Abstract
Environmental changes, both natural and anthropogenic, mainly related to rising temperatures and water scarcity, are clearly visible around the world. Climate change is important for crop production and is a major issue for the growth and productivity of cucumbers. Processes such as sex determination, flower morphogenesis and fruit development in cucumbers are highly sensitive to various forms of stress induced by climatic changes. It is noteworthy that many factors, including genetic factors, transcription factors, phytohormones and miRNAs, are crucial in regulating these processes and are themselves affected by climate change. Changes in the expression and activity of these factors have been observed as a consequence of climatic conditions. This review focuses primarily on exploring the effects of climate change and abiotic stresses, such as increasing temperature and drought, on the processes of sex determination, reproduction, and fruit development in cucumbers at the molecular level. In addition, it highlights the existing research gaps that need to be addressed in order to improve our understanding of the complex interactions between climate change and cucumber physiology. This, in turn, may lead to strategies to mitigate the adverse effects and enhance cucumber productivity in a changing climate.
1. Introduction
Agriculture in many ways is closely linked to changes in climatic conditions, whether natural or anthropogenic, as both have a significant impact on the yield of economically important vegetable crops, such as cucumbers. Due to the high concentration of nutrients, micronutrients, vitamins and minerals, cucumbers are essential sources for human nutrition. Favorable environmental growing conditions, which depend on many factors, are the most important criteria for good crop quality. These factors include good growing conditions, soil, cultivation techniques, irrigation and fertilization, as well as biotic and abiotic conditions, all of which have a major influence on quality of the cucumber. Due to continuous changes in climatic conditions, the quality of cucumbers could be affected [1,2]. Climate change is caused by increased emissions of greenhouse gases (carbon dioxide, nitric oxide and methane). Rising greenhouse gases not only increase global temperatures, but also alter rainfall patterns, humidity, drought and CO2 concentration. This could lead to reduced yields, which may not be enough to meet food needs as the world’s population continues to grow. Predicting and understanding the effects of climate change on plant developmental processes, from the molecular basis to the desired phenotype, is increasingly important.
Crops are exposed to abiotic and biotic stresses as a result of climate change. Abiotic stresses (temperature, drought and CO2 concentration) can have a direct effect on plant development. Increasing temperatures affect physiological and biochemical pathways that are essential for respiration, cellular mechanisms, photosynthetic activity and enzymatic activity, and they also influence the action of various genetic factors, transcription factors and hormones. This can affect changes in the genome and epigenetic regulation, which affect changes in the regulation of transcription and translation, causing physical and biochemical changes. Such a self-perpetuating condition can lead to the production of various plant defense mechanisms in response to changing climatic conditions. As a result, this affects the development of the plant and leads to changes in crop and yield productivity (Figure 1).
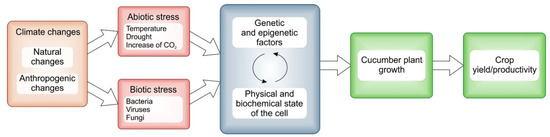
Figure 1.
Schematic model of factors correlated with climate change and their impact on plant development and crop productivity.
In addition, abiotic stresses can also affect biotic stresses. Biotic stresses resulting from pathogen attack can have direct or indirect effects on crop species. The direct effects of pests on plants include reproduction survival, development and spread of the pest, while the indirect effects include the relationship between the pest/pathogen to the environment and to other pest/organisms, such as competitors, enemyspecies, vectors and mutualists. Some common pest/pathogen of cucumbers include Podosphaera xanthii, Tetranychus urticae and Aphis gossypii, and some viruses include cucumber mosaic virus (CMV), cucumber green mottle mosaic virus (CGMMV), cucurbit yellow stunting disorder (CYSDV), potyviruses zucchini yellow mosaic virus (ZYMV), papaya ringspot virus (PRSV), watermelon mosaic virus (WMV) and Moroccan watermelon mosaic virus (MWMV) are very well studied for their interaction with cucumbers in changing climatic situation [3,4]. A coevolution of plants and pathogens can be observed in the environment. Changes in climatic conditions have accelerated this process and led to the evolution of new pests and pathogens, resulting in the introduction of new plant diseases [5]. Therefore, climate change poses a higher risk to vegetable crops, such as cucumbers.
The cucumber (Cucumis sativus, n = 7) belongs to the Cucurbitaceae family, which includes 825 species in 118 genera and is one of the most important economic crops [6,7]. Cucumbers are susceptible to various stresses, either biotic or abiotic, which severely affect agronomic factors, such as plant growth and productivity. The productivity of cucumber is largely dependent on the formation of good-quality fruits. Fruit development depends on the completion of two stages of the plant life cycle: the vegetative and the reproductive stages. Both of these stages are highly sensitive to environmental stress conditions. In cucumber cultivation, variable growth patterns are observed. This includes high fruit production with delayed growth as well as low fruit production with rapid growth [8]. Kahlen (2007) conducted a study that emphasized the effect of environmental conditions on plant growth [9]. The research highlighted the environmental factors, including temperature, light, humidity and nutrient availability, that significantly affected plant development and growth patterns. In addition, the architecture of a plant plays a crucial role in determining its growth and development which is largely dependent on the technical ability to cultivate.
