Performance and Stability Analysis of Selected Durum Wheat Genotypes Differing in Their Kernel Characteristics
Abstract
1. Introduction
2. Results
2.1. Field Data
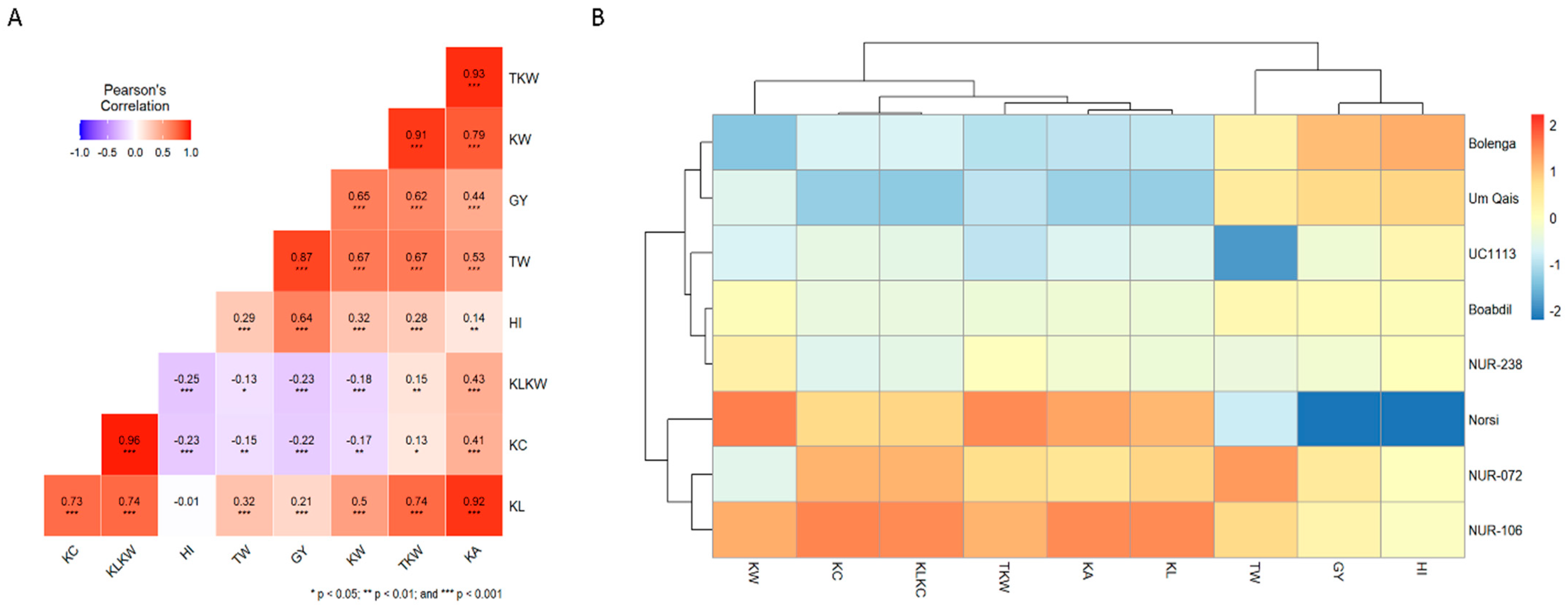
