Cloning and Expression of Class I Chitinase Genes from Four Mangrove Species under Heavy Metal Stress
Abstract
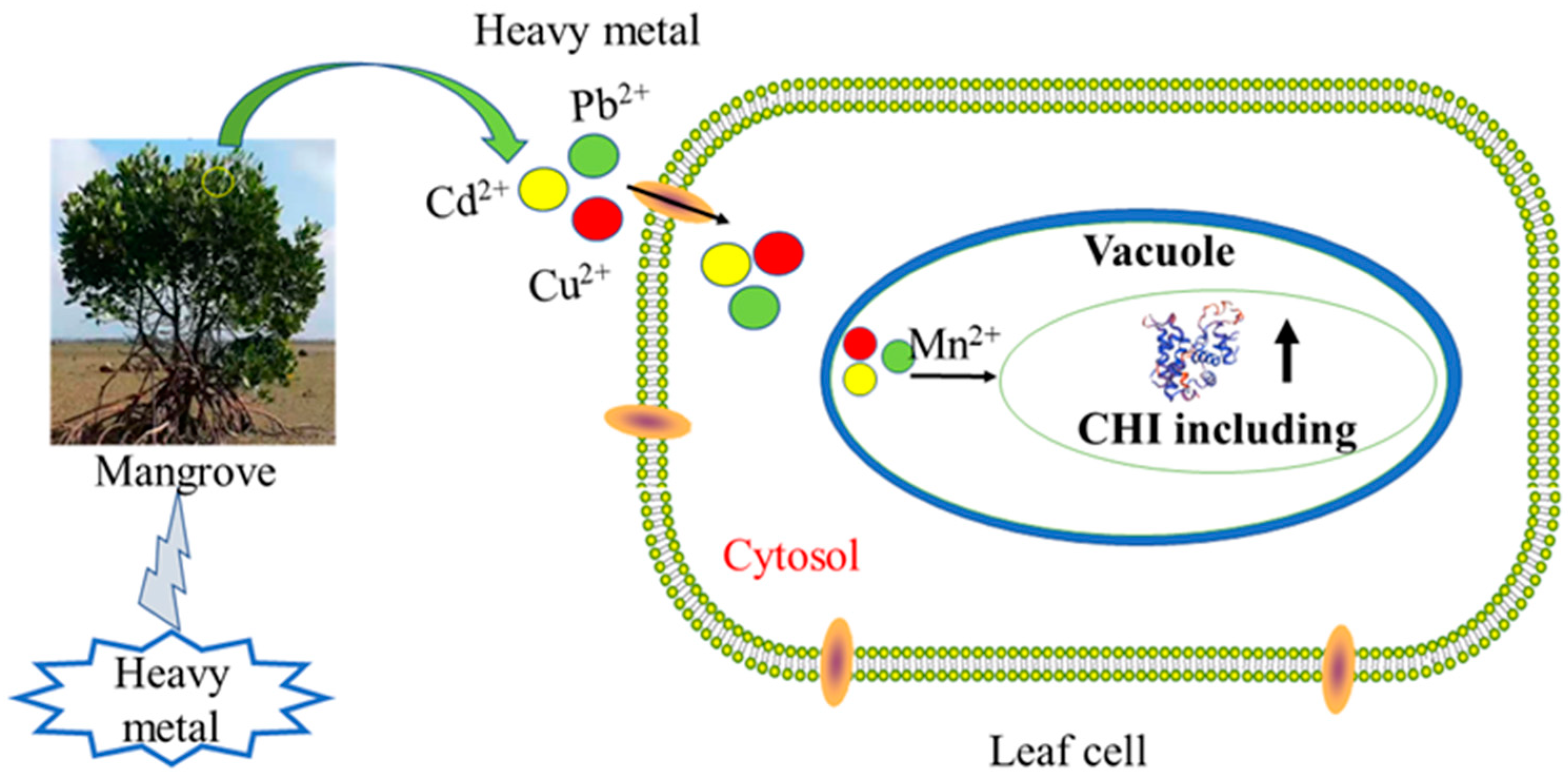
1. Introduction
2. Results
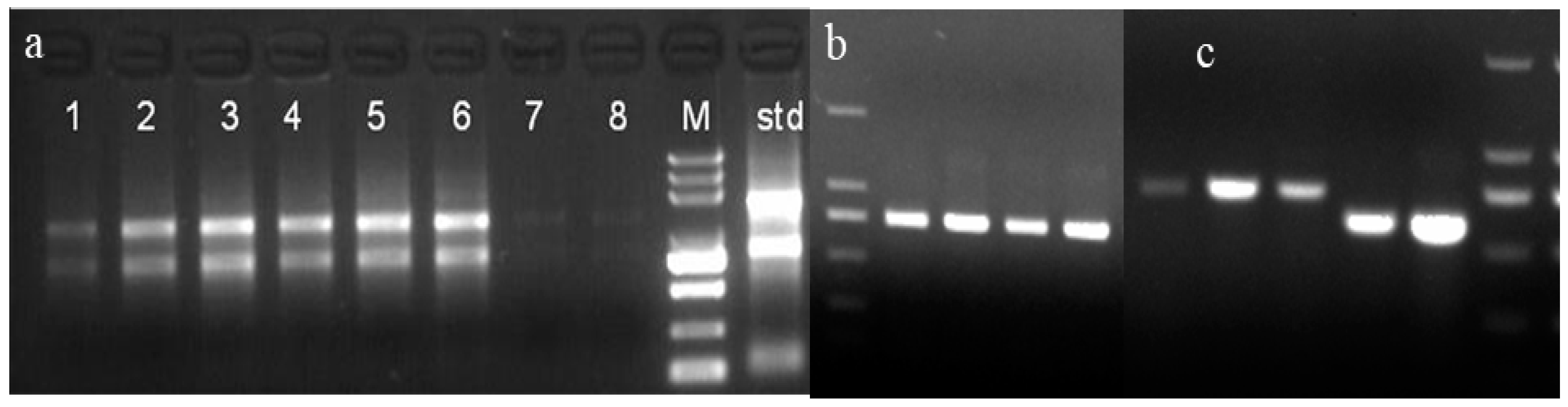
2.1. The Full-Length cDNA of CHI I Gene Cloning
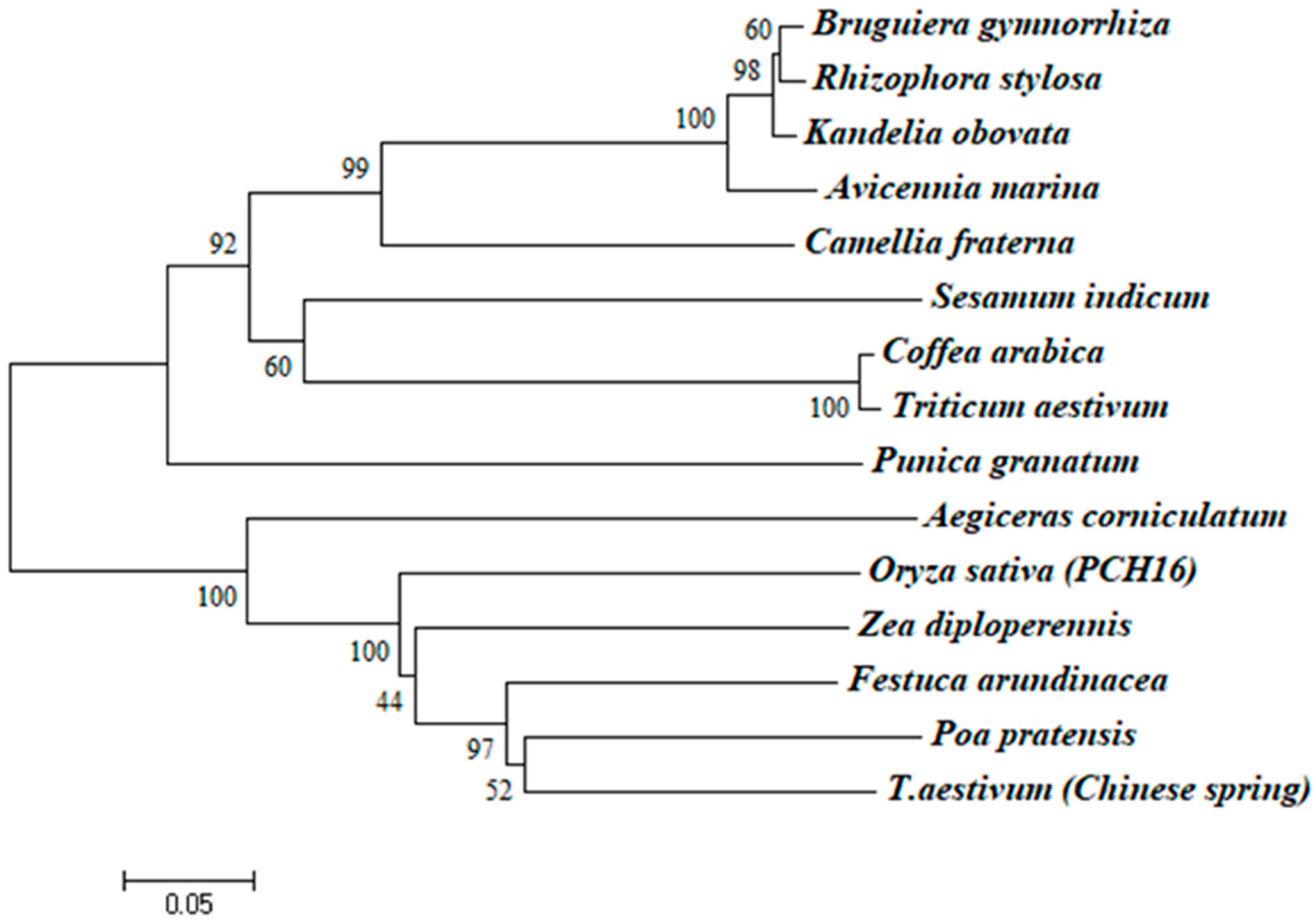
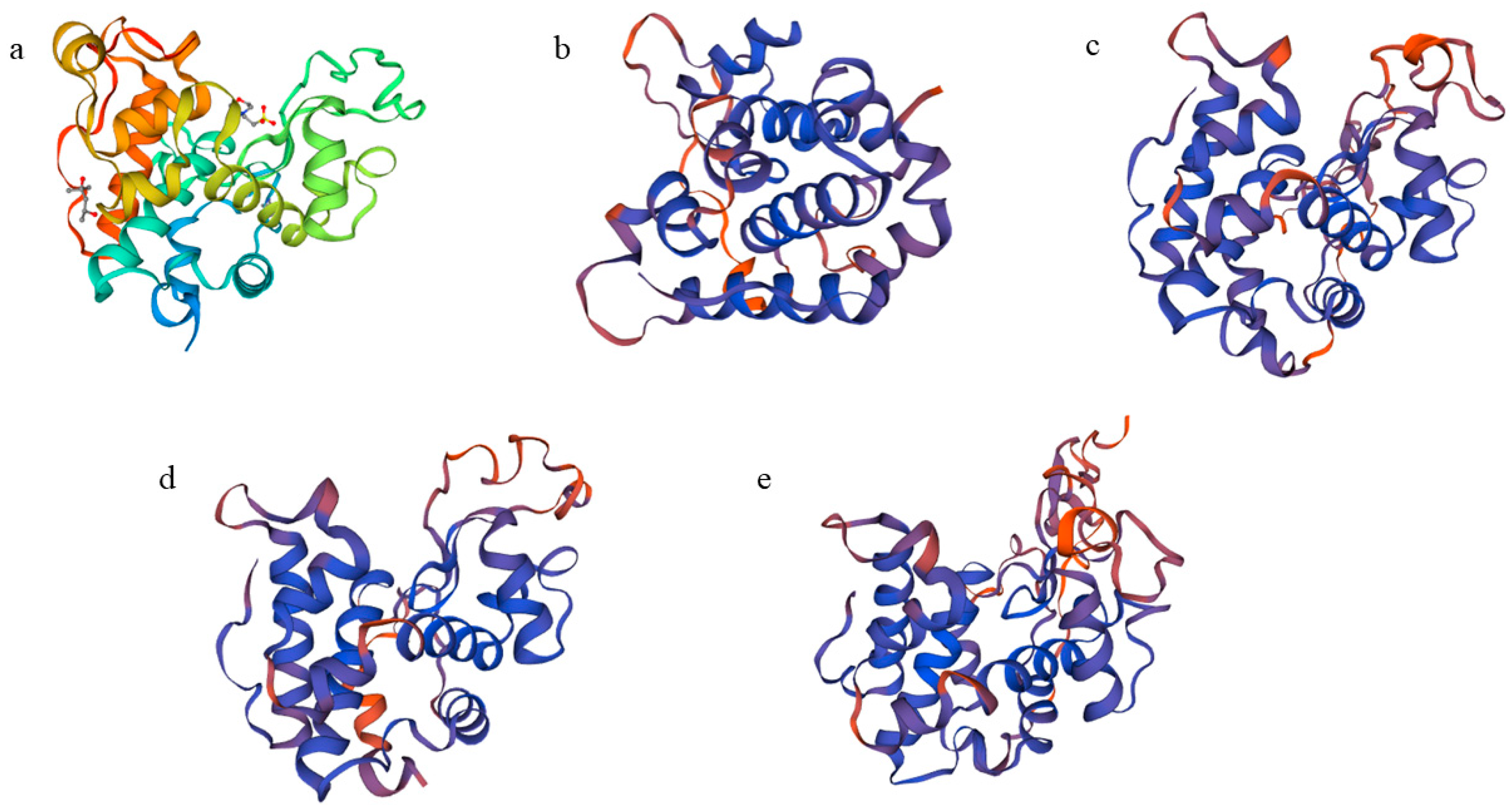
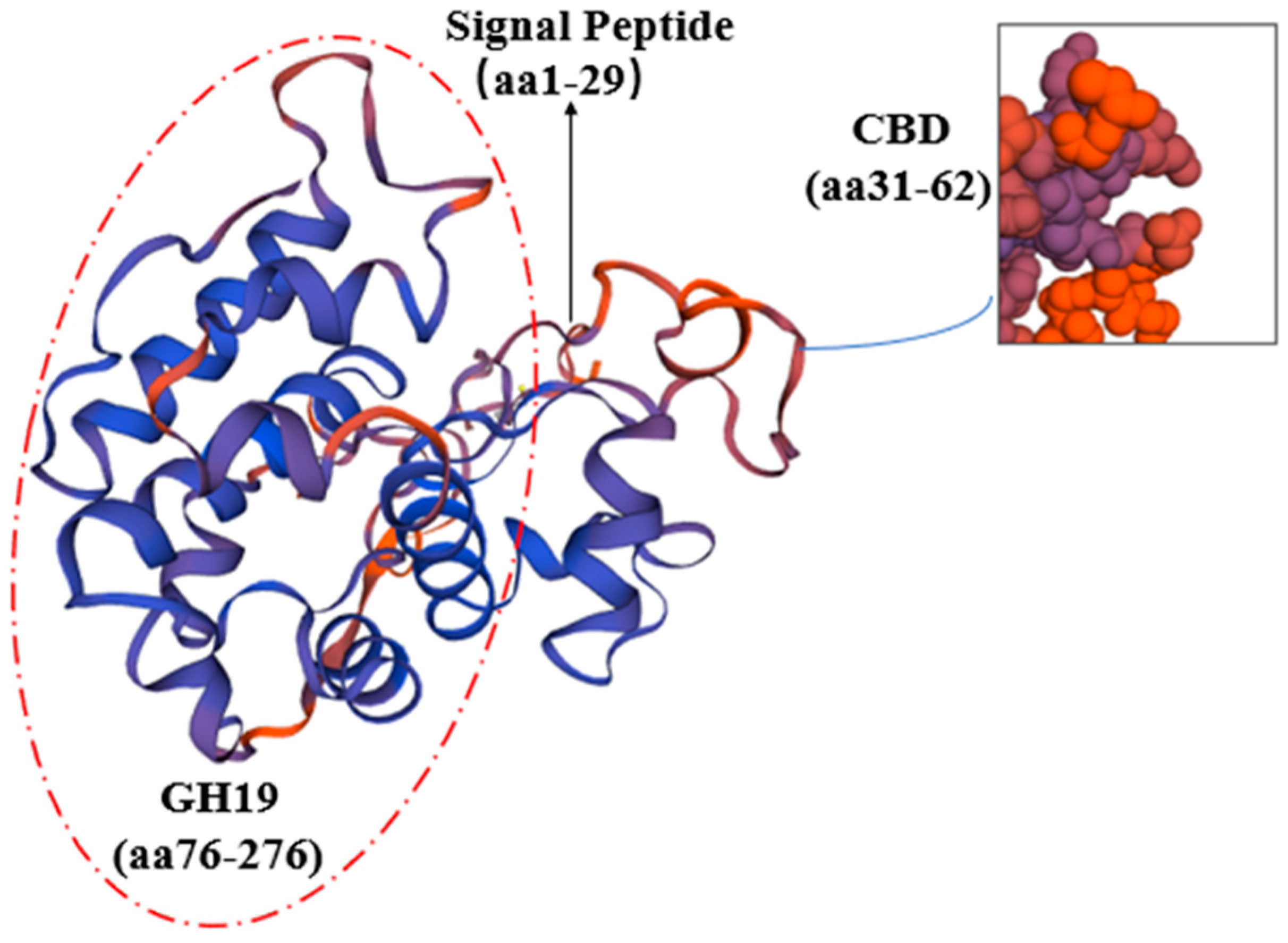
2.2. Sequence and Structure Analysis of the Full-Length cDNA Sequence of CHI I
2.3. CHI I mRNA Expression in Leaf in Response to Heavy Metal
3. Discussion
3.1. Cloning and Structural Characterization Analysis of CHI I
3.2. Expression of CHI I in Leaves in Response to Heavy Metal
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Total RNA Isolation and First-Strand cDNA Synthesis
4.3. Cloning the Full-Length cDNA of Chitinase Gene
4.4. Bioinformatic Analysis
4.5. Analysis of CHI I Gene Expression by Real-Time Quantitative PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.S. Molecular Ecology of Mangroves; The Science Publishing Company: Beijing, China, 2019; pp. 3–15. [Google Scholar]
- Wang, Y.S.; Gu, J.D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to the global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105–248. [Google Scholar] [CrossRef]
- Wang, Y.S. Restoration and Evaluation Technology of Mangrove Ecosystem; The Science Publishing Company: Beijing, China, 2013; pp. 1–20. [Google Scholar]
- Kamala, K.S.; Batvari, B.; Lee, K.J.; Kannan, N.; Krishnamoorthy, R.; Shanthi, K.; Jayaprakash, M. Assessment of heavy metals (Cd, Cr, and Pb) in water, sediment, and seaweed (Ulva lactuca) in the Pulicat Lake, Southeast India. Chemosphere 2008, 71, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.; Lorenzo, V.D. Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, G.; Burchett, M. Zinc distribution and excretion in the leaves of the grey mangrove, Avicennia marina (Forsk.) Vierh. Environ. Exp. Bot. 1999, 41, 167–175. [Google Scholar] [CrossRef]
