Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System
Abstract
1. Introduction
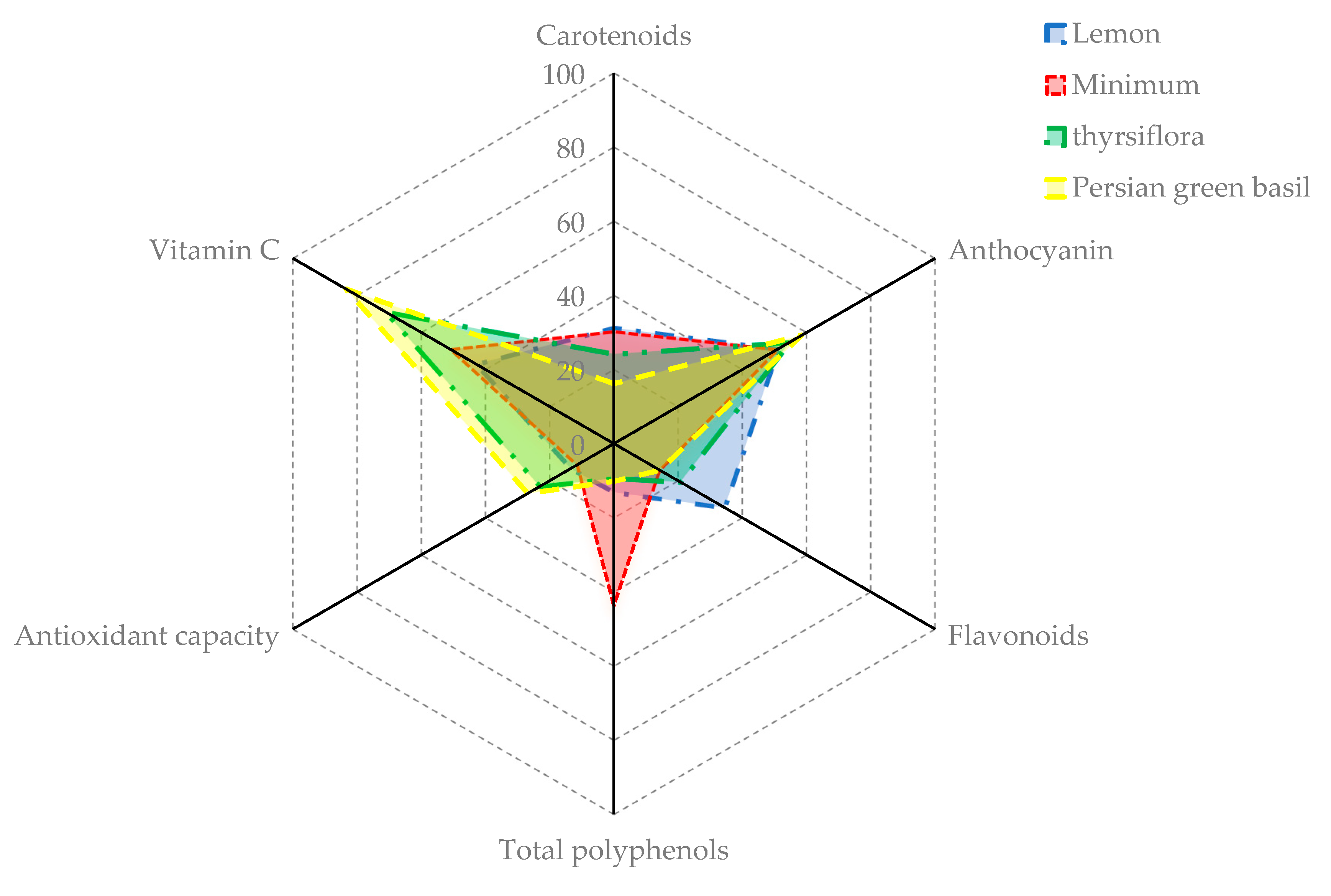
2. Results
2.1. Chlorophyll and Carotenoid Content
2.2. Vitamin C
2.3. Antioxidant Capacity (AC)
2.4. Total Phenolic Compounds (TPC)
2.5. Total Flavonoid Contents (TFC)
2.6. Anthocyanin
2.7. Nitrate
| 9 | Chla | Chlb | Chla + b | Car | Vit C | AC | TPC | TFC | ACNs | Nit | a* | L* | b* | Hue | Ch | AI | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chla | 1 | ||||||||||||||||
| Chlb | 0.987 ** | 1 | |||||||||||||||
| Chla + b | 0.999 ** | 0.992 ** | 1 | ||||||||||||||
| Car | 0.308 | 0.350 | 0.326 | 1 | |||||||||||||
| Vit C | 0.250 | 0.292 | 0.260 | 0.215 | 1 | ||||||||||||
| AC | 0.547 * | 0.544 * | 0.544 * | 0.161 | 0.385 | 1 | |||||||||||
| TPC | 0.476 * | 0.466 * | 0.481 * | 0.113 | 0.186 | 0.669 ** | 1 | ||||||||||
| TFC | −0.064 | −0.096 | −0.071 | 0.137 | −0.120 | 0.066 | −0.210 | 1 | |||||||||
| ACNs | 0.084 | 0.047 | 0.080 | 0.060 | −0.040 | 0.130 | 0.295 | 0.199 | 1 | ||||||||
| Nit | 0.497 * | 0.494 * | 0.500 * | 0.221 | −0.066 | 0.434 * | 0.367 | 0.495 * | −0.059 | 1 | |||||||
| a* | −0.123 | −0.180 | −0.140 | −0.163 | 0.019 | −0.129 | 0.234 | 0.250 | 0.472 * | −0.116 | 1 | ||||||