Temperature is a major factor that changes continuously and is an important determinant of cucumber growth, development and yield. Higher temperature, both air and soil, reduces overall growth by affecting various physiological processes, such as reduced photosynthesis rate and increased transpiration rate [10,11]. Decreased photosynthesis results in reduced biomass and smaller fruit size, while increased transpiration rate results in increased plant water demand, leading to loss of soil water reserves and thus plant water stress. Combined temperature and water stress significantly reduce crop productivity, affecting flowering onset, male to female flower ratio, health and viability of male and female reproductive elements, reduced fruit yield and accelerated fruit ripening. The key factors, including temperature, light and relative humidity, also influence plant stomatal conductance, vapor pressure deficit and transpiration, which also have an impact on the production [12]. The temperature change also affects phytohormone (ethylene, auxin, GA and ABA) biosynthesis and signaling factors, thus altering processes at the molecular level, resulting in altered gene expression and further modification of the composition of the proteome and metabolome. This leads to developmental changes that affect yield quality. To cope with stress, plants have developed various stress response mechanisms, that activate molecular factors by regulating gene expression, involving specific transcription factors and signaling miRNA molecules.
The aim of this study is to identify possible pathways through which climate change may affect sex determination at the molecular level, which in turn affects fruit development and crop yield. Understanding how growth development and sex determination are regulated may lead to increased yields and improved agricultural practices. Our goal is to identify sensitive and critical points in the biology of cucumber flower development that may occur under changing climatic conditions and reduce yield productivity and quality. Finding solutions to increase crop productivity is essential to combat environmental impacts and meet the food needs of a rapidly growing population. Therefore, there is a need to focus on linking gene function to important agronomic traits as well as the development of genetic transformation techniques and gene-editing technologies for cucumbers, which are essential for functional studies on cucumbers.
2. Cucumber Growth, Flower and Fruit Development under the Impact of Climatic Changes
2.1. Basics of Cucumbers
The cucumber originates from tropical and subtropical regions; therefore, it prefers warm weather, and the optimal temperature for growth is 25–30 °C during the day and 18–21 °C at night. Nowadays, cucumbers are cultivated worldwide, but it requires a minimum of 15 °C for its development [13] and is sensitive to chilling and frost [14,15]. For good yield, cucumbers require high light intensity [16] and well-draining soils rich in organic matter at a pH of 6.0–7.0. The supply of cucumbers is limited due to several factors affecting production [17]. In cucumbers, the female flower development is particularly important, as it leads to fruit production and increases crop yield and productivity.
There are several sex morphotypes in cucumbers, including monoecious, dioecious, andromonoecious, trimonoecious, gynoecious and hermaphrodite [18]. These sex types are determined by the presence and arrangement of male and female flowers on the plant, making cucumber a good model for studying sex determination, fruit development and the vascular system in plants [19,20]. Flower development begins with the initiation of floral meristems and ends with blooming. In cucumbers, flower buds initially contain the potential to develop both male and female reproductive structures. However, the expression of certain genes and their interactions ultimately determine whether the flower will develop as male, female or hermaphroditic. Specifically, the inhibition of either the stamens or the carpels development results in the formation of unisexual flowers. In the absence of inhibition, the flower develops with both stamens and carpels, resulting in a bisexual flower [18,21].
The initiation of flowering and subsequent fruit development in cucumber plants is a complex process involving the interplay of multiple factors. Genetic elements, including transcription factors, repressors, genes and phytohormonal signals, play a crucial role in these processes. However, it is important to note that the expression and regulation of these genetic factors are controlled by the dynamic environmental conditions in which the plants grow. The combined action of these factors results in changes at multiple levels, including the physiological, morphological and molecular levels, which ultimately control the intricate processes involved in flower and fruit formation in cucumber plants. Both external and internal factors are important in determining how cucumber plants grow and develop.
2.2. Climatic Impact
Several interrelated phenomena affecting plant growth and yield are associated with the observed climatic changes. Increasing temperatures are correlated with a decrease in rainfall, which is associated with a decrease in the amount of water available, leading to drought stress. In addition, it affects the change of seasons and can lead to rapid changes in weather that negatively affect crops (frost, heavy or prolonged rainfall leading to flooding, long periods of drought). Therefore, in the present study, we paid more attention to two climatic factors, increasing temperature and drought, and their effects on the cucumber plants development, mainly in relation to sex determination.
2.2.1. Effect of Temperature Increase on Cucumber
Climate change can be described as a long-term, significant difference in average weather [22]. Like other agricultural crops, cucumber production also depends on environmental/climatic conditions for a better yield. In general, farmers face losses in the form of low productivity due to climate change [22,23,24]. This temperature variation can affect vegetable crops. As the optimum temperature for cucumber growth is between 20 and 25 °C, temperatures of around 35 °C can induce heat stress in the plants [25]. Stress has been well studied for reducing crop production and accelerating fruit ripening, which affects fruit quality [26]. Elevated temperature suppresses photosynthetic processes by modulating enzymatic activity, mainly Rubisco and other related enzymes [10] and the electron transport chain [27], resulting in the impairment of chlorophyll biosynthesis [11]. High temperature can also indirectly affect the photosynthetic process by increasing leaf surface temperature and affecting stomatal conductance [28,29]. Elevated temperatures not only affect the above ground parts of plants, but also significantly affect the root system. Heat in the root zone significantly reduces plant height, stem diameter, shoot fresh weight, shoot dry mass, and shoot water content of cucumbers.