- MacFarlane, G.; Burchett, M. Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquat. Bot. 2000, 68, 45–59. [Google Scholar] [CrossRef]
- Qin, T. Soluble sugar and proline contents of Kandelia obovata seedling leaves on Cd responses to stress. J. Ecol. 2006, 26, 3366–3371. [Google Scholar]
- Huang, G.Y.; Wang, Y.S. Expression and characterization analysis of type 2 metallothionein from grey mangrove species (Avicennia marina) in response to metal stress. Aquat. Toxicol. 2010, 99, 86–92. [Google Scholar] [CrossRef]
- Sharma, C.; Irudayaraj, V. Studies on heavy metal (Arsenic) tolerance in a mangrove fern Acrostichum aureum L. J. Basic Appl. Biol. 2010, 4, 143–152. [Google Scholar]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Wang, Y.S.; Inyang, A.I. Ecophysiological differences between five mangrove seedlings under heavy metal stress. Mar. Pollut. Bull. 2021, 172, 112900. [Google Scholar] [CrossRef]
- Hall, J. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 366, 1–11. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, E.N. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef]
- Huang, G.Y.; Wang, Y.S. Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J. Hazard. Mater. 2010, 182, 848–854. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Sun, C.C.; Lou, Z.P.; Dong, J.D. A novel metallothionein gene from a mangrove plant Kandelia candel. Ecotoxicology 2012, 21, 1633–1641. [Google Scholar] [CrossRef]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad, K. Minireview: Plant chitinases. Plant J. 1993, 3, 31–409. [Google Scholar] [CrossRef]