| L* | 0.119 | 0.157 | 0.126 | −0.050 | −0.171 | 0.083 | −0.203 | −0.367 | −0.372 | −0.027 | −0.814 ** | 1 | |||||
| b* | 0.163 | 0.212 | 0.174 | 0.039 | −0.133 | 0.056 | −0.210 | −0.187 | −0.546 * | 0.168 | −0.884 ** | 0.924 ** | 1 | ||||
| Hue | −0.189 | −0.219 | −0.201 | −0.243 | −0.014 | −0.187 | 0.288 | 0.101 | 0.309 | −0.135 | 0.910 ** | −0.666 ** | −0.716 ** | 1 | |||
| Ch | 0.155 | 0.209 | 0.168 | 0.082 | −0.090 | 0.047 | −0.190 | −0.224 | −0.543 * | 0.155 | −0.927 ** | 0.908 ** | 0.990 ** | −0.762 ** | 1 | ||
| AI | 0.536 * | 0.537 * | 0.543 * | 0.579 ** | 0.459 * | 0.667 ** | 0.561 ** | 0.379 | 0.452 * | 0.529 * | 0.181 | −0.320 | −0.259 | 0.055 | −0.242 | 1 | |
| Y | 0.301 | 0.302 | 0.302 | 0.112 | 0.067 | 0.127 | 0.448 * | −0.268 | 0.071 | 0.058 | 0.160 | 0.171 | 0.010 | 0.233 | −0.037 | 0.188 | 1 |
| Cultivars and Genotypes | a* | L* | b* | Hue | Chroma | APCI Index | Yield (kg m−2) | APCI Index × Yield |
|---|---|---|---|---|---|---|---|---|
| (−60/+60) | (0–100) | (−60/+60) | (0–360)° | √(a2 + b2) | ||||
| Persian Ablagh | −22.41 g | 51.55 d | 28.30 f | 179.10 b | 36.10 g | 70.30 a | 3.34 ab | 234.80 a |
| Dark Opal | −13.24 e | 42.94 j | 21.05 h | 178.99 b | 24.87 i | 46.05 g | 1.42 hi | 65.39 fg |
| Amethyst Improved | 6.41 b | 30.85 l | 13.00 j | 181.11 a | 14.49 l | 55.59 d | 2.32 cdefgh | 128.97 cd |
| Red Rubin | 8.82 a | 39.74 k | 16.58 i | 181.08 a | 18.78 k | 55.57 d | 3.16 abcd | 175.60 bc |
| Italian large leaf | −26.34 j | 50.02 def | 37.42 bc | 179.04 b | 45.76 abc | 56.33 d | 1.98 ghi | 111.53 e |
| Thyrsiflora | −25.05 hij | 57.79 b | 36.35 bc | 179.03 b | 44.15 c | 33.88 l | 3.60 a | 121.97 d |
| Cinnamon | −26.02 j | 55.03 c | 36.92 bc | 179.04 b | 45.17 bc | 36.71 jk | 3.01 abcde | 110.50 e |
| Persian green basil | −24.11 ghi | 49.34 efg | 30.77 e | 179.09 b | 39.09 e | 34.91 | 1.79 ghi | 62.49 g |
| Persian purple basil | 5.46 b | 45.47 i | 25.11 g | 181.36 a | 25.70 i | 38.18 ij | 3.03 abcde | 115.69 de |
| Basilico Rosso | 2.66 c | 39.28 k | 14.37 j | 181.39 a | 14.61 l | 45.64 g | 3.39 a | 154.72 c |
| Kapoor | −25.55 hij | 48.15 gh | 35.90 c | 179.05 b | 44.06 c | 63.71 b | 3.24 abc | 206.42 ab |
| lettuce leaf basil | −24.54 hij | 50.82 de | 33.51 d | 179.06 b | 41.53 d | 51.15 f | 2.19 efghi | 112.02 e |
| Classic Italiano | −25.95 ij | 60.91 a | 39.25 a | 179.01 b | 47.05 a | 42.43 h | 3.54 a | 150.20 c |
| Genovese | −26.26 j | 55.00 c | 37.84 ab | 179.04 b | 46.06 ab | 42.44 h | 3.77 a | 160.00 c |
| Lemon | −24.00 gh | 51.45 d | 34.06 d | 179.04 b | 41.67 d | 31.05 m | 1.35 i | 41.92 h |
| Mobarake | −26.37 j | 53.51 c | 37.05 bc | 179.05 b | 45.48 abc | 58.15 d | 2.05 fghi | 119.21 d |
