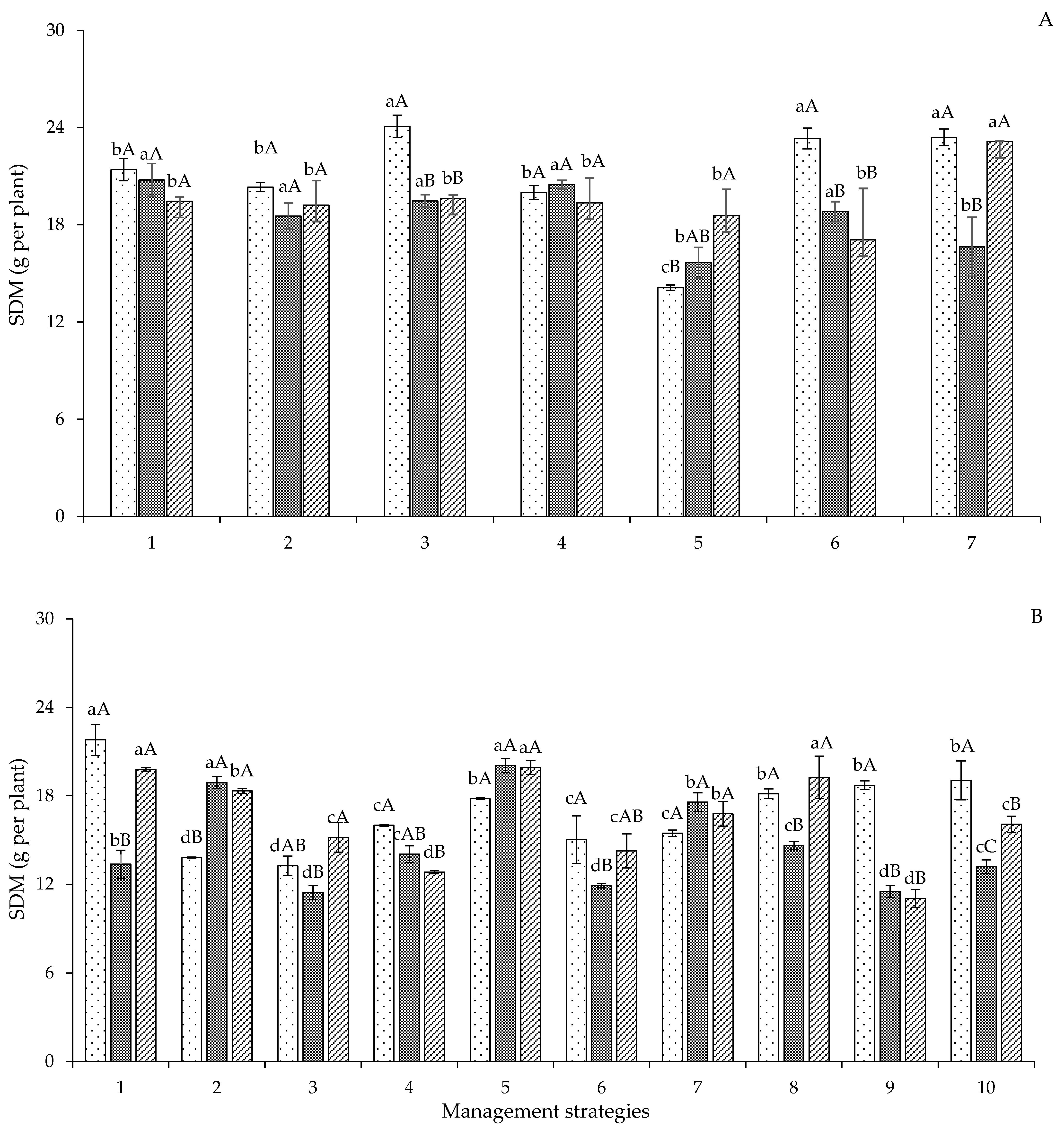
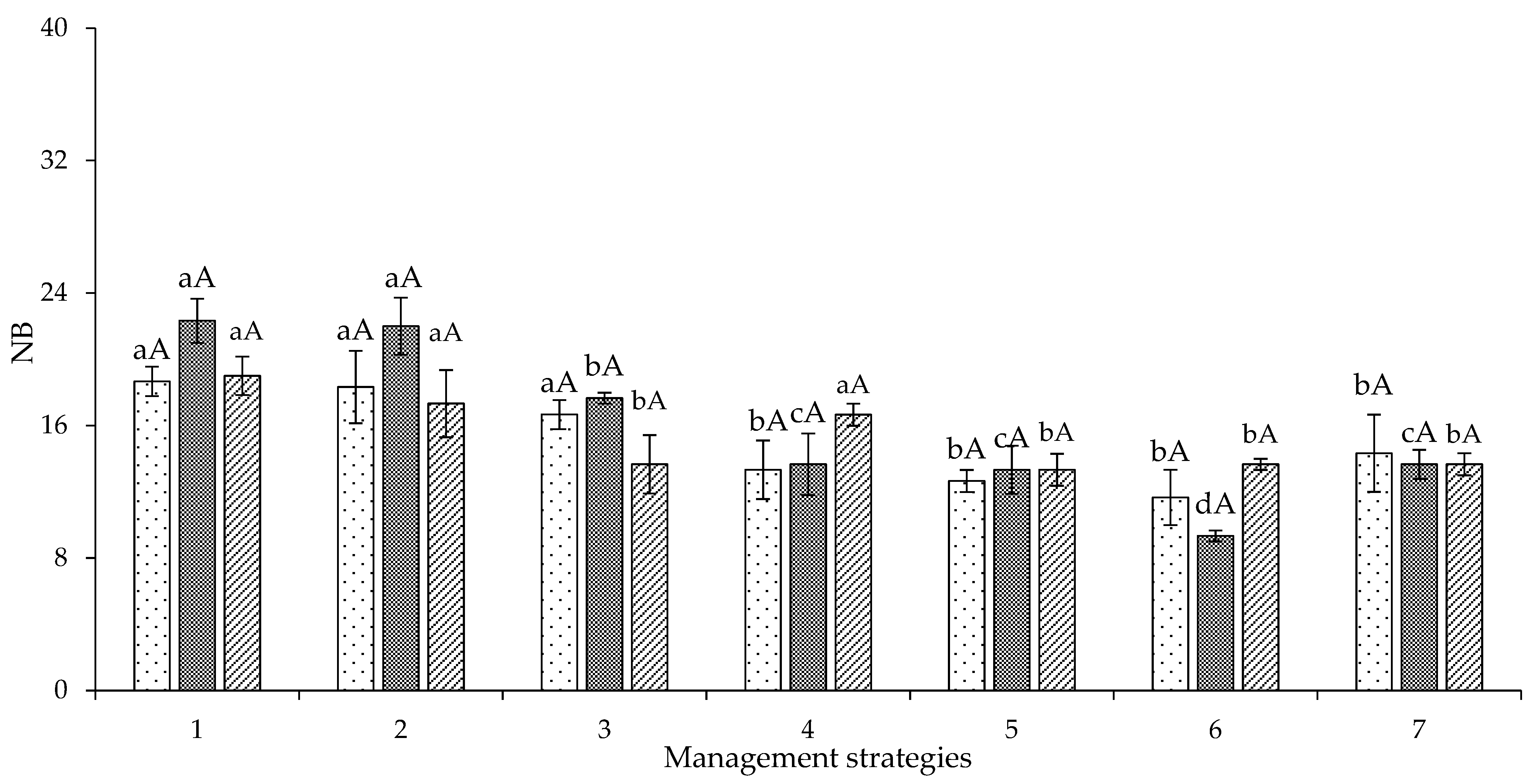
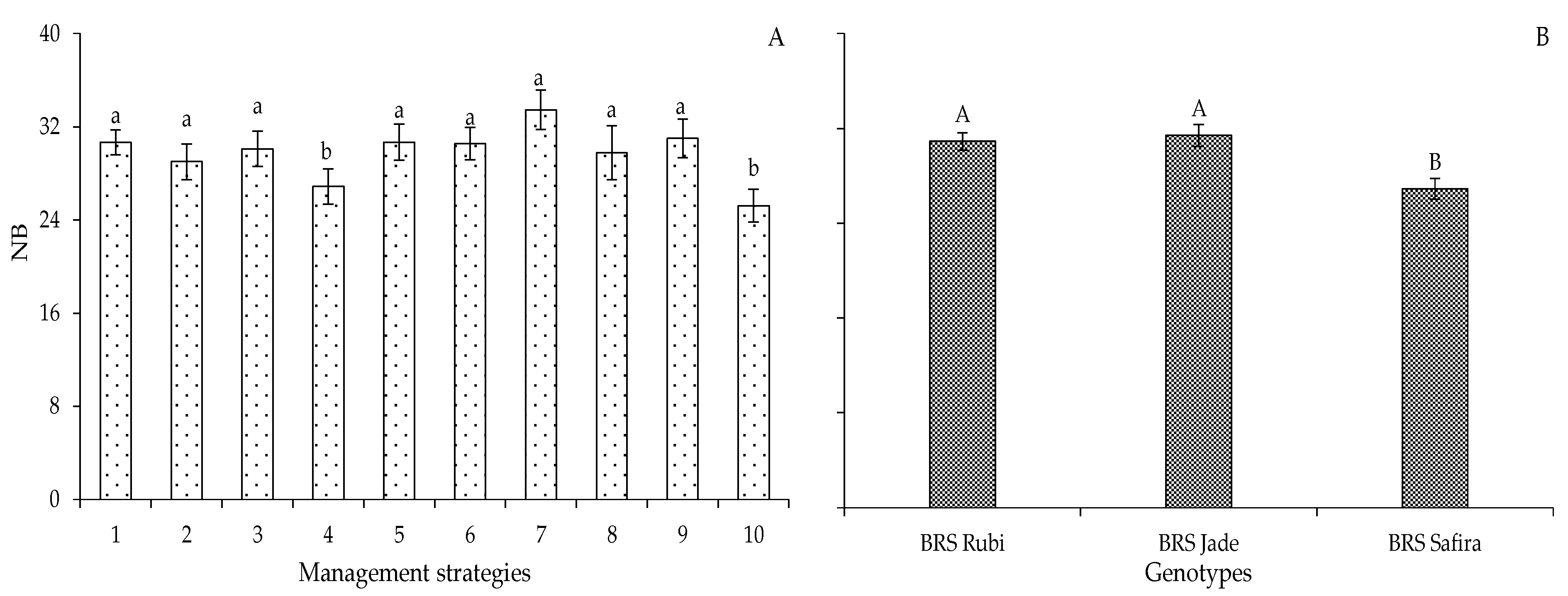



| Variables | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Experiment I | |||||||
| PH | SD | NL | LA | LDM | SDM | ||
| Strategies (S) | 6 | 57.88 ** | 1.77 ** | 85.70 ** | 306,614.41 ns | 42.37 ** | 26.06 ** |
| Genotypes (G) | 2 | 1216.38 ** | 5.77 ** | 127.70 * | 1,533,690.29 ** | 14.41 * | 28.91 ** |
| S × G | 12 | 13.52 ns | 0.25 ns | 10.90 ns | 131,676.48 ns | 21.95 ** | 14.78 ** |
| Blocks | 2 | 801.49 ** | 0.26 ns | 2.35 ns | 172,268.89 ns | 4.62 ns | 1.52 ns |
| Residual | 40 | 17.59 | 0.23 | 34.45 | 148,975.87 | 3.28 | 4.08 |
| CV (%) | 6.03 | 4.74 | 13.50 | 4.74 | 9.80 | 10.27 | |
| Experiment II | |||||||
| Strategies (S) | 9 | 69.83 * | 2.63 ** | 70.87 ** | 573,779.38 ** | 35.73 ** | 45.22 ** |
| Genotypes (G) | 2 | 1695.18 ** | 3.80 ** | 18.85 ns | 62,178.84 ns | 49.57 ** | 30.03 ** |
| S × G | 18 | 28.47 ns | 0.71 ns | 66.54 ** | 43,513.00 ns | 19.97 ** | 16.56 ** |
| Blocks | 2 | 62.25 ns | 1.08 ns | 1.30 ns | 503,244.40 ** | 1.08 ns | 1.71 ns |
| Residual | 58 | 35.80 | 0.73 | 12.88 | 41,845.31 | 4.59 | 1.48 |