- Melchers, L.S.; Apothekerde, G.M.; Knaap, J.A. A new class of tobacco chitinases homologous to bacterial exo-chitinases displays antifungal activity. Plant J. 1994, 5, 469–480. [Google Scholar] [CrossRef]
- Chen, C.S.; Zhu, X.F.; Yu, Z.F. Effect of cowpea chitinase pure enzyme solution on different fungi. Plant Prot. 2000, 27, 375–376. [Google Scholar]
- Zou, X.H. Characterization of Chitinase Activity and Gene Expression in Muskmelon Seeds. Bachelor’s Thesis, The Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2000. [Google Scholar]
- Kasprzewska, A. Plant chitinases-regulation and function. Cell. Biol. Lett. 2003, 8, 809–824. [Google Scholar]
- Corrales, I.; Poschenrieder, C.; Barcelo, J. Boron-induced amelioration of aluminium toxicity in a monocot and a dicot species. J. Plant Physiol. 2008, 165, 504–513. [Google Scholar] [CrossRef]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum. J. Exp. Bot. 2005, 56, 167–178. [Google Scholar] [CrossRef]
- Beata, P.; Ildiko, M.; Awaad, A.S.; Kaushik, G.; Govil, J.N. Plant defense against heavy metals: The involvement of pathogenesis-related (PR) proteins. In Recent Progress in Medicinal Plant: Mechanism and Action of Phytoconstituents; Awaad, A.S., Kaushik, G., Govil, J.N., Eds.; Studium Press LLC: New Delhi, India, 2011; pp. 179–205. [Google Scholar]
- Békésiová, B.; Hraška, Š.; Libantová, J.; Moravčíková, J.; Matušíková, I. Heavy-metal stress induced accumulation of chitinase isoforms in plants. Mol. Biol. Rep. 2008, 35, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Dana, M.M.; Pintor-Toro, J.A.; Cubero, B. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol. 2006, 142, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Rivera, B.F.; Metwally, A.; Martin, L.F.; Van Tuinen, D.; Dietz, K.J.; Gianinazzi, S.; Gianinazzi-Pearson, V. Molecular responses to cadmium in roots of Pisum sativum L. Water Air Soil Pollut. 2003, 168, 171–186. [Google Scholar] [CrossRef]