| Clove | −24.04 gh | 48.59 fg | 29.98 ef | 179.11 b | 38.43 ef | 39.78 i | 2.42 bcdefg | 96.27 ef |
| Minimum | −22.68 g | 46.60 hi | 29.18 ef | 179.09 b | 36.96 fg | 33.57 l | 2.45 bcdefg | 82.25 f |
| Blue Spice | −25.96 ij | 54.21 c | 37.26 bc | 179.04 b | 45.41 abc | 35.25 kl | 1.95 ghi | 68.74 fg |
| Violetto | −8.99 d | 42.47 j | 21.41 h | 178.83 b | 23.22 j | 60.97 c | 2.98 abcdef | 181.69 b |
| Hoary | −20.25 f | 45.52 i | 25.23 g | 179.11 b | 32.35 h | 50.64 f | 2.29 defgh | 115.97 de |
| Species | *** | *** | *** | *** | *** | *** | *** | *** |
2.8. Leaf Color Profile
2.9. Antioxidant Potential Composite Index (APCI)
2.10. Yield
2.11. APCI Index × Yield (AY Index)
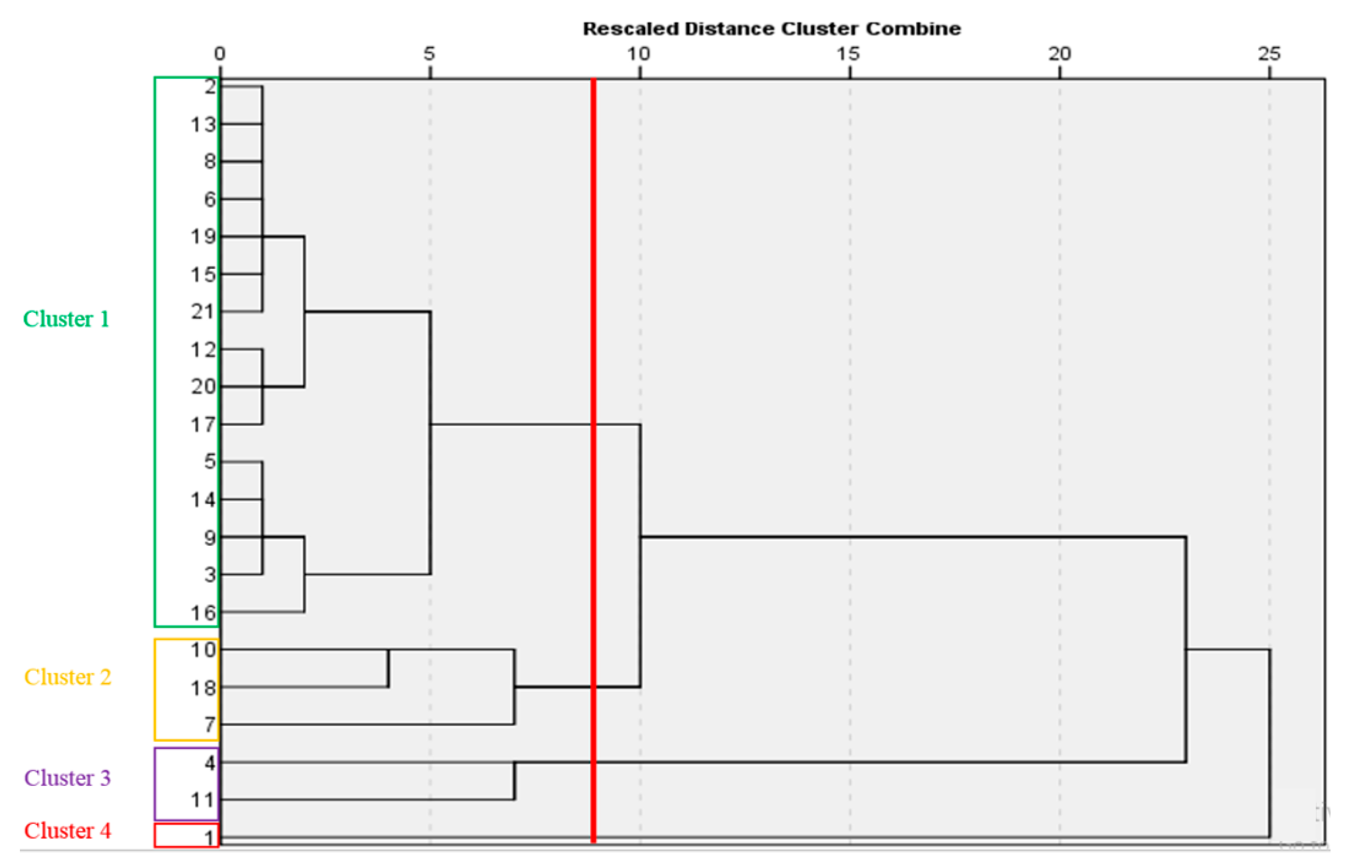
2.12. Cluster Analysis
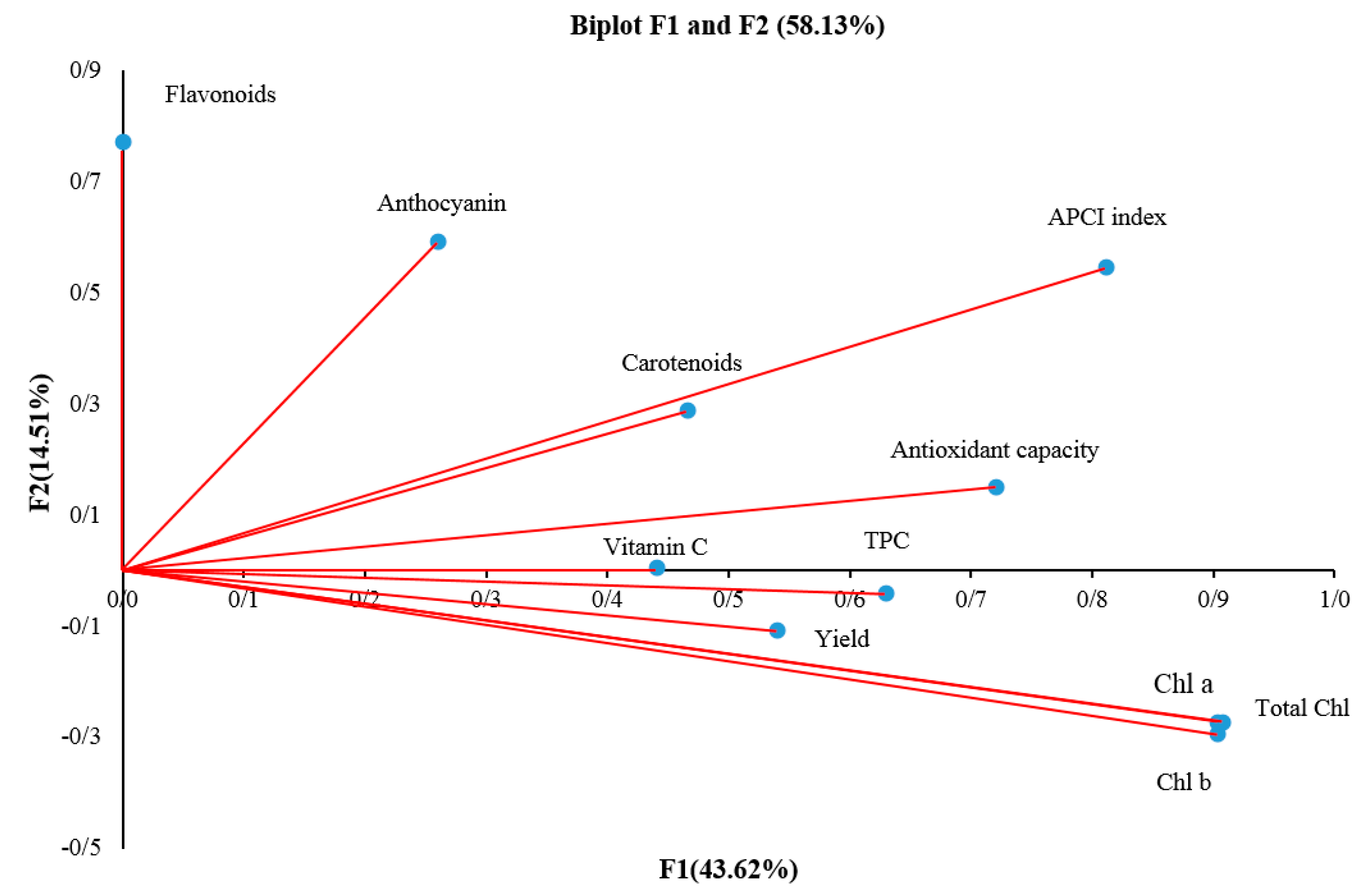
2.13. Principle Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation System
4.2. Chlorophyll and Carotenoid Content
4.3. Vitamin C
4.4. Antioxidant Capacity, Polyphenols, Flavonoids, and Anthocyanin
4.5. Nitrate Concentration
4.6. Leaf Color Profile
4.7. Antioxidant Potential Composite Index (APCI)
4.8. Yield of Basil Microgreen
4.9. APCI Index × Yield (AY Index)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhasin, M. Ocimum-Taxonomy, medicinal potentialities and economic value of essential oil. J. Biosph. 2012, 1, 48–50. [Google Scholar]