2.2. Correlation and Cluster Analysis
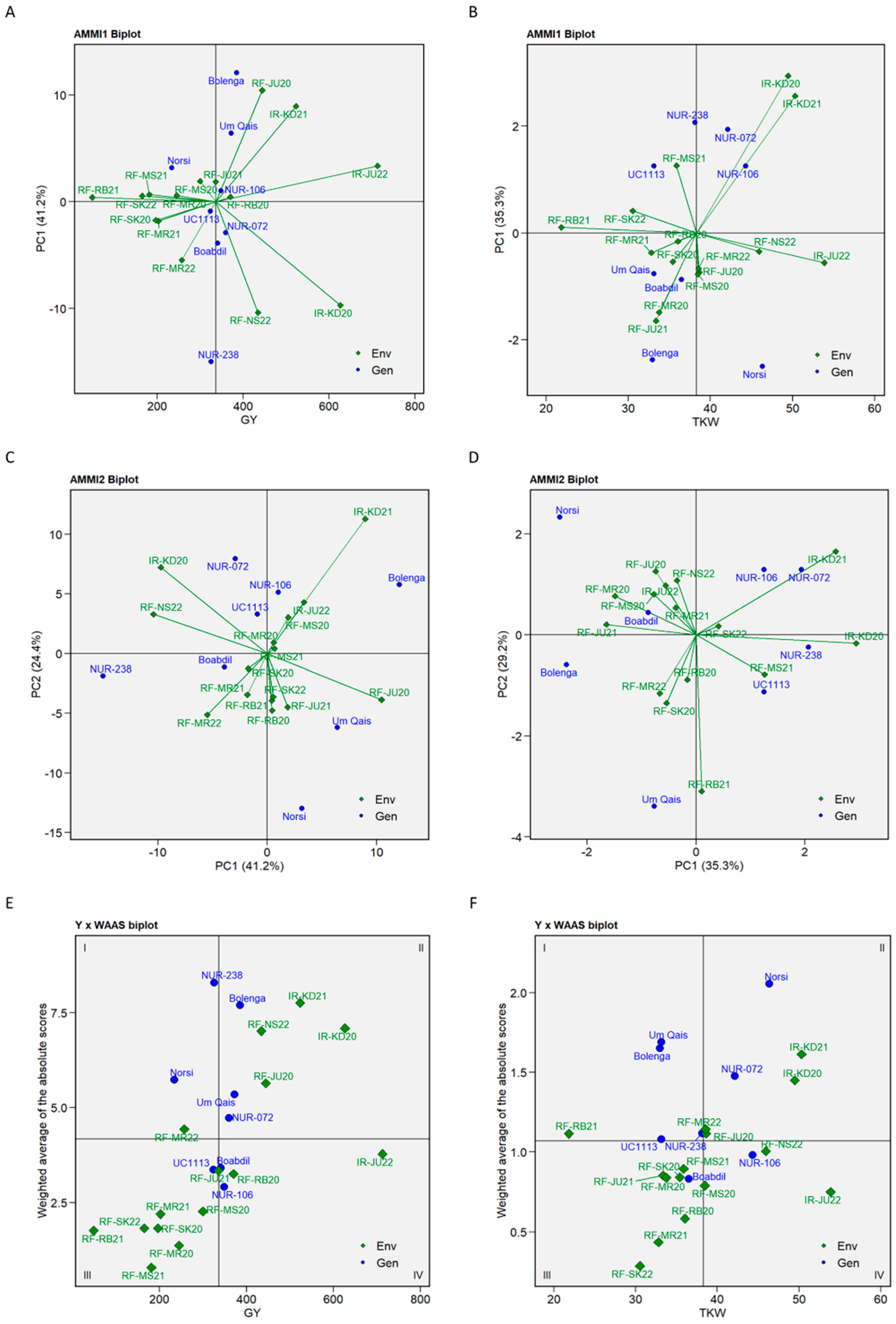
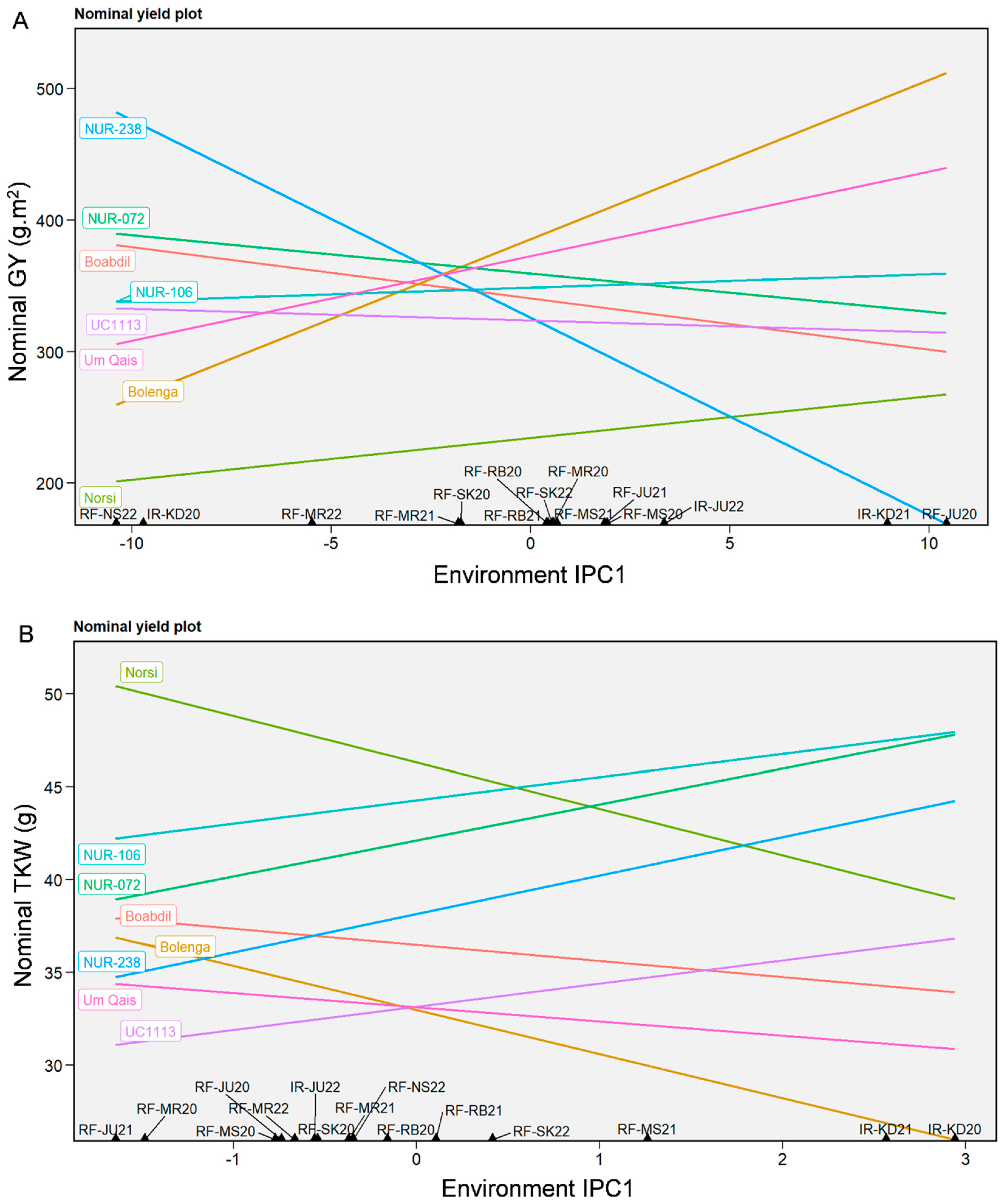
2.3. Genotypes Performance and Stability Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Experiments
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Grains Council [IGC], Online Database: World Grain Statistics. 2016. Available online: https://www.igc.int/en/subscriptions/subscription.aspx (accessed on 21 May 2023).