- Rivera, B.F.; Metwally, A.; Martin, L.F.; Van, T.D.; Dietz, K.J.; Gianinazzi, S.; Gianinazzi, P.V. Molecular changes in Pisum sativum L. roots during arbuscular mycorrhiza buffering of cadmium stress. Mycorrhiza 2005, 16, 51–60. [Google Scholar] [CrossRef]
- Hossain, M.A.; Noh, H.N.; Kim, K.I. Mutation of the chitinase-like protein-encoding AtCTL2 gene enhances lignin accumulation in dark-grown Arabidopsis seedlings. J. Plant Physiol. 2010, 167, 650–658. [Google Scholar] [CrossRef]
- Hermans, C.; Porco, S.; Verbruggen, N.; Bush, D.R. Chitinase-like protein CTL1 plays a role in altering root system architecture in response to multiple environmental conditions. Plant Physiol. 2010, 152, 904–917. [Google Scholar] [CrossRef]
- Keulen, H.V.; Wei, R.; Cutright, T.J. Arsenate-induced expression of a class Ⅲ chitinase in the dwarf sunflower Helianthus annuus. Environ. Exp. Bot. 2008, 63, 281–288. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Fang, W.C.; Kao, C.H. Enhanced peroxidase activity in rice leaves in response to excess iron, copper and zinc. Plant Sci. 2000, 158, 71–76. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N.; Bisht, S.S. Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci. 2002, 162, 381–388. [Google Scholar] [CrossRef]
- Shinshi, H.; Neuhas, J.M.; Ryals, J.; Meins, F. Structure of a tobacco endochitinase gene: Evidence that different chitinase genes can arise by trans-position of sequences encoding a cysteine rich domain. Plant Mol. Biol. 1990, 14, 357–368. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Li, X.B.; Yang, X.Y.; Luo, M.; Hou, L.; Guo, S.H.; Luo, X.-Y.; Pei, Y. Cloning and characterization of a balsam pear class I chitinase gene (Mcchit1) and its ectopic expression enhances fungal resistance in transgenic plants. Biosci. Biotechnol. Biochem. 2007, 71, 1211–1219. [Google Scholar] [CrossRef]
