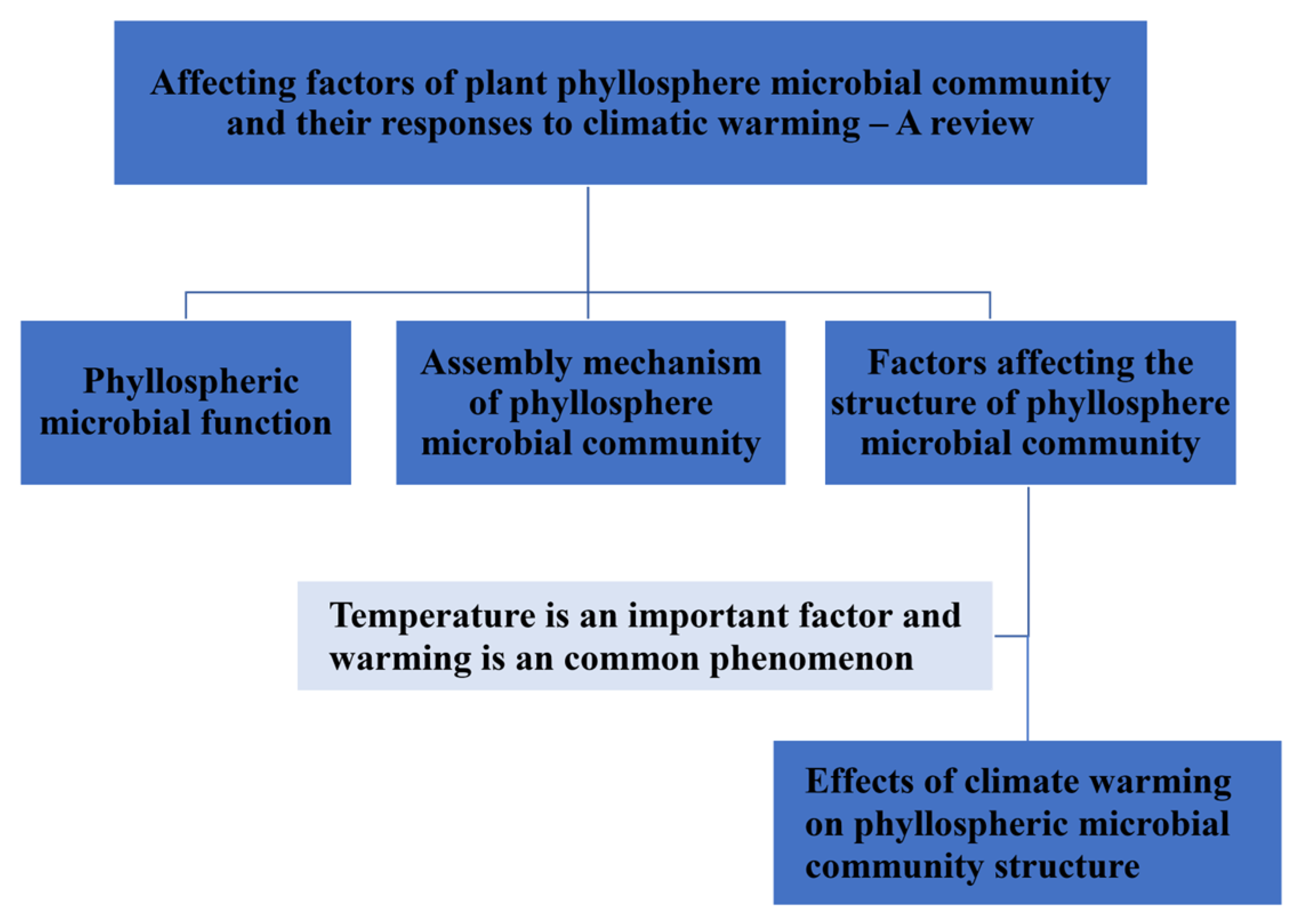
Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review
Abstract
1. Introduction
2. Phyllospheric Microbial Function
3. Assembly Mechanism of Phyllosphere Microbial Community
4. Factors Affecting the Structure of the Phyllosphere Microbial Community
5. Effects of Climate Warming on Phyllospheric Microbial Community Structure
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordier, T.; Robin, C.; Capdevielle, X.; Fabreguettes, O.; Desprez-Loustau, M.-L.; Vacher, C. The composition of phyllosphere fungal assemblages of European beech (Fagus sylvatica) varies significantly along an elevation gradient. New Phytol. 2012, 196, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Pati, B.R. Effect of spraying nitrogen-fixing phyllospheric bacterial isolates on rice plants. Zentralbl. Mikrobiol. 1992, 147, 441–446. [Google Scholar] [CrossRef]
- Meena, K.K.; Kumar, M.; Kalyuzhnaya, M.G.; Yandigeri, M.S.; Singh, D.P.; Saxena, A.K.; Arora, D.K. Epiphytic pink-pigmented methylotrophic bacteria enhance germination and seedling growth of wheat (Triticum aestivum) by producing phytohormone. Anton. Leeuw. Int. J. G. 2012, 101, 777–786. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Senthilkumar, M.; Seshadri, S.; Chung, H.Y.; Yang, J.C.; Sundaram, S.; Sa, T.M. Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Bot. Bull. Acad. Sin. 2004, 45, 315–324. [Google Scholar]
- Pedraza, R.O.; Bellone, C.H.; de Bellone, S.; Sorte, P.M.B.; Teixeira, K.R.D. Azospirillum inoculation and nitrogen fertilization effect on grain yield and on the diversity of endophytic bacteria in the phyllosphere of rice rainfed crop. Eur. J. Soil Biol. 2009, 45, 36–43. [Google Scholar] [CrossRef]
- Smyth, E.M.; McCarthy, J.; Nevin, R.; Khan, M.R.; Dow, J.M.; O’Gara, F.; Doohan, F.M. In vitro analyses are not reliable predictors of the plant growth promotion capability of bacteria; a Pseudomonas fluorescens strain that promotes the growth and yield of wheat. J. Appl. Microbiol. 2011, 111, 683–692. [Google Scholar] [CrossRef]
- Agha, S.I.; Jahan, N.; Azeem, S.; Parveen, S.; Tabassum, B.; Raheem, A.; Ullah, H.; Khan, A. Characterization of broad-spectrum biocontrol efficacy of Bacillus velezensis against Fusarium oxysporum in Triticum aestivum L. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12590. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.Y.; Khan, N.; Tan, L.L.; Yang, S. Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Tian, X.L.; Xu, D.; Sun, T.T.; Zhao, S.Y.; Li, Y.; Wang, D.D. Plant resistance and leaf chemical characteristic jointly shape phyllosphere bacterial community. World J. Microbiol. Biotechnol. 2020, 36, 139. [Google Scholar] [CrossRef]
- Vadivukkarasi, P.; Bhai, R.S. Phyllosphere-associated Methylobacterium: A potential biostimulant for ginger (Zingiber officinale Rosc.) cultivation. Arch. Microbiol. 2020, 202, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Mahawar, H.; Dubey, G.; Atoliya, N.; Parmar, R.; Devi, M.H.; Kollah, B.; Mohanty, S.R. Prospect of pink pigmented facultative methylotrophs in mitigating abiotic stress and climate change. J. Basic Microbiol. 2022, 62, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Schauer, S.; Kutschera, U. A novel growth-promoting microbe, Methylobacterium funariae sp. nov., isolated from the leaf surface of a common moss. Plant Signal. Behav. 2011, 6, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Li, P.D.; Zhu, Z.R.; Zhang, Y.Z.; Xu, J.P.; Wang, H.K.; Wang, Z.Y.; Li, H.Y. The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.Y.; Su, Y.; Cheng, C.; Shang, B.; Agathokleous, E.; Feng, Z.Z. Effects of elevated ozone on bacterial communities inhabiting the phyllo- and endo-spheres of rice plants. Sci. Total Environ. 2022, 830, 154705. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis thaliana against Leaf-Pathogenic Pseudomonas syringae by Sphingomonas Strains in a Controlled Model System. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef]
- Zhou, L.S.; Li, H.; Zhang, Y.; Han, S.Q.; Xu, H. Sphingomonas from petroleum-contaminated soils in Shenfu, China and their PAHs degradation abilities. Braz. J. Microbiol. 2016, 47, 271–278. [Google Scholar] [CrossRef]
- van der Wal, A.; Leveau, J.H.J. Modelling sugar diffusion across plant leaf cuticles: The effect of free water on substrate availability to phyllosphere bacteria. Environ. Microbiol. 2011, 13, 792–797. [Google Scholar] [CrossRef]
- Schiro, G.; Verch, G.; Grimm, V.; Muller, M.E.H. Alternaria and Fusarium Fungi: Differences in Distribution and Spore Deposition in a Topographically Heterogeneous Wheat Field. J. Fungi 2018, 4, 63. [Google Scholar] [CrossRef]
- Stassen, M.J.J.; Hsu, S.H.; Pieterse, C.M.J.; Stringlis, I.A. Coumarin Communication Along the Microbiome-Root-Shoot Axis. Trends Plant Sci. 2021, 26, 169–183. [Google Scholar] [CrossRef]
- Cortes-Patino, S.; Vargas, C.; Alvarez-Florez, F.; Bonilla, R.; Estrada-Bonilla, G. Potential of Herbaspirillum and Azospirillum Consortium to Promote Growth of Perennial Ryegrass under Water Deficit. Microorganisms 2021, 9, 91. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. For. Pathol. 2011, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Xing, L.; Zhi, Q.Q.; Hu, X.; Liu, L.L.; Xu, H.; Zhou, T.; Yin, H.Q.; Yi, Z.X.; Li, J. Influence of Association Network Properties and Ecological Assembly of the Foliar Fugal Community on Crop Quality. Front. Microbiol. 2022, 13, 783923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Ning, D.L. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef]
- Chave, J. Neutral theory and community ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Davies, T.J.; Grytnes, J.-A.; Harrison, S.P.; et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.; Hampe, A.; Porte, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The Phyllosphere: Microbial Jungle at the Plant-Climate Interface. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2016; Volume 47, pp. 1–24. [Google Scholar]
- Carlstroem, C.I.; Field, C.M.; Bortfeld-Miller, M.; Mueller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; He, X.; Otto, J.-C.; Ruiz-Hernandez, V.; Hanusch, M. Divergent assembly processes? A comparison of the plant and soil microbiome with plant communities in a glacier forefield. FEMS Microbiol. Ecol. 2021, 97, fiab135. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Veach, A.M.; Muchero, W.; Wahl, T.; Stegen, J.C.; Schadt, C.W.; Cregger, M.A. Assembly of the Populus Microbiome Is Temporally Dynamic and Determined by Selective and Stochastic Factors. Msphere 2021, 6, e01316-20. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Montoya, L.; Xu, L.; Madera, M.; Hollingsworth, J.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; Dahlberg, J.A.; et al. Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat. Commu. 2020, 11, 34. [Google Scholar] [CrossRef]
- Bell, J.K.; Mamet, S.D.; Helgason, B.; Siciliano, S.D. Brassica napus Bacterial Assembly Processes Vary with Plant Compartment and Growth Stage but Not between Lines. Appl. Environ. Microbiol. 2022, 88, e00273-22. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, X.; Xu, J.; Xie, H.; Cai, Y.; Liu, Y.; Ding, X. Strategies and Structure Feature of the Aboveground and Belowground Microbial Community Respond to Drought in Wild Rice (Oryza longistaminata). Rice 2021, 14, 79. [Google Scholar] [CrossRef]
- Yan, K.; Han, W.H.; Zhu, Q.L.; Li, C.R.; Dong, Z.; Wang, Y.P. Leaf surface microtopography shaping the bacterial community in the phyllosphere: Evidence from 11 tree species. Microbiol. Res. 2022, 254, 126897. [Google Scholar] [CrossRef]
- Hu, J.J. Effects of Warming, Altered Precipitation and Fertilization on Phyllosphere Bacterial Community Structure in an Alpine Meadow; Lanzhou University: Lanzhou, China, 2022. [Google Scholar]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Lan, G.Y.; Wu, Z.X.; Chen, B.Q.; Quan, F.; Li, M.M.; Sun, S.Q.; Du, H.N. Phyllosphere fungal communities of rubber trees exhibited biogeographical patterns, but not bacteria. Environ. Microbiol. 2022, 24, 3777–3790. [Google Scholar] [CrossRef]
- Glushakova, A.M.; Chernov, I.Y. Seasonal dynamics of the structure of epiphytic yeast communities. Microbiology 2010, 79, 830–839. [Google Scholar] [CrossRef]
- Finkel, O.M.; Burch, A.Y.; Lindow, S.E.; Post, A.F.; Belkin, S. Geographical Location Determines the Population Structure in Phyllosphere Microbial Communities of a Salt-Excreting Desert Tree. Appl. Environ. Microbiol. 2011, 77, 7647–7655. [Google Scholar] [CrossRef]
- Tang, G.J.; Xu, L.X.; Wang, X.Y.; Zhang, J.G. Effects of Leaf Morphological and Chemical Properties on the Population Sizes of Epiphytes. Microb. Ecol. 2023, 85, 157–167. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Messier, C.; Kembel, S.W. Host species identity, site and time drive temperate tree phyllosphere bacterial community structure. Microbiome 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Shen, Z.X.; Sun, W.; Zhong, Z.M.; Zhang, X.Z.; Zhou, Y.T. A meta-analysis of the effects of experimental warming on plant physiology and growth on the Tibetan Plateau. J. Plant Growth Regul. 2015, 34, 57–65. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.X. Effects of enhanced UV-B radiation on plant physiology and growth on the Tibetan Plateau: A meta-analysis. Acta Physiol. Plant. 2017, 39, 85. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Shen, Z.X.; Fu, G. A meta-analysis of the effects of experimental warming on soil carbon and nitrogen dynamics on the Tibetan Plateau. Appl. Soil Ecol. 2015, 87, 32–38. [Google Scholar] [CrossRef]
- Wang, S.; Fu, G. Modelling soil moisture using climate data and normalized difference vegetation index based on nine algorithms in alpine grasslands. Front. Environ. Sci. 2023, 11, 1130448. [Google Scholar] [CrossRef]
- Mechan-Llontop, M.E.; Tian, L.; Sharma, P.; Heflin, L.; Bernal-Galeano, V.; Haak, D.C.; Clarke, C.R.; Vinatzer, B.A. Experimental Evidence Pointing to Rain as a Reservoir of Tomato Phyllosphere Microbiota. Phytobiomes J. 2021, 5, 382–399. [Google Scholar] [CrossRef]
- Al Ashhab, A.; Meshner, S.; Alexander-Shani, R.; Dimerets, H.; Brandwein, M.; Bar-Lavan, Y.; Winters, G. Temporal and Spatial Changes in Phyllosphere Microbiome of Acacia Trees Growing in Arid Environments. Front. Microbiol. 2021, 12, 656269. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, Z.; Fu, G. Geo-Distribution Patterns of Soil Fungal Community of Pennisetum flaccidum in Tibet. J. Fungi 2022, 8, 1230. [Google Scholar] [CrossRef]
- Wu, J.S.; Zhang, X.Z.; Shen, Z.X.; Shi, P.L.; Yu, C.Q.; Chen, B.X. Effects of livestock exclusion and climate change on aboveground biomass accumulation in alpine pastures across the Northern Tibetan Plateau. Chin. Sci. Bull. 2014, 59, 4332–4340. [Google Scholar] [CrossRef]
- Faticov, M.; Abdelfattah, A.; Roslin, T.; Vacher, C.; Hamback, P.; Blanchet, F.G.; Lindahl, B.D.; Tack, A.J.M. Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. New Phytol. 2021, 231, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Moszczynska, E.; Plaskowska, E.; Matkowski, K.; Biesiada, A.; Weber, R. Fungi assemblages of the phyllosphere of eastern purple coneflower (Echinacea purpurea (L.) Moench.) depending on the rate of nitrogen. Acta Sci. Pol.-Hortorum Cultus 2013, 12, 153–162. [Google Scholar]
- Fu, G.; Shen, Z.X. Response of alpine plants to nitrogen addition on the Tibetan Plateau: A meta-analysis. J. Plant Growth Regul. 2016, 35, 974–979. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Suslow, T.; Allende, A. Impact of chlorine dioxide disinfection of irrigation water on the epiphytic bacterial community of baby spinach and underlying soil. PLoS ONE 2018, 13, e0199291. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.S. Seasonal Community Succession of the Phyllosphere Microbiome. Mol. Plant-Microbe Interact. 2015, 28, 274–285. [Google Scholar] [CrossRef]
- Leveau, J.H.J.; Lindow, S.E. Appetite of an epiphyte: Quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 2001, 98, 3446–3453. [Google Scholar] [CrossRef] [PubMed]
- Bunster, L.; Fokkema, N.J.; Schippers, B. Effect of surface-active Pseudomonas spp. on leaf wettability. Appl. Environ. Microbiol. 1989, 55, 1340–1345. [Google Scholar] [CrossRef]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef]
- Mechaber, W.L.; Marshall, D.B.; Mechaber, R.A.; Jobe, R.T.; Chew, F.S. Mapping leaf surface landscapes. Proc. Natl. Acad. Sci. USA 1996, 93, 4600–4603. [Google Scholar] [CrossRef]
- Mercier, J.; Lindow, S.E. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 2000, 66, 369–374. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.K.; Jeon, J.; Harris, W.A.; Lee, Y.-H. Domestication of Oryza species eco-evolutionarily shapes bacterial and fungal communities in rice seed. Microbiome 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Hawkes, C.V. Plant and Soil Drivers of Whole-Plant Microbiomes: Variation in Switchgrass Fungi from Coastal to Mountain Sites. Phytobiomes J. 2021, 5, 69–79. [Google Scholar] [CrossRef]
- Forero-Junco, L.M.; Alanin, K.W.S.; Djurhuus, A.M.; Kot, W.; Gobbi, A.; Hansen, L.H. Bacteriophages Roam the Wheat Phyllosphere. Viruses-Basel 2022, 14, 244. [Google Scholar] [CrossRef]
- Schreiber, L.; Krimm, U.; Knoll, D.; Sayed, M.; Auling, G.; Kroppenstedt, R.M. Plant-microbe interactions: Identification of epiphytic bacteria and their ability to alter leaf surface permeability. New Phytol. 2005, 166, 589–594. [Google Scholar] [CrossRef]
- Thapa, S.; Prasanna, R.; Ranjan, K.; Velmourougane, K.; Ramakrishnan, B. Nutrients and host attributes modulate the abundance and functional traits of phyllosphere microbiome in rice. Microbiol. Res. 2017, 204, 55–64. [Google Scholar] [CrossRef]
- Wulff, E.G.; van Vuurde, J.W.L.; Hockenhull, J. The ability of the biological control agent Bacillus subtilis, strain BB, to colonise vegetable brassicas endophytically following seed inoculation. Plant Soil 2003, 255, 463–474. [Google Scholar] [CrossRef]
- Mittelstrass, J.; Sperone, F.G.; Horton, M.W. Using transects to disentangle the environmental drivers of plant-microbiome assembly. Plant Cell Environ. 2021, 44, 3515–3525. [Google Scholar] [CrossRef]
- Sapkota, R.; Jorgensen, L.N.; Nicolaisen, M. Spatiotemporal Variation and Networks in the Mycobiome of the Wheat Canopy. Front. Plant Sci. 2017, 8, 1357. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Peng, C.; Sun, W.T.; Dong, R.; Hao, J. Effects of Variety, Plant Location, and Season on the Phyllosphere Bacterial Community Structure of Alfalfa (Medicago sativa L.). Microorganisms 2022, 10, 2023. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Yu, C.; Fu, G. The change in environmental variables linked to climate change has a stronger effect on aboveground net primary productivity than does phenological change in alpine grasslands. Front. Plant Sci. 2022, 12, 798633. [Google Scholar] [CrossRef]
- Li, S.; Fu, G. Impacts of Anthropogenic Activities and Climate Change on Forage Nutrition Storage in Tibetan Grasslands. Plants 2023, 12, 2735. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yu, C.; Fu, G. Non-growing/growing season non-uniform-warming increases precipitation use efficiency but reduces its temporal stability in an alpine meadow. Front. Plant Sci. 2023, 14, 1090204. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Shen, Z.X.; Zhang, X.Z. Increased precipitation has stronger effects on plant production of an alpine meadow than does experimental warming in the Northern Tibetan Plateau. Agr. Forest Meteorol. 2018, 249, 11–21. [Google Scholar] [CrossRef]
- Yu, C.Q.; Han, F.S.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Li, J.; Bates, K.A.; Hoang, K.L.; Hector, T.E.; Knowles, S.C.L.; King, K.C. Experimental temperatures shape host microbiome diversity and composition. Glob. Chang. Biol. 2023, 29, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant-microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Xiong, C.; Wei, Z.; Chen, Q.-L.; Ma, B.; Zhou, S.-Y.-D.; Tan, J.; Zhang, L.-M.; Cui, H.-L.; Duan, G.-L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Kazenel, M.R.; Kivlin, S.N.; Taylor, D.L.; Lynn, J.S.; Rudgers, J.A. Altitudinal gradients fail to predict fungal symbiont responses to warming. Ecology 2019, 100, e02740. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.P.; Luo, L.Y.; Liu, Y.; Jin, D.C.; Zhang, Z.; Li, X.J.; Fu, W.; Tang, T.; Xiao, Y.L.; et al. Scale-Dependent Effects of Growth Stage and Elevational Gradient on Rice Phyllosphere Bacterial and Fungal Microbial Patterns in the Terrace Field. Front. Plant Sci. 2022, 12, 766128. [Google Scholar] [CrossRef]
- Ikeda, S.; Tokida, T.; Nakamura, H.; Sakai, H.; Usui, Y.; Okubo, T.; Tago, K.; Hayashi, K.; Sekiyama, Y.; Ono, H.; et al. Characterization of leaf blade- and leaf sheath-associated bacterial communities and assessment of their responses to environmental changes in CO2, temperature, and nitrogen levels under field conditions. Microbes Environ. 2015, 30, 51–62. [Google Scholar] [CrossRef]
- Magan, N.; Baxter, E.S. Effect of increased CO2 concentration and temperature on the phyllosphere mycoflora of winter wheat flag leaves during ripening. Ann. Appl. Biol. 1996, 129, 189–195. [Google Scholar] [CrossRef]
- Ren, G.D.; Zhu, C.W.; Alam, M.S.; Tokida, T.; Sakai, H.; Nakamura, H.; Usui, Y.; Zhu, J.G.; Hasegawa, T.; Jia, Z.J. Response of soil, leaf endosphere and phyllosphere bacterial communities to elevated CO2 and soil temperature in a rice paddy. Plant Soil 2015, 392, 27–44. [Google Scholar] [CrossRef]
- Khlifa, R.; Houle, D.; Morin, H.; Kembel, S.W. Inconsistent effects of nitrogen canopy enrichment and soil warming on black spruce epiphytic phyllosphere bacterial communities, taxa, and functions. Can. J. For. Res. 2021, 51, 1199–1207. [Google Scholar] [CrossRef]
- Perazzolli, M.; Vicelli, B.; Antonielli, L.; Longa, C.M.O.; Bozza, E.; Bertini, L.; Caruso, C.; Pertot, I. Simulated global warming affects endophytic bacterial and fungal communities of Antarctic pearlwort leaves and some bacterial isolates support plant growth at low temperatures. Sci. Rep.-Uk 2022, 12, 18839. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Aydogan, E.L.; Moser, G.; Mueller, C.; Kampfer, P.; Glaeser, S.P. Long-Term Warming Shifts the Composition of Bacterial Communities in the Phyllosphere of Galium album in a Permanent Grassland Field-Experiment. Front. Microbiol. 2018, 9, 144. [Google Scholar] [CrossRef]
- Balint, M.; Bartha, L.; O’Hara, R.B.; Olson, M.S.; Otte, J.; Pfenninger, M.; Robertson, A.L.; Tiffin, P.; Schmitt, I. Relocation, high-latitude warming and host genetic identity shape the foliar fungal microbiome of poplars. Mol. Ecol. 2015, 24, 235–248. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Rudgers, J.A. Direct and Indirect Influences of Warming on Leaf Endophytic Fungi: A Physiological and Compositional Approach; Academic Press: Cambridge, MA, USA, 2019; pp. 125–140. [Google Scholar] [CrossRef]
- Aydogan, E.L.; Budich, O.; Hardt, M.; Choi, Y.H.; Jansen-Willems, A.B.; Moser, G.; Mueller, C.; Kaempfer, P.; Glaeser, S.P. Global warming shifts the composition of the abundant bacterial phyllosphere microbiota as indicated by a cultivation-dependent and -independent study of the grassland phyllosphere of a long-term warming field experiment. FEMS Microbiol. Ecol. 2020, 96, fiaa087. [Google Scholar] [CrossRef]
- Campisano, A.; Albanese, D.; Yousaf, S.; Pancher, M.; Donati, C.; Pertot, I. Temperature drives the assembly of endophytic communities’ seasonal succession. Environ. Microbiol. 2017, 19, 3353–3364. [Google Scholar] [CrossRef]
- Carrell, A.A.; Kolton, M.; Glass, J.B.; Pelletier, D.A.; Warren, M.J.; Kostka, J.E.; Iversen, C.M.; Hanson, P.J.; Weston, D.J. Experimental warming alters the community composition, diversity, and N-2 fixation activity of peat moss (Sphagnum fallax) microbiomes. Glob. Chang. Biol. 2019, 25, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zha, X.; Fu, G. Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review. Plants 2023, 12, 2891. https://doi.org/10.3390/plants12162891
Huang S, Zha X, Fu G. Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review. Plants. 2023; 12(16):2891. https://doi.org/10.3390/plants12162891
Chicago/Turabian StyleHuang, Shaolin, Xinjie Zha, and Gang Fu. 2023. "Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review" Plants 12, no. 16: 2891. https://doi.org/10.3390/plants12162891
APA StyleHuang, S., Zha, X., & Fu, G. (2023). Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review. Plants, 12(16), 2891. https://doi.org/10.3390/plants12162891