The duration of initiation and expansion of floral organs and leaves is shortened by warming, leaving less time for biomass accumulation, ultimately resulting in reduced plant size [30,31]. Higher temperatures (42 °C) also suppress seed germination in cucumbers, thereby reducing plant growth and reproductive traits. In cucumbers, increasing temperature has a detrimental effect on sex expression, flowering, pollination and fruit set. High temperatures and long days tend to keep the vines in the male phase, while short days and low temperatures encourage more female flowers. Thus, an increase in temperature may reduce the number of female flowers, which indicates lower productivity [32,33]; male flowers are increased, but they are smaller and have reduced nectar, which will affect pollination, and pollen per flower also decline. In addition, early flowers in cucumber may drop when exposed to extremely high temperatures [34,35]. At the stage of fruit development, if the cucumber plant is exposed to high temperature, it will cause bitterness in the fruit [34]. High temperatures exert significant effects on various physiological characteristics in cucumbers, including changes in malondialdehyde (MDA) content and the activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) enzymes [11,36]. These studies provide scientific evidence for the effects of high temperature on cucumber physiology [37,38]. In addition, high temperatures have been found to disrupt normal flower development in cucumbers by inducing pollen sterility [36]. Leaf wilting, physical damage to plant shoot and root growth, physiological disorders, biochemical changes and reproductive problems also result in a significant reduction in crop yield at high temperatures [39,40].
2.2.2. Impact of Drought on Cucumber
Drought is another major limiting factor for agricultural crops and, when combined with high temperatures, can affect the vitality of crops [41]. Depending on the species, yield losses under drought stress can range from 30 to 90 percent for sensitive crops. [42]. If plants can adapt their physiology to a drought environment, they will have a better chance of surviving. Higher temperatures may increase the ability of the environment to absorb more water vapor, resulting in more evapotranspiration by plants, which will lead to higher water requirements. Increase plant water demand could deplete the reservoir of water in the cultivating soil, creating a state of plant water stress (EPA, 2021). In dry and semidry climates, drought stress is the major factor that negatively affects plant growth and production, and undoubtedly reduces crop productivity [43,44,45,46,47]. Drought stress can reduce agricultural production by decreasing the activity of enzymes involved in the Calvin cycle [48,49,50].
The presence of drought results in numerous physiological, biochemical, morphological and molecular changes [43,44], including vascular tissue contraction, decreased water uptake [51] and impaired photo assimilate translocation [52]. In addition, drought inhibits ion uptake, impairs ATP biosynthesis and ROS accumulation, which accelerates oxidative damage and ultimately reduces plant development [53]. According to González Villagra et al. [54], drought stress disrupts the production of endogenous phytohormones by increasing ABA concentrations, decreasing IAA and GAs, and rapidly decreasing zeatin concentrations. The hormonal imbalance slows down the growth of plant cells by reducing their turgor, elongation and volume, leading to a decrease in growth characteristics [51]. Liu et al. (2018) conducted a study that demonstrated the negative effects of drought on cucumber seedlings, specifically showing a decrease in leaf thickness [55]. This can be attributed to a simultaneous decrease in the thickness of both the palisade and spongy layers. The study provides evidence of drought effects on cucumber seedling morphology. In addition, an Indian research group conducted a descriptive study on different cucumber genotypes and showed that drought also leads to a reduction in fruit yield per vine [56]. The study highlighted that different cucumber genotypes have different responses to drought stress. Farag et al. (2019) conducted a study that focused on the effect of drought stress on cucumber yield and its components [56]. The results of the study showed a significant decrease in yield, including a reduction in the number of fruits per plant, fruit weight per plant, and total yield, when compared to well-watered plants.
In addition, severe drought stress was found to have extensive detrimental effects on various aspects of cucumber growth and development. Metwaly et al. (2022) reported that severe drought significantly reduced vine length, fresh leaf weight, number of branches per plant, leaf number per plant, photosynthetic pigment content, and leaf area per plant [57]. These results provide evidence for the widespread negative effects of severe drought on cucumber plants. The very first response of plants to drought is stomatal closure. Further, as drought stress continues, plants induce other acclimation responses, such as cell wall modification and antioxidant production [58,59].
3. Molecular Regulation of Sex Determination, Flower and Fruit Development
Cucumbers are a species within the angiosperm family with the advantage of having perfect flowers with separate carpels and stamen coexisting together. The regulation of flower development, particularly of the female flower, has a significant impact on cucumber yield.
3.1. Sex Determination and Flower Morphogenesis Processes
Flowering is a critical trait in plants as it plays an important role in increasing crop productivity. Identifying and studying the genes involved in this process enables targeted approaches to crop improvement and agricultural sustainability.
Several studies have reported the effects of phytohormones, genetic factors, and environmental factors on sex determination in cucumbers (Figure 2). At the phytohormonal level, ethylene serves as the primary hormone that facilitates sex determination in cucumber flowers [60,61,62]. Auxin, cytokinin and brassinosteroids have a feminizing effect by interacting with ethylene signaling [63,64]. Although several ethylene synthesis genes involved in sex determination in cucurbits have been described, the focus is on elucidating the molecular link between the transition from male to female flowers or vice versa, which is still unknown.