- Huang, J.K.; Wen, L.; Swegle, M.; Tran, H.-C.; Tin, T.H.; Naylor, H.M.; Muthukrisnan, S.; Reeck, G.R. Nucleotide sequence of a rice genomic clone that encodes a class I chitinase. Plant Molecuar Biol. 1991, 16, 479–480. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Q.; Panbangred, W.; Shirasu, K.; Lamb, C. Regulation, expression and function of a new basic chitinase gene in rice (Oryza sativa L.). Plant Mol. Biol. 1996, 30, 387–401. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, Y.S.; Cheng, H.; Ping, L.; Foong, Y.S. Cloning the Aegiceras corniculatum class Ⅰ chitinase gene (AcCHI Ⅰ) and the response of AcCHI Ⅰ mRNA Expression to cadmium stress. Ecotoxicology 2015, 24, 1705–1713. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, Y.S.; Zhang, J.P.; Cheng, H.; Ping, L. Molecular cloning of class Ⅲ chitinase gene from Avicennia marina and its expression analysis in response to cadmium and lead stress. Ecotoxicology 2015, 24, 1697–1704. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Cell-PLoc: A package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Kezuka, Y.; Kojima, M.; Mizuno, R.; Suzuki, K.; Watanabe, T.; Nonaka, T. Structure of full-length class I chitinase from rice revealed by X-ray crystallography and small-angle X-ray scattering. Proteins Struct. Funct. Bioinf. 2010, 78, 2295–2305. [Google Scholar] [CrossRef]
- Graham, L.S.; Sticklen, M.B. Plant chitinases. Can. J. Bot. 1994, 72, 1057–1083. [Google Scholar] [CrossRef]
- Santos, P.; Fortunato, A.R.A.; Pawlowski, K. Chitinases in root nodules. Plant Biotechnol. 2008, 25, 299–307. [Google Scholar] [CrossRef]