| CV (%) | 8.57 | 8.01 | 6.27 | 15.00 | 8.24 | 7.57 | |
| Variables | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Experiment I | |||||||
| NB | SCW | %Fiber | HI | WC | WUE | ||
| Strategies (S) | 6 | 93.32 ** | 60,333.53 ** | 13.04 ns | 1.68 * | 34.77 ** | 44.43 ** |
| Genotypes (G) | 2 | 6.33 ns | 14,702.10 ** | 378.35 ** | 17.96 ** | 2.8 ns | 177.99 ** |
| S × G | 12 | 11.70 ** | 754.43 ** | 14.48 ns | 0.58 ns | 9.47 ns | 3.59 ns |
| Blocks | 2 | 28.42 * | 311.08 * | 11.49 ns | 0.25 ns | 2.84 ns | 3.50 ns |
| Residual | 40 | 5.74 | 80.40 | 15.64 | 0.55 | 5.68 | 2.45 |
| CV (%) | 15.44 | 6.52 | 12.67 | 21.63 | 5.1 | 12.22 | |
| Experiment II | |||||||
| Strategies (S) | 9 | 46.91 ** | 264.38 ns | 43.92 ns | 0.46 * | 6201.3 ** | 6.74 ** |
| Genotypes (G) | 2 | 182.50 ** | 6628.82 ** | 307.34 ** | 6.47 ** | 0.0004 ns | 23.67 ** |
| S × G | 18 | 14.61 ns | 256.38 ns | 48.08 ns | 0.28 ns | 0.0004 ns | 0.85 ns |
| Blocks | 2 | 158.70 ** | 2484.06 ns | 72.84 ns | 1.07 ** | 0.0004 ns | 7.76 ** |
| Residual | 58 | 15.13 | 283.03 | 46.63 | 0.20 | 0.0004 | 1.00 |
| CV (%) | 13.08 | 18.52 | 19.93 | 20.38 | 5.00 | 18.19 | |