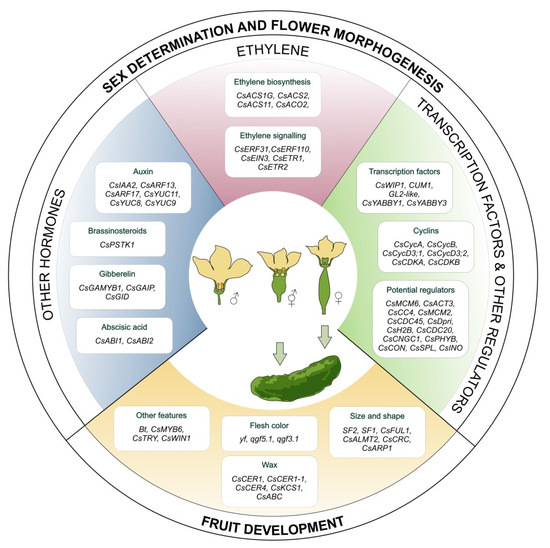
Figure 2.
Genes and transcription factors related to the sex determination, flower morphogenesis and fruit development processes in cucumbers.
Ethylene synthesis is a two-step process that begins with the conversion of S-adenosyl-L-methionine (SAM) to 1-aminocyclopropane-1-carboxylic acid (ACC) by the enzyme ACC synthase (ACS) (Figure 3). ACC is then converted to ethylene by ACC oxidase (ACO) enzymes [65,66,67]. This conversion is regulated by three main genes: female (F, encoding an extra copy of the CsACS1G gene), androecious (a, encoding ACS11, which blocks the female flower developmental pathway), and monoecious (M, encoding ACS2 in the carpel primordia) [68,69,70,71,72]. These genes encode ACC synthases and oxidases, that together regulate a complex ethylene synthesis process, leading to female flower development [73,74].
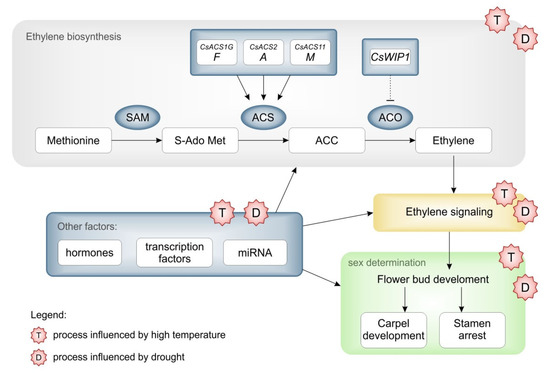
Figure 3.
The hypothetical scheme of how the temperature and drought influence the sex determination in cucumbers. The scheme of ethylene biosynthesis and signaling in sex determination pathway in cucumbers, major molecular regulators of these processes and possible influence of climatic factors.
CsACS1G, CsACS2, CsACS11 and CsACO2 are some of the important genes involved in the synthesis of the ethylene in cucumber [75]. The F locus is responsible for femaleness and determines the female phenotype among the three genetic loci. In cucumber, the F gene refers to CsACS1, indicating a gynoecious plant [76]. A duplication of the F gene leads to the development of the ACS1G gene, which has a new promoter and is expressed early in the development of floral buds [77,78,79]. Even in the absence of ACS11, ACS1G regulates ethylene development in conjunction with ACO2 [77,78]. Additionally, CsWIP1, which encodes a C2H2 zinc finger transcription factor, inhibits female flower development and leads to the formation of male flowers in cucumbers [80,81]. CsWIP1 binds to the promoter of CsACO2 and causes a decrease in its expression [74].
Yamasaki et al. (2017) observed a correlation between sex determination and cell cycle pathways in cucumbers [82]. They examined the expression of six cell-cycle-related genes: four cyclins, CsCycA, CsCycB, CsCycD3;1 and CsCycD3;2; and two cyclin-dependent kinases, A (CsCDKA) and CsCDKB in male and female flower buds. Wang et al. (2019) performed RNA sequencing on young apical buds of gynoecious and female cucumber at three different growth stages [83]. They identified nine genes as potential candidates for sex differentiation regulators in cucumber: Cs-MCM6, Cs-ACT3, Cs-XRCC4, Cs-MCM2, Cs-CDC45, Cs-Dpri, Cs-H2B, Cs-CDC20 and Cs-CNGC1. Five of them (Cs-MCM6, Cs-MCM2, Cs-CDC45, Cs-Dpri and Cs-CDC20) were found to be involved in the cell cycle pathway, suggesting that they may play an important role in cucumber sex determination [83]. A recent discovery has revealed the presence of an insertional LTR-RT mutation within the first exon of the CsPHYB gene, which is responsible for the long hypocotyl and early flowering phenotype observed in cucumbers [84].