- Arakane, Y.; Koga, D. Purification and characterization of an ovel chitinase isozyme from yam tuber. Biosci. Biotechnol. Biochem. 1999, 63, 1895–1901. [Google Scholar] [CrossRef]
- Iseli, B.; Boller, T.; Neuhaus, J.M. The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity. Plant Physiol. 1993, 103, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Diaz, P.A.; Collada, C.; Sánchez-Monge, R.; Aragoncillo, C.; Castillo, R.; Ortega, N.; Alvarez, M.; Carrillo, T.; Salcedo, G. Class I chitinases as potential panaller gens involved in the latex-fruit syndrome. J. Allergy Clin. Immunol. 1999, 103, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Sakaguchi, M.; von Heijne, G.; Hamasaki, N.; Mihara, K. Forced transmembrane orientation of hydrophilic polypeptide segments in multispanning membrane proteins. Mol. Cell 1998, 2, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, T.; Jin, X. Lead induced changes in the growth and antioxidant metabolism of the lead accumulating and non-accumulating ecotypes of Sedum alfredii. J. Integr. Plant Biol. 2008, 50, 129–140. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; Berglund, L.; Nielsen, K.K. Structure of endochitinase genes from sugar beets. In Advances in Chitin and Chitosan; Brine, C.J., Sandford, P.A., Zikakis, J.P., Eds.; Elsevier Applied Science: London, UK, 1992. [Google Scholar]
- Loon, L.C.V.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Kim, J.K.; Jang, I.C.; Wu, R.; Zuo, W.N.; Boston, R.S.; Lee, Y.H.; Ahn, I.-P.; Nahm, B.H. Co-expression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res. 2003, 12, 475–484. [Google Scholar] [CrossRef]