- Bhamra, S.K.; Heinrich, M.; Johnson, M.R.; Howard, C.; Slater, A. The Cultural and Commercial Value of Tulsi (Ocimum tenuiflorum L.): Multidisciplinary Approaches Focusing on Species Authentication. Plants 2022, 11, 3160. [Google Scholar] [CrossRef] [PubMed]
- Gaddamwar, A.G.; Rajput, P.R.; Parsodkar, V.J. Extraction of basil, padina, ajwain and development of oxygen garden in the school yard as a preventive measure for COVID-19. Int. J. Appl. Sci. Eng. 2020, 8, 1408–1411. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens species. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef]
- Frąszczak, B.; Kula-Maximenko, M. The Biometric Parameters of Microgreen Crops Grown under Various Light Conditions. Agriculture 2022, 12, 576. [Google Scholar] [CrossRef]
- Rizvi, A.; Sharma, M.; Saxena, S. Microgreens: A Next Generation Nutraceutical for Multiple Disease Management and Health Promotion. Genet. Resour. Crop Evol. 2023, 70, 311–332. [Google Scholar] [CrossRef]
- Treadwell, D.; Robert, H.; Linda, L.; Wanda, L. Microgreens: A New Specialty Crop: HS1164, Rev. 9/2020. Edis 2020. Available online: https://edis.ifas.ufl.edu/publication/HS1164 (accessed on 25 June 2023).