- Martínez-Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, İ.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum wheat in the Mediterranean Rim: Historical evolution and genetic resources. Genet. Resour. Crop. Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- Gonzalez-Segura, E.; Magaña-Barajas, E.; Torres-Chávez, P.I.; Manthey, F.; Ramírez-Wong, B. Characterization of the Dynamic Viscoelastic Behavior of Semolina Dough Obtained from Mexican Durum Wheat Cultivars. Adv. Chem. Eng. 2014, 3, 58–63. [Google Scholar]
- Kadkol, G.P.; Sissons, M. Durum Wheat: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 117–124. [Google Scholar] [CrossRef]
- Ermişer, D.; Yalçın, E. Dietary fibre, protein profile and technological characteristics of durum spaghetti enriched with refined/whole grain hull-less barley flour. J. Cereal Sci. 2021, 102, 103315. [Google Scholar] [CrossRef]
- De Vita, P.; Taranto, F. Durum Wheat (Triticum turgidum ssp. durum) Breeding to Meet the Challenge of Climate Change. In Advances in Plant Breeding Strategies: Cereals, 1st ed.; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2019; Volume 5, pp. 471–524. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Van Hoorn, J.W.; Lahmer, F.Z.; Hamdy, A.; Oweis, T. Durum wheat and barley productivity in saline–drought environments. Eur. J. Agron. 2009, 31, 1–9. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Dube, E.; Laing, M.D.; Tsilo, T.J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agric. 2016, 15, 935–943. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P. Changes in grain weight as a consequence of de-graining treatments at pre-and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum × T. tauschii). Funct. Plant Biol. 2000, 27, 183–191. [Google Scholar] [CrossRef]
- Kobata, T.; Koç, M.; Barutçular, C.; Tanno, K.I.; Inagaki, M. Harvest index is a critical factor influencing the grain yield of diverse wheat species under rain-fed conditions in the Mediterranean zone of south-eastern Turkey and northern Syria. Plant Prod. Sci. 2018, 21, 71–82. [Google Scholar] [CrossRef]
- Semenov, M.A.; Stratonovitch, P.; Alghabari, F.; Gooding, M.J. Adapting wheat in Europe for climate change. J. Cereal Sci. 2014, 59, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A systematic review of durum wheat: Enhancing production systems by exploring genotype, environment, and management (G × E × M) synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef]
- Bassi, F.M.; Nachit, M.M. Genetic gain for yield and allelic diversity over 35 years of durum wheat breeding at ICARDA. Crop. Breed. Genet. Genom. 2019, 1, e190004. [Google Scholar] [CrossRef]
- Li, W.; Yang, B. Translational genomics of grain size regulation in wheat. Theor. Appl. Genet. 2017, 130, 1765–1771. [Google Scholar] [CrossRef]
- Mangini, G.; Blanco, A.; Nigro, D.; Signorile, M.A.; Simeone, R. Candidate genes and quantitative trait loci for grain yield and seed size in durum wheat. Plants 2021, 10, 312. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Wu, Q.; Chen, Y.; Zhang, P.; Zhang, Y.E.; Hu, W.; Wang, X.; Zhao, H.; Dong, L.; et al. Molecular characterization of a novel TaGL3-5A allele and its association with grain length in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2019, 132, 1799–1814. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Meng, Y.; Hao, Y.; Xu, H.; Hao, M.; Lan, S.; Zhang, Y.; Lv, L.; Zhang, K.; et al. Dissection of genetic basis underpinning kernel weight-related traits in common wheat. Plants 2021, 10, 713. [Google Scholar] [CrossRef]
- Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Longin, C.F.H. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theor. Appl. Genet. 2018, 131, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integr. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Desiderio, F.; Zarei, L.; Licciardello, S.; Cheghamirza, K.; Farshadfar, E.; Virzi, N.; Sciacca, F.; Bagnaresi, P.; Battaglia, R.; Guerra, D.; et al. Genomic regions from an Iranian landrace increase kernel size in durum wheat. Front. Plant Sci. 2019, 10, 448. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2009, 49, 184–189. [Google Scholar] [CrossRef]
- Kaur, V.; Behl, R.; Singh, S.; Madaan, S. Endosperm and pericarp size in wheat (Triticum aestivum L.) grains developed under high temperature and drought stress conditions. Cereal Res. Commun. 2011, 39, 515–524. [Google Scholar] [CrossRef]
- Xiao, Y.; He, S.; Yan, J.; Zhang, Y.; Zhang, Y.; Wu, Y.; Xia, X.; Tian, J.; Ji, W.; He, Z. Molecular mapping of quantitative trait loci for kernel morphology traits in a non-1BL. 1RS × 1BL. 1RS wheat cross. Crop. Pasture Sci. 2011, 62, 625–638. [Google Scholar] [CrossRef]
- Adjabi, A.; Bouzerzour, H.; Benmahammed, A. Stability analysis of durum wheat (Triticum durum Desf.) grain yield. J. Agron. 2014, 13, 131–139. [Google Scholar] [CrossRef]
- Sehgal, D.; Mondal, S.; Guzman, C.; Garcia Barrios, G.; Franco, C.; Singh, R.; Dreisigacker, S. Validation of candidate gene-based markers and identification of novel loci for thousand-grain weight in spring bread wheat. Front. Plant Sci. 2019, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Armion, M.; Sadeghzadeh, B.; Golkari, S.; Khalilzadeh, G.R.; Ahmadi, H.; Abedi-Asl, G.R.; Eskandari Torbaghan, M. Assessment of grain yield stability and adaptability of rainfed durum wheat breeding lines. Appl. Field Crop. Res. 2017, 29, 25–42. [Google Scholar] [CrossRef]
- Mohammadi, R.; Sadeghzadeh, B.; Poursiahbidi, M.M.; Ahmadi, M.M. Integrating univariate and multivariate statistical models to investigate genotype × environment interaction in durum wheat. Ann. Appl. Biol. 2021, 178, 450–465. [Google Scholar] [CrossRef]
- Rawashdeh, N.K.; Haddad, N.I.; Al-Ajlouni, M.M.; Turk, M.A. Phenotypic diversity of durum wheat (Triticum durum Desf.) from Jordan. Genet. Resour. Crop. Evol. 2007, 54, 129–138. [Google Scholar] [CrossRef]
- Ayed, S.; Bouhaouel, I.; Othmani, A.; Bassi, F.M. Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia. Agronomy 2021, 11, 1782. [Google Scholar] [CrossRef]
- Frankin, S.; Roychowdhury, R.; Nashef, K.; Abbo, S.; Bonfil, D.J.; Ben-David, R. In-field comparative study of landraces vs. modern wheat genotypes under a Mediterranean climate. Plants 2021, 10, 2612. [Google Scholar] [CrossRef] [PubMed]
- Nazco, R.; Villegas, D.; Ammar, K.; Peña, R.J.; Moragues, M.; Royo, C. Can Mediterranean durum wheat landraces contribute to improved grain quality attributes in modern cultivars? Euphytica 2012, 185, 1–17. [Google Scholar] [CrossRef]
- Soriano, J.M.; Villegas, D.; Sorrells, M.E.; Royo, C. Durum wheat landraces from east and west regions of the Mediterranean basin are genetically distinct for yield components and phenology. Front. Plant Sci. 2018, 9, 80. [Google Scholar] [CrossRef]
- Dencic, S.; Kastori, R.; Kobiljski, B.; Duggan, B. Evaluation of grain yield and its components in wheat cultivars and landraces under near optimal and drought conditions. Euphytica 2000, 113, 43–52. [Google Scholar] [CrossRef]