- Yu, L.M. Cloning and Expression Analysis of Chitinase Gene from Larix gmelinii. Master’s Thesis, Inner Mongolia Agricultural University, Huhehot, China, 2014. [Google Scholar]
- Liu, X.W. Molecular Cloning and Expression Analysis of ZaCHIT1 in Zanthoxylum piperitum DC.var. inerme Makino. Master’s Thesis, Guizhou University, Guiyang, China, 2017. [Google Scholar]
- Spisso, A.; Verni, E.; Nahan, K.; Martinez, L.; Landero, J.; Pacheco, P. The metabolic effects of mercury during the biological cycle of vines (Vitis vinifera). Biol. Met. 2018, 31, 243–254. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, W.S. Mechanism of plant tolerance to heavy metal cadmium. J. Plant Physiol. Mol. Biol. 2006, 32, 1–8. [Google Scholar]
- Gu, J.G.; Zhou, Q.X.; Wang, X. Ways to control soil heavy metal pollution and its research progress. Appl. Base J. Basic Eng. Sci. 2003, 11, 143–151. [Google Scholar]
- Yang, S.Y.; Wang, F.; Xie, J.C. Toxicity of heavy metals to plants and mechanism of plant tolerance. J. Anhui Norm. Univ. 2004, 27, 71–74. [Google Scholar]
- Zhou, Y.Y.; Wang, Y.S.; Sun, C.C. Molecular Cloning and Expression Analysis of the Typical class III chitinase genes from three mangrove species under heavy metal stress. Plants 2023, 12, 1681. [Google Scholar] [CrossRef]


| Species | Name of Gence | Number of Amino Acids | Molecular Weight | pI | The High Content of Amino Acid | Instability Index | Stable or Not | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|---|---|
| Rhizophora stylosa | Rs Chi | 276aa | 29.47 kDa | 4.65 | Gly11.2% Ala9.1% Ser 9.1% | 27.79 | Y | −0.186 |
| Bruguiera gymnorrhiza, | Bg Chi | 276aa | 29.50 kDa | 4.74 | Gly11.2% Ser 9.1% Ala9.1% | 27.79 | Y | −0.189 |
| Kandelia obovata, | Ko Chi | 276aa | 29.59 kDa | 4.69 | Gly11.2% Ser 9.1% Ala8.7% | 27.16 | Y | −0.218 |
| Avicennia marina | Am Chi | 276aa | 25.57 kDa | 4.66 | Gly10.9% Ser 9.1% Ala8.3% | 28.75 | Y | −0.155 |