| Irrigation Management Strategies | Phenological Stages | |||
|---|---|---|---|---|
| Vegetative (A) | Flowering (B) | Yield Formation (C) | ||
| Experiment I 1 | Experiment II 2 | ETr (%) | ||
| A1B1C1 | A-E0 | 100 | 100 | 100 |
| A2B1C1 | B-E0 | 100 | 100 | 100 |
| B-EV | 40 | 100 | 100 | |
| B-EFL | 100 | 40 | 100 | |
| A1B2C1 | C-E0 | 100 | 100 | 100 |
| C-EV | 40 | 100 | 100 | |
| C-EFR | 100 | 100 | 40 | |
| A1B2C2 | BC-E0 | 100 | 100 | 100 |
| BC-EV | 40 | 100 | 100 | |
| BC-EFF | 100 | 40 | 40 | |
| Experiment I | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bulk Density | Total Porosity | Moisture (%) | Available Water | Sorption Complex | ||||||
| Ca2+ | Mg2+ | Na+ | K+ | pHsp | ECse | |||||
| kg dm−3 | % | 0.33 atm | 15 atm | % | cmolc kg−1 | - | dS m−1 | |||
| 1.37 | 48.88 | 15.01 | 5.81 | 9.20 | 6.4 | 4.11 | 0.1 | 0.8 | 7.76 | 0.22 |
| Experiment II | ||||||||||
| 1.33 | 49.81 | 16.75 | 6.50 | 10.25 | 6.57 | 5.28 | 0.22 | 5.41 | 7.72 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, W.A.; Nobre, R.G.; Soares, L.A.d.A.; de Lima, G.S.; Gheyi, H.R.; Fernandes, P.D.; Ferreira, A.P.N.; da Silva, A.A.R.; de Azevedo, C.A.V.; Silva, D.V.; et al. Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton. Plants 2023, 12, 2892. https://doi.org/10.3390/plants12162892