- Royo, C.; Maccaferri, M.; Álvaro, F.; Moragues, M.; Sanguineti, M.C.; Tuberosa, R.; Maalouf, F.; del Moral, L.F.G.; Demontis, A.; Rhouma, S.; et al. Understanding the relationships between genetic and phenotypic structures of a collection of elite durum wheat accessions. Field Crop. Res. 2010, 119, 91–105. [Google Scholar] [CrossRef]
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic diversity within a global panel of durum wheat (Triticum durum) landraces and modern germplasm reveals the history of alleles exchange. Front. Plant Sci. 2017, 8, 1277. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, A.A. Phenotypic divergence in the meta-population of the Hourani durum wheat landrace. J. Food Agric. Environ. 2006, 4, 186–191. [Google Scholar]
- Aberkane, H.; Amri, A.; Belkadi, B.; Filali-Maltouf, A.; Kehel, Z.; Tahir, I.S.; Meheesi, S.; Tsivelikas, A. Evaluation of durum wheat lines derived from interspecific crosses under drought and heat stress. Crop. Sci. 2021, 61, 119–136. [Google Scholar] [CrossRef]
- Chamekh, Z.; Karmous, C.; Ayadi, S.; Sahli, A.; Hammami, Z.; Fraj, M.B.; Benaissa, N.; Trifa, Y.; Slim-Amara, H. Stability analysis of yield component traits in 25 durum wheat (Triticum durum Desf.) genotypes under contrasting irrigation water salinity. Agric. Water Manag. 2015, 152, 1–6. [Google Scholar] [CrossRef]
- Haouari, C.C.; Nasraoui, A.H.; Carrayol, E.; Gouia, H. Variations in a-, β-amylase and a-glycosidase activities in two genotypes of wheat under NaCl salinity stress. Afr. J. Agric. Res. 2013, 8, 2038–2043. [Google Scholar] [CrossRef]
- Bassi, F.M.; Sanchez-Garcia, M. Adaptation and stability analysis of ICARDA durum wheat elites across 18 countries. Crop. Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
- Martinez, F.; Sillero, J.C.; Rubiales, D. Resistance to leaf rust in cultivars of bread wheat and durum wheat grown in Spain. Plant Breed. 2007, 126, 13–18. [Google Scholar] [CrossRef]
- Royo, A.; Abió, D. Salt tolerance in durum wheat cultivars. Span. J. Agric. Res. 2003, 1, 27–35. [Google Scholar] [CrossRef]
- Zaim, M.; Kehel, Z.; Garcia, M.S.; Belkadi, B.; Maltouf, A.F.; Al Abdallat, A.; Bassi, F.M. Genomic regions of durum wheat involved in water productivity. BioRxiv 2023, 2023-06. [Google Scholar] [CrossRef]
- Benkadja, S.; Maamri, K.; Guendouz, A.; Oulmi, A.; Frih, B. Stability analysis for grain yield and thousand kernel weight of durum wheat (Triticum durum Desf.) genotypes growing under semi-arid conditions. Agric. Sci. Technol. 2022, 14, 34–43. [Google Scholar] [CrossRef]
- Sall, A.T.; Cisse, M.; Gueye, H.; Kabbaj, H.; Ndoye, I.; Filali-Maltouf, A.; Belkadi, B.; El-Mourid, M.; Ortiz, R.; Bassi, F.M. Heat tolerance of durum wheat (Triticum durum desf.) elite germplasm tested along the Senegal river. J. Agric. Sci. 2018, 10, 217. [Google Scholar] [CrossRef]
- Chicaiza, O.; Khan, I.A.; Zhang, X.; Brevis, J.C.; Jackson, L.; Chen, X.; Dubcovsky, J. Registration of five wheat isogenic lines for leaf rust and stripe rust resistance genes. Crop. Sci. 2006, 46, 485–487. [Google Scholar] [CrossRef]
- Houshmand, S.; Arzani, A.; Mirmohammadi-Maibody, S.A.M. Effects of salinity and drought stress on grain quality of durum wheat. Commun. Soil Sci. Plant Anal. 2014, 45, 297–308. [Google Scholar] [CrossRef]
- Mohler, V.; Albrecht, T.; Castell, A.; Diethelm, M.; Schweizer, G.; Hartl, L. Considering causal genes in the genetic dissection of kernel traits in common wheat. J. Appl. Genet. 2016, 57, 467–476. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; You, J.; Tang, H.; Mu, Y.; Jiang, Q.; Liu, Y.; Chen, G.; Wang, J.; Qi, P.; et al. Genetic identification and characterization of chromosomal regions for kernel length and width increase from tetraploid wheat. BMC Genom. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, M.; Alqudah, A.M.; Lohwasser, U.; Tarawneh, R.A.; Simón, M.R.; Börner, A. Genetic dissection of grain architecture-related traits in a winter wheat population. BMC Plant Biol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Kumar, A.; Mantovani, E.E.; Seetan, R.; Soltani, A.; Echeverry-Solarte, M.; Jain, S.; Simsek, S.; Doehlert, D.; Alamri, M.S.; Elias, E.M.; et al. Dissection of Genetic Factors underlying Wheat Kernel Shape and Size in an Elite × Nonadapted Cross using a High Density SNP Linkage Map. Plant Genome 2016, 9, 2015-09. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, X.; Zhang, W.; Zhang, N.; Song, L.; Liu, L.; Xue, X.; Liu, G.; Liu, J.; Meng, D.; et al. QTL Detection for Kernel Size and Weight in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP and SSR-Based Linkage Map. Front. Plant Sci. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; Zhang, P.; Chen, T.; Tian, T.; Wang, P.; Che, Z.; Shahinnia, F.; Yang, D. Identification of quantitative trait loci (QTL) and meta-QTL analysis for kernel size-related traits in wheat (Triticum aestivum L.). BMC Plant Biol. 2022, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. META-R: A software to analyze data from multi-environment plant breeding trials. Crop. J. 2020, 8, 745–756. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Gauch, H.G., Jr. Statistical analysis of yield trials by AMMI and GGE. Crop. Sci. 2006, 46, 1488–1500. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.; da Silva, J.A.; Sari, B.G.; Diel, M.I. Mean performance and stability in multi-environment trials II: Selection based on multiple traits. Agron. J. 2019, 111, 2961–2969. [Google Scholar] [CrossRef]
- Rocha, J.R.D.A.S.D.C.; Machado, J.C.; Carneiro, P.C.S. Multitrait index based on factor analysis and ideotype-design: Proposal and application on elephant grass breeding for bioenergy. GCB Bioenergy 2017, 10, 52–60. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sayaydeh, R.; Shtaya, M.J.; Qubbaj, T.; Al-Rifaee, M.K.; Alabdallah, M.A.; Migdadi, O.; Gammoh, I.A.; Al-Abdallat, A.M. Performance and Stability Analysis of Selected Durum Wheat Genotypes Differing in Their Kernel Characteristics. Plants 2023, 12, 2664. https://doi.org/10.3390/plants12142664
Al-Sayaydeh R, Shtaya MJ, Qubbaj T, Al-Rifaee MK, Alabdallah MA, Migdadi O, Gammoh IA, Al-Abdallat AM. Performance and Stability Analysis of Selected Durum Wheat Genotypes Differing in Their Kernel Characteristics. Plants. 2023; 12(14):2664. https://doi.org/10.3390/plants12142664
Chicago/Turabian StyleAl-Sayaydeh, R., M. J. Shtaya, T. Qubbaj, M. K. Al-Rifaee, M. A. Alabdallah, O. Migdadi, I. A. Gammoh, and A. M. Al-Abdallat. 2023. "Performance and Stability Analysis of Selected Durum Wheat Genotypes Differing in Their Kernel Characteristics" Plants 12, no. 14: 2664. https://doi.org/10.3390/plants12142664
APA StyleAl-Sayaydeh, R., Shtaya, M. J., Qubbaj, T., Al-Rifaee, M. K., Alabdallah, M. A., Migdadi, O., Gammoh, I. A., & Al-Abdallat, A. M. (2023). Performance and Stability Analysis of Selected Durum Wheat Genotypes Differing in Their Kernel Characteristics. Plants, 12(14), 2664. https://doi.org/10.3390/plants12142664