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Viera, I.; Chen, K.; Ríos, J.J.; Benito, I.; Pérez-Gálvez, A.; Roca, M. First-pass metabolism of chlorophylls in mice. Mol. Nutr. Food Res. 2018, 62, 1800562. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Ciriello, M.; Formisano, L.; Pannico, A.; Giordano, M.; Gentile, B.R.; Fusco, G.M.; Kyriacou, M.C.; Carillo, P.; Rouphael, Y. Protein hydrolysate combined with hydroponics divergently modifies growth and shuffles pigments and free amino acids of carrot and dill microgreens. Horticulturae 2021, 7, 279. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive Composition and Nutritional Profile of Microgreens Cultivated in Thailand. Appl. Sci. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Mezeyová, I.; Hegedűsová, A.; Golian, M.; Andrejiová, A.; Šlosár, M.; Mezey, J. Influence of Microgreens Biofortification with Selenium on Their Quantitative and Qualitative Parameters. Agronomy 2022, 12, 1096. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compost Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Georgios, A.; Soteriou, G.G.; Kyratzis, A.; Antoniou, C.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Preharvest nutrient deprivation reconfigures nitrate, mineral, and phytochemical content of microgreens. Foods 2021, 10, 1333. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Completing a pathway to plant vitamin C synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 9109–9110. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Giordano, M.; Pannico, A.; Carillo, P.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Cultivar-specific performance and qualitative descriptors for butterhead Salanova lettuce produced in closed soilless cultivation as a candidate salad crop for human life support in space. Life Sci. J. 2019, 9, 61. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant properties and sensory evaluation of microgreens from commercial and local farms. Food Sci. Hum. Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Kapur, A.A.; Hasković, A.; Čopra-Janićijević Klepo, L. Spectrophotometric analysis of total ascorbic acid contetnt in various fruits and vegetables. Glas. Chem. Tehnol. Bosne Herceg. 2012, 38, 39–42. [Google Scholar]
- Dhaka, A.S.; Dikshit, H.K.; Mishra, G.P.; Tontang, M.T.; Meena, N.L.; Kumar, R.R.; Praveen, S. Evaluation of Growth Conditions, Antioxidant Potential, and Sensory Attributes of Six Diverse Microgreens Species. Agriculture 2023, 13, 676. [Google Scholar] [CrossRef]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A route from bioavailability to bioactivity addressing potential health benefits to tackle human chronic diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health efects—A review. J. Func. Foods 2015, 18, 820–889. [Google Scholar] [CrossRef]
- Saini, R.; Mehra, H.; Goyal, T.; Dhiman, N.K. Dietary Antioxidants and their Potential Role in Human Disease Management. Curr. Nutr. Food Sci. 2023, 19, 262–281. [Google Scholar]
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Func. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT—Food Sci. Technol. 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, Y.; Lester, G.E.; Kou, L.; Yang, T.; Wang, Q. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. J. Food Sci. Technol. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Nemeth, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary favonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Scalbert, A. Ellagitannins–nature, occurrence and dietary burden. J Sci Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Keutgen, N.; Hausknecht, M.; Tomaszewska-Sowa, M.; Keutgen, A.J. Nutritional and sensory quality of two types of cress microgreens depending on the mineral nutrition. Agronomy 2021, 11, 1110. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzade, A.; Sadeghi, O.; Biregani, A.N.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory effects of flavonoids: Possible induction of TCD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front. Immunol. 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. Plant Physiol. 2002, 9, 172–174. [Google Scholar]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant. Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hort. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of Crisphead ‘Iceberg’ and Romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 37–45. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D.; Khadka, A.; Ojha, P. Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Func. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [PubMed]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hort. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs setting. Offic. J. Europ. Union 2011, 320, 15–17. [Google Scholar]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 2006, 201, 163–177. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Kariñho-Betancourt, E.; Hernández-Soto, P.; Rendón-Anaya, M.; Calderón-Cortés, N.; Oyama, K. Differential expression of genes associated with phenolic compounds in galls of Quercus castanea induced by Amphibolips michoacaensis. J. Plant Intract. 2019, 14, 177–186. [Google Scholar] [CrossRef]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as Plant Protective Companion against Abiotic Stress. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 277–308. [Google Scholar]
- Žlabur, J.Š.; Opačić, N.; Žutić, I.; Voća, S.; Poštek, M.; Radman, S.; Uher, S.F. Valorization of nutritional potential and specialized metabolites of basil cultivars depending on cultivation method. Agronomy 2021, 11, 1048. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ferreira, I.C.; Rosskopf, E.N. Microgreens: From trendy vegetables to functional food and potential nutrition security resource. In Proceedings of the III International Symposium on Soilless Culture and Hydroponics: Innovation and Advanced Technology for Circular Horticulture, Lemesos, Cyprus, 19–22 March 2021; 1321, pp. 235–242. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef]
- Arnon, A.N. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var Sheva). Sci. Horti. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Okamoto, Y. Simple spectroscopic determination of nitrate, nitrite, and ammonium in Arabidopsis thaliana. Bio Protoc. 2017, 7, e2280. [Google Scholar] [CrossRef] [PubMed]
- Minolta. Precise Color Communication; Minolta Camera, Ltd.: Ramsey, NJ, USA, 1993. [Google Scholar]


| Cultivars and Genotypes | Chl a | Chl b | Chl a + b | Carotenoids | Vitamin C | Antioxidant Capacity (%) | Polyphenols | Flavonoids | Anthocyanin | Nitrate |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg GA 100 g−1 FW) | (mg CAE g−1 FW) | (mg 100 g−1 FW( | (mg kg−1 FW) | ||
| Persian Ablagh | 0.65 bcd | 0.17 bc | 0.83 bc | 0.20 abcd | 1.69 bcde | 60.28 b | 1463.79 a | 1.68 g | 26.19 a | 533.29 defg |
| Dark Opal | 0.41 hij | 0.10 hij | 0.51 gh | 0.06 d | 1.56 bcdef | 35.14 cd | 146.64 g | 3.94 cde | 24.74 a | 155.98 i |
| Amethyst Improved | 0.27 k | 0.08 j | 0.35 i | 0.15 bcd | 1.77 bcd | 8.26 g | 486.17 de | 7.95 a | 18.78 bcde | 551.31 cdef |
| Red Rubin | 0.66 bcd | 0.16 cd | 0.82 bcd | 0.17 abcd | 1.71 bcd | 23.56 cdefg | 1210.21 b | 1.72 g | 21.05 b | 484.37 efgh |