Although ethylene-synthesizing genes are known to be involved in sex determination, some ethylene-signaling genes are also essential for this process. Following ethylene biosynthesis, little information is known about downstream ethylene-signaling to regulate female flower development. After the synthesis of ethylene, its signaling is regulated by several receptor proteins presented on the membrane of the endoplasmic reticulum (ER). In the presence of ethylene, several ethylene-responsive factors (ERFs) are activated and initiate the expression of several downstream ethylene-responsive genes [85,86,87]. An increase in the promoter activity of CsACS11 through interactions with CsERF110 in cucumbers was confirmed using yeast one-hybrid assay. This interaction also regulates ethylene signaling [88]. CsEIN3 activates CsERF31, which stimulates CsACS2 and acts as a positive feedback loop to increase ethylene and female-flower production in the ethylene signaling. CsERF31 also responds to the F-derived ethylene signal and positively regulates ethylene feedback in cucumbers by activating M expression [89]. Ethylene perception interferes with stamen growth in female cucumber flowers through DNA damage. The ethylene receptor CsETR1 is downregulated in the stamen of female flowers, causing stamen development arrest [90,91]. Under increased amount of endogenous ethylene, CsETR2 and CsERS play role in female flower development in gynoecious cucumber plants. Molecular and functional characterization of two C. pepo mutants with altered ethylene receptors, CpETR1A and CpETR2B, indicated that mutations in these genes leads to the change of sex morphotypes, converting monoecious plants to andromonoecious plants. In addition to the initial hermaphroditization of female flowers, the number of male flowers also increase significantly [92,93]. Another ethylene-inducible DNase gene, CsCaN, functions in the stamen primordia of female floral buds [94]. This could be the reason for the inhibition of stamen formation in female flowers [91].
3.2. Factors Regulating Sex Determination
Ethylene is the main sex-related hormone, but other phytohormones, such as cytokinin, brassinosteroids and auxin, promote carpel development through interactions with ethylene, while gibberellins promote stamen development. In addition to ethylene, auxin indirectly influences the sex of cucumbers. Exogenous IAA can increase ACS gene expression and promote ACC and ethylene production, thereby influencing female flower formation [63]. CsIAA2 and other auxin-inducible genes have been proposed to play a role in the promoting ethylene and thus regulating sex in cucumbers [95]. More female flowers were produced in both female and male lines directly due to an increase in exogenous auxin concentration. The auxin-induced binding of ESR2 to the ACS2 promoter increases the expression of ACS2. This binding increases more ethylene production, which in turn makes the cucumber more feminine [96]. In some cases, reduction in auxin biosynthesis had no effect on sex determination in cucumber, which may indicate that auxin synthesis does not play a direct role in sex expression [62]. Analysis of the cucumber MADS box (CUM1) suggests that auxin and ethylene regulate the development of male and female flowers by altering CUM1 expression. This regulation of sex determination by auxins is mediated by the auxin response factors CsARF13 and CsARF17, which act as upstream regulators of CUM1 [97]. Auxin biosynthesis is regulated by YUCs, which catalyze the rate limiting step, and CsYUC11, which have specific expression in the stamen of male flower development [98]. The endogenous concentration of indole-3-acetic acid (IAA) serves as a defense mechanism against heat-induced damage by triggering the activation of CsYUC8 and CsYUC9 genes in response to high temperatures [98].
Cucumber plants treated with exogenous GA inhibit ethylene biosynthesis by suppressing the expression of the CsACS1G gene [62], which inhibits female flower production and promotes maleness. CsGAMYB1, a positive regulator of gibberellin signaling, is thought to produce male flowers while inhibiting female flowers [99]. CsGAIP, a negative regulator of GA signaling, inhibits B-class floral homeotic genes, thereby preventing the formation of male flowers. The ubiquitin proteosomal proteolysis of CsGAIP reduces B-class homeotic gene inhibition and promotes staminate development [100]. In addition, transcriptome analysis revealed that GA regulates cucumber sex expression in an ethylene-dependent manner, probably involving the genes CsACS2, CsETR1 and ERFs, or in an ethylene-independent manner, involving the C-class floral homeotic gene CAG2 [101]. High temperatures induce GA and GA-biosynthesis-related genes in Arabidopsis and soybeans. It is possible that high temperature induces male flower development by increasing GA, but this needs to be investigated [102]. Fukuda (2009) reported that elevated temperature also promotes bolting in lettuce by increasing the expression of LsGA3ox1, which is a key gene responsible for stem elongation [103].
Brassinosteroids also have an ethylene-dependent effect. Female flower production was increased by the application of epibrassinolide in cucumbers, melons and zucchinis [63,64]. Exogenous brassinosteroids increase cucumber femaleness and cause increased ethylene production [104]. BAK1, a receptor in the BR signaling pathway, and CsPSTK1, a putative serine/threonine kinase, are correlated, suggesting that CsPSTK1 may be involved in BR signaling [105].
The role of ABA in sex determination has not been widely reported, but one study has identified CsABI1 and CsABI2 genes (members of the protein phosphatase 2C gene family) that are differentially expressed in male and female floral buds [106]. This finding suggests that ABA signaling is involved in the formation of male and female flowers and has a regulatory role during floral morphogenesis and a role in the selective development of specific whorls in unisexual flowers [106]. In addition to these parameters, hormones have complex interactions with sugars to maintain signaling pathways, particularly ABA and ethylene [107,108].