Guedes WA, Nobre RG, Soares LAdA, de Lima GS, Gheyi HR, Fernandes PD, Ferreira APN, da Silva AAR, de Azevedo CAV, Silva DV, et al. Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton. Plants. 2023; 12(16):2892. https://doi.org/10.3390/plants12162892
Chicago/Turabian StyleGuedes, Wellinghton Alves, Reginaldo Gomes Nobre, Lauriane Almeida dos Anjos Soares, Geovani Soares de Lima, Hans Raj Gheyi, Pedro Dantas Fernandes, Ana Paula Nunes Ferreira, André Alisson Rodrigues da Silva, Carlos Alberto Vieira de Azevedo, Daniel Valadão Silva, and et al. 2023. "Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton" Plants 12, no. 16: 2892. https://doi.org/10.3390/plants12162892
APA StyleGuedes, W. A., Nobre, R. G., Soares, L. A. d. A., de Lima, G. S., Gheyi, H. R., Fernandes, P. D., Ferreira, A. P. N., da Silva, A. A. R., de Azevedo, C. A. V., Silva, D. V., & de Medeiros, J. F. (2023). Irrigation Strategies with Controlled Water Deficit in Two Production Cycles of Cotton. Plants, 12(16), 2892. https://doi.org/10.3390/plants12162892