| Italian large leaf | 0.48 efghi | 0.13 efg | 0.62 efg | 0.28 ab | 1.13 def | 31.42 cd | 432.52 de | 6.23 b | 16.58 cdefgh | 740.96 ab |
| Thyrsiflora | 0.59 cde | 0.15 cde | 0.74 cde | 0.08 cd | 1.71 bcd | 20.24 defg | 135.88 g | 1.64 g | 14.35 fgh | 147.11 i |
| Cinnamon | 0.50 efgh | 0.13 efg | 0.63 efg | 0.07 cd | 1.06 ef | 25.06 cdef | 848.10 c | 1.21 ghi | 14.18 fgh | 607.09 bcde |
| Persian green basil | 0.34 jk | 0.10 hij | 0.44 hi | 0.05 d | 2.00 abc | 23.52 cdefg | 148.76 g | 1.14 ghi | 15.32 efgh | 95.62 i |
| Persian purple basil | 0.43 fghij | 0.11 ghi | 0.53 fgh | 0.05 d | 1.19 def | 13.08 efg | 404.46 def | 3.58 e | 19.84 bcd | 142.82 i |
| Basilico Rosso | 0.41 hij | 0.11 ghi | 0.52 gh | 0.08 cd | 1.45 cdef | 54.49 b | 723.69 c | 0.63 i | 18.30 bcdef | 358.22 gh |
| Kapoor | 0.93 a | 0.24 a | 1.18 a | 0.24 abc | 2.40 a | 57.49 b | 1085.53 b | 1.58 g | 12.83 h | 721.22 abc |
| lettuce leaf basil | 0.73 b | 0.19 b | 0.92 b | 0.20 abcd | 1.27 def | 37.89 c | 253.05 efg | 4.53 c | 20.15 bc | 866.54 a |
| Classic Italiano | 0.40 hij | 0.12 fgh | 0.52 gh | 0.21 abcd | 1.46 cdef | 31.34 cd | 148.23 g | 1.46 g | 17.21 bcdefg | 126.51 i |
| Genovese | 0.36 ijk | 0.09 ij | 0.45 hi | 0.14 bcd | 1.29 def | 19.60 defg | 445.00 de | 3.79 de | 15.23 efgh | 651.71 bcde |
| Lemon | 0.40 hij | 0.10 hij | 0.50 gh | 0.10 bcd | 1.03 f | 11.78 efg | 191.29 fg | 2.74 f | 13.38 gh | 327.33 h |
| Mobarake | 0.55 def | 0.14 def | 0.68 def | 0.10 bcd | 1.95 abc | 86.76 a | 479.11 de | 4.30 cd | 13.01 gh | 675.46 bcd |
| Clove | 0.54 defg | 0.14 def | 0.68 def | 0.24 abc | 1.25 def | 20.57 defg | 291.53 efg | 0.77 hi | 15.71 defgh | 130.81 i |
| Minimum | 0.39 hijk | 0.11 ghi | 0.50 gh | 0.10 bcd | 1.22 def | 10.08 fg | 638.72 cd | 1.13 ghi | 13.32 gh | 403.70 fgh |
| Blue Spice | 0.42 ghij | 0.12 fgh | 0.54 fgh | 0.08 cd | 1.52 bcdef | 26.45 cde | 153.17 g | 2.36 f | 14.08 gh | 601.94 bcde |
| Violetto | 0.69 bc | 0.17 bc | 0.86 bc | 0.23 abcd | 1.45 cdef | 61.86 b | 281.64 efg | 6.03 b | 18.36 bcdef | 744.39 ab |
| Hoary | 0.31 jk | 0.09 ij | 0.40 hi | 0.33 a | 2.11 ab | 22.31 cdefg | 180.53 fg | 1.38 gh | 15.93 defgh | 108.40 i |
| Species | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | |
|---|---|---|---|
| Total | % of Variance | Cumulative % | |
| 1 | 4.80 | 43.62 | 43.62 |
| 2 | 1.60 | 14.51 | 58.13 |
| 3 | 1.17 | 10.66 | 68.79 |
| 4 | 1.03 | 9.33 | 78.12 |
| F1 | F2 | F3 | F4 | |
|---|---|---|---|---|
| Chlorophyll a | 0.90 | −0.27 | −0.07 | −0.28 |
| Chlorophyll b | 0.90 | −0.29 | −0.11 | −0.24 |
| Total chlorophyll | 0.90 | −0.27 | −0.07 | −0.27 |
| Carotenoids | 0.46 | 0.28 | −0.46 | 0.04 |
| Vitamin C | 0.44 | 0.00 | −0.30 | 0.75 |
| Antioxidant capacity | 0.72 | 0.15 | −0.06 | 0.16 |
| Total Polyphenols | 0.63 | −0.04 | 0.49 | 0.16 |
| Total flavonoid content | 0.00 | 0.77 | −0.28 | −0.39 |
| Anthocyanin | 0.26 | 0.59 | 0.61 | −0.04 |
| APCI index | 0.81 | 0.54 | −0.03 | 0.15 |
| Yield | 0.54 | −0.10 | 0.36 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants 2023, 12, 2652. https://doi.org/10.3390/plants12142652
Fayezizadeh MR, Ansari NA, Sourestani MM, Hasanuzzaman M. Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants. 2023; 12(14):2652. https://doi.org/10.3390/plants12142652
Chicago/Turabian StyleFayezizadeh, Mohammad Reza, Naser Alemzadeh Ansari, Mohammad Mahmoudi Sourestani, and Mirza Hasanuzzaman. 2023. "Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System" Plants 12, no. 14: 2652. https://doi.org/10.3390/plants12142652
APA StyleFayezizadeh, M. R., Ansari, N. A., Sourestani, M. M., & Hasanuzzaman, M. (2023). Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants, 12(14), 2652. https://doi.org/10.3390/plants12142652