Plant growth and development is highly dependent on the coordinated expression of all above mentioned genes and many others that are regulated by plant transcription factors (TFs). These TFs can influence the expression of downstream genes by directly or indirectly binding to cis-regulatory elements in the promoter region of the target genes. They can upregulate or downregulate gene expression, and possess various motifs, including DNA-binding and activation motifs. There are several families of transcription factors with different motifs in their protein structure, such as bZIP, HD-ZIP III, NAC, AP2/EREBP, WRKY, bHLH, TCP, MADS Box, ARF and MYB families [109]. For example, the HD-ZIP IV family transcription factor GL2-LIKE interacts with CsJAZ1 to form a complex that regulates male flower development, seed viability and pollen intensity by regulating the expression of the FT gene expression [110]. In addition, in cucumbers, CsSPOROCYTELESS (CsSPL) directly interacts with CsYABBY1, CsYABBY3 and CsINO to regulate integument development in cucumber ovules, as evidenced by yeast two-hybrid (Y2H) assay [111].
The research reported above provides strong evidence for the association of phytohormones and transcription factors with variation in climate conditions for the regulation of flower morphogenesis in plants. Considering this, it can be said that factors such as temperature and drought are important in regulating flowering in plants.
3.3. Climate Effect on Flowering
The above mentioned genes may be related to climate change. Climatic conditions (drought, heat stress and humidity) have a major impact on the molecular mechanisms of the cell. This, in turn, changes the phenotypes of the flower [112,113,114].
Not many genes are identified in cucumber that could be affected by stress but are reported in other plant species. Temperature may play a role in activating or repressing ACS activity, resulting in either induced or suppressed ethylene production. It is accelerated in kiwis [115] and gets suppressed in tomatoes [116]. For cucumbers, whether it is suppressed or enhanced is still an unexplored, but it would be interesting to find out whether ethylene is suppressed because a reduction of female flowering is reported for cucumbers at high temperatures.
Not only high temperature, but also drought stress, is also related to ethylene signaling. Under drought conditions, ethylene signaling is severely affected in many plants [117,118,119]. Drought-induced ethylene biosynthesis leads to the de novo synthesis of ACS, which accumulates ACC and increases the ethylene levels [120]. In rice, OsERF101 is reported to be induced by drought stress especially in reproductive structures [121]. Other genes, such as OsERF109 and OsERF3, act as a potential genetic factor that improves drought tolerance by repressing ethylene signaling and the expression of ethylene biosynthetic genes, respectively [122,123]. A study on sugarcane demonstrated the role of ERF3 in increasing tolerance to drought stress [124]. OsARD1, a metalloenzyme known as ACIREDUCTONE DIOXYGENASE, interacts with Fe2+ and mediates the synthesis of methionine, a critical precursor in the ethylene biosynthetic pathway. The overexpression of OsARD1 in rice plants significantly increased endogenous ethylene levels, improved water capacity and reduced susceptibility to drought stress [125]. The effects of drought on sex expression and flower have not been well studied in cucumbers, so it would be very interesting to investigate the effects of drought on the sex expression and flower development in cucumbers. In addition, investigating the relationship between climatic conditions and the regulatory mechanisms of sex-related-genes are of a great interest in the context of cucumber research.
High temperatures are usually an inducer of high ethylene production due to the acceleration of all plant metabolic processes. High temperatures induce ethylene production in several agricultural crops, such as kiwi (Actinidia deliciosa (A. Chev.) [115,126], soybean (Glycine max) [127] and wheat (Triticum aestivum L.) [128]. In addition, molecular studies on soybeans have shown that an increase in ethylene production induced by high temperatures is associated with a decrease in photosynthetic activity and an increase in lipid peroxidation and reactive oxygen species accumulation [129].
Drought-tolerant plants respond to stress by increasing ethylene production. Alfalfa (Medicago sativa L.) [130], cotton [131] and rice [120] have been shown to increase ethylene production during a water deficit. Furthermore, a water deficit in wheat resulted in the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) [120].
Since it has been shown in plants that drought stress can induce increased ethylene production, it is very likely that in cucumbers, depending on the genotype, this stress can affect the distribution of flowers on the plant or even the proportion in their appearance, with a tendency towards female flowers. In other plants, considering the regulation at the molecular level, studies of the transcriptome carried out under drought conditions allowed for the observation of an increased activity of genes related to ethylene biosynthesis and signaling in soybeans [132].
Ethylene can be considered as a marker of stress conditions. However, it can also have negative effects on production quality, for example, in ornamental plants. Ethylene induced by water stress during cultivation has been shown to increase the malformation of buds or flowers and negatively affect the quality of freesia [133,134].
3.4. Fruit Development and Regulators of this Process
The initiation of female flower formation marks a crucial moment in fruit development, and it is essential to note that the growing conditions during this phase are of a great importance. The interplay between environmental factors and the intricate biological mechanisms involved in fruit growth can significantly affect the final outcome. In addition to flower development, fruit growth depends on the interaction of genetic factors that can act as a growth inhibitors or stimulators. The interaction of various factors results in fruit diversity in terms of most basic morphological characteristics, such as shape, size and color, which are important criteria to improve fruit quality.