| Heavy Metal (mg/L) | Control Group (CK) | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|
| Cu2+ | 0 | 5.0 | 25.0 | 50.0 | 75.0 |
| Pb2+ | 0 | 1.0 | 5.0 | 10.0 | 15.0 |
| Cd2+ | 0 | 0.2 | 1.0 | 2.0 | 3.0 |
| Primers | Sequence (5′–3′) |
|---|---|
| F1 | GGCTCCTTCACTTATTCACG |
| R1 | ATTGTCTCCCCAAACCCT |
| GSP1 | ATTGTCTCCCCAAACCCT |
| GSP2 | GCTCCTTCACTTATTCACG |
| NGSP1 | GCAAGAGTGAGAGATAGCGAAGGTT |
| NGSP2 | GATACAACTGTCCTGGAACTT |
| qF | GTGGCACAGGCAGTGAATAC |
| qR | CCTTCCCCTCGCAACTAG |
| Bg18S (F) | CGGGGGCATTCGTATTTC |
| Bg18S (R) | CCTGGTCGGCATCGTTTAT |
| Ko18S (F) | CCTGAGAAACGGCTACCACATC |
| Ko18S (R) | ACCCATCCCAAGGTCCAACTAC |
| Am18S (F) | CCCGTTGCTGCGATGAT |
| Am18S (R) | GCTGCCTTCCTTGGATGTG |
| Rs18S (F) | ACCATAAACGATGCCGACC |
| Rs18S (R) | CCTTGCGACCATACTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-Y.; Wang, Y.-S.; Sun, C.-C.; Fei, J. Cloning and Expression of Class I Chitinase Genes from Four Mangrove Species under Heavy Metal Stress. Plants 2023, 12, 2772. https://doi.org/10.3390/plants12152772
Zhou Y-Y, Wang Y-S, Sun C-C, Fei J. Cloning and Expression of Class I Chitinase Genes from Four Mangrove Species under Heavy Metal Stress. Plants. 2023; 12(15):2772. https://doi.org/10.3390/plants12152772
Chicago/Turabian StyleZhou, Yue-Yue, You-Shao Wang, Cui-Ci Sun, and Jiao Fei. 2023. "Cloning and Expression of Class I Chitinase Genes from Four Mangrove Species under Heavy Metal Stress" Plants 12, no. 15: 2772. https://doi.org/10.3390/plants12152772
APA StyleZhou, Y.-Y., Wang, Y.-S., Sun, C.-C., & Fei, J. (2023). Cloning and Expression of Class I Chitinase Genes from Four Mangrove Species under Heavy Metal Stress. Plants, 12(15), 2772. https://doi.org/10.3390/plants12152772