The size and shape of the fruit are remarkable features of cell division, which increases the number and proliferation of the cells. Cucumber fruits can have different shapes like long or short and oblong/oval/cylindrical, but the majority of cucumbers has an elongated shape. Various genetic factors involved in cell cycle pathway have a direct impact on the size of cucumber fruit. Recent research has shed light on the vital role of the SF2 gene, which encodes a histone deacetylase HDC1 that plays a crucial role in regulating the proliferation of fruit cells by controlling the synthesis and metabolism of polyamines and cytokinins. SF2 mutations result in a significant decrease in cell proliferation rate, which causes shorter cucumber fruit production [135]. Another gene that has been shown to affect cucumber fruit development is SF1, a RING-type E3 ligase. SF1 mutations result in increased self-ubiquitination and degradation. This also leads to the accumulation of ACS2, which is a rate-limiting enzyme in ethylene biosynthesis. This overproduction of ethylene, in turn, leads to the development of shorter cucumbers in these mutants, showing a dose-dependent effect of ethylene [136]. CsFUL1a, a MADS-box like gene that binds to the promoter of Superman (SUP) and represses its expression, which can affect cell division and inhibit auxin transport during fruit elongation, causes the development of shorter fruits [137]. A newly discovered gene, CsALMT2, has been identified as being responsible for the formation of the hollow trait in cucumber. This gene is mainly expressed in the ovule development zone, which is located inside the fruit. The expression of CsALMT2 in this specific zone suggests its involvement in regulating cellular processes related to ovule development, potentially affecting the overall fruit morphology, but the molecular mechanisms are still unidentified [138].
Fruit development in plants is influenced by phytohormones. GA and auxin positively regulate fruit development, while it is negatively regulated by ethylene [139,140,141]. The commercial value of cucumber fruit depends on its size, color and curvature. The overproduction of ethylene results in long fruits, while decreased ethylene production suppresses cell division, resulting in shorter fruits. Therefore, maintaining the correct ethylene dosage is critical to properly regulate cell division in the developing fruit [136]. The GA receptor gene, CsGID1a, is associated with fruit locule formation and therefore promotes cell expansion to control fruit shape in cucumber [142]. The amount of IAA affects fruit size at different developmental stages in cucumber. CsYUC10b, an auxin biosynthesis gene, is involved in the formation of fruit curvature, and its overexpression promotes straight fruit development [143].
In addition to their role in sex determination, the effects of bZIP family transcription factors have been reported in many processes, such as organ and tissue differentiation, hormone signaling, light response and pathogen defense in plants. They also include developmental processes, physiological systems and stress situations in different plants, such as maize [144], cucumbers [145] and tomatoes [146]. Two MYB genes, CsMYB6 and CsTRY, negatively regulate trichome initiation on cucumber fruits [147], while CsMYB60 regulates fruit spine color in cucumber [148]. In Arabidopsis, WIN1 was identified as a wax-regulator gene and was found to be responsible for the glossy phenotype. CsWIN1, another AP2/ERF-type transcription factor, is associated with pericarp formation in cucumbers and increases the expression of wax biosynthetic genes, such as CsCER1, CsCER1-1, CsCER4, CsKCS1 and CsABC, the wax transporter gene [149]. It has been observed that a member of the YABBY family called CRC (CRABS CLAW) influences fruit length in cucumbers. This gene has two alleles, namely CsCRCA and CsCRCG, which contribute to different phenotypes associated with short and long fruit length, respectively [150].
The downstream effects of CsCRC on fruit length involve the regulation of several genes involved in cucumber development. CsARP1, which encodes an auxin-responsive protein, serves as a target gene of CsCRC and plays an important role in promoting fruit elongation, primarily by facilitating cell expansion [150].
Fruits in cucumber are comprised of three distinct layers: epicarp, mesocarp and endocarp. The mesocarp and endocarp are responsible for the flesh of the fruit, which has a variety of colors ranging from orange to yellow and green or white. The main pigments that give the fruit its color are chlorophylls and carotenoids. β-carotene, as a vital nutrient, contributes significantly to maintaining healthy eyes. According to Cuevas (2010), the amount of β-carotene in the mesocarp and endocarp was controlled by two recessive genes and a single recessive gene, respectively. In cucumbers, the accumulation of β-carotene to high levels in the endocarp causes a natural genetic variant in a β-carotene hydroxylase gene [151]. Lu et al. (2015) identified a single recessive gene, yf, which is responsible for the yellow color of flesh in cucumber [152]. In addition, two loci, qgf5.1 and qgf3.1, responsible for the formation of green flesh in cucumber were identified through QTL mapping and GWAS. The candidate gene for qgf5.1 was reported to be Csa5G021320 [153]. However, very little information has been reported on the molecular mechanism underlying the genetic factors that govern the regulation of fruit flesh color in cucumbers.
The effect of bitterness in fruits not only reduces their flavor but also diminishes their marketability, which ultimately leads to a decrease in demand for the crop and loss of profit for the farmers. The major component responsible for the bitterness in cucumber fruit is cucurbitacin C (CuC) [154,155], which is an oxygenated tetracyclic triterpenoid. The Bt (bitter fruit) gene, a transcription factor that regulates CuC biosynthesis, has been shown to control the levels of cucurbitacin only in cucumber fruits [156]. CuC is rapidly synthesized in response to biotic or environmental stresses, such as very low or very high temperature, drought or insufficient light intensity. Therefore, changes in the environmental conditions can affect fruit flavor and further the quality of market product [157]. Climatic conditions have a significant impact on the proper development of flowers and fruits. Thus, understanding the influence of climatic conditions on this critical stage can have impressive results on fruit quality and yield. However, little is known about the effects of climate change on fruit formation and development at the molecular level.
3.5. Regulation of mRNA and miRNA during Growth and Development by Climatic Factors
Transcriptome analysis revealed several key genes that are involved in the environmental stress response in cucumbers [158,159]. A high expression of NAC, abscisic acid 80-hydroxylase1, ethylene responsive genes, three WRKYs TFs, CsCaM3 and bHLH96 are reported to have higher expression in heat stress condition to provide tolerance to high temperature in cucumbers [160]. It has been reported that most of the genes activated during heat stress encode transcription factors or coactivators involved in thermo-tolerance regulation. The key regulators of plant response to elevated temperature are heat shock factors (HSFs) [161]. In addition to these reported TFs, lncRNAs, circRNAs and miRNAs also play an important role in the heat stress response in cucumbers [162]. Antioxidant enzymes, such as SOD, CAT, APX and POD, also play a role in the heat stress response as well. They are more activated under stress and are involved in thermo-tolerance in cucurbits [163,164], and genes encoding these enzymes show higher expression under heat stress conditions also in cucumbers [165]. The HSP gene family, which encodes heat shock proteins, is of great importance in the response to heat stress, which has been well described in cucurbits. The role of the HSP20 genes in response to elevated temperature has been well studied in pumpkins, cucumbers, melons and watermelons [166,167,168,169]. Thermo-tolerance is an important quantitative trait in cucumbers and is controlled by QTLs. Several articles have reported the role of QTLs in cucumbers under heat stress. QTLs-qHT3.2 and qHT1.1 in cucumber seedlings have been reported to tolerate heat stress [170,171].
As key regulatory molecules, miRNAs play a pivotal role in modulating gene expression or repression by directly binding to their target genes. The expression of miRNAs is also affected by abiotic and biotic stresses, such as drought, heat and CO2 [172]. In various plant species, miRNAs have been reported to be directly or indirectly associated with key factors involved in flower and fruit development processes, including phytohormones, in particular ethylene in cucumbers. In Poncirus trifoliate, the positive regulation of cold tolerance by miR396b occurs through the downregulation of ACO transcripts, resulting in the inhibition of ethylene synthesis and the facilitation of polyamine synthesis. This dual effect ultimately enhances the ability to scavenge reactive oxygen species (ROS) [173].
A comprehensive understanding of miRNA contribution to flower development was obtained by profiling miRNA expression in tomatoes. Potential miRNAs and their target genes that can be correlated with heat stress regulation were identified. miRNA–target pairs, such as miR398b-3p/SlCSD1, miR393-5p/SlTIR1, miR160a/SlARF10/16, miR156e-5p/SlSPL15 and miR397-5p/LACs, were shown to be correlated with the regulation of heat stress response and metabolic pathways in stamens and pistils in tomatoes [174]. The other study showed the differential expression of miR156/157 in cucumbers under higher temperature conditions and negative correlation with the expression of its target genes, encoding SBP-box transcription factors [175]. It has been reported that SBP-box genes are crucial in the regulation of plant growth, flowering, fruit development and other processes [176]. Therefore, it can be hypothesized that miR156/157 regulates the physiological processes involved in plant growth and development through the SBP-box TFs. In addition to temperature, the effect of CO2 stress on flowering was shown to be mediated via miRNA in leaves and bolters of Arabidopsis. The expression of miR156/157 and miR172 is differentially regulated by high CO2 levels and elevated temperatures, showing different responses. High CO2 level facilitates an enhanced flowering process by suppressing the expression of miR156/157, which targets SPL9, while concurrently activating the expression of miR172, which controls the expression of AP2. Conversely, high temperature exerts an opposite effect on these miRNAs compared to high CO2 levels [177,178]. Furthermore, it has been observed that the expression of miR156 and miR172 is affected by temperature, leading to temperature-dependent effects on the DOG1 gene. The DOG1 gene plays a crucial role in regulating seed germination and flowering time, which are important developmental milestones in plants. In the case of lettuce, the downregulation of LsDOG1 expression at high temperatures has been shown to facilitate seed germination and also promote early flowering. These effects are accompanied by a decrease in miR156 levels and an increase in miR172 levels [179].
Extensive research has revealed the important role of miR159 in Arabidopsis, rice and wheat, particularly in the regulation of the GAMYB-like family of transcription factors. These transcription factors play a critical role not only in flower development but also in the intricate gibberellin (GA) signaling pathways. In addition, GAMYB transcription factors were found to be involved in anther development. This shows that TamiR159 in wheat is involved in heat stress signaling, which in turn also affects anther development [180,181]. An analysis in cucumbers showed that csa-miR159b plays a critical role in ABA-mediated heat tolerance. During heat stress, ABA accumulates in plants and represses the csa-miR159b expression, which in turn causes the induction of the CsGAMYB1 gene, which promotes male flowers development in cucumber (in an ethylene-dependent or -independent manner) [182].
In the study by Zhang et al., the expression of a novel miR153, was found to be influenced by both temperature and photoperiod in cucumber shoot apices. This novel miRNA was shown to target ERFs (ethylene responsive factors), which are key components of ethylene signaling pathways. Based on these findings, it is hypothesized that the novel miR153 may be involved in the intricate process of flower sex determination, potentially modulating the expression and activity of ERFs to regulate floral development and reproductive processes in cucumbers [183].
However, there is a limited understanding of the specific miRNAs involved in the regulation of flowering and fruit ripening in cucumbers, particularly in response to climatic factors. This knowledge gap represents a significant research opportunity to identify key miRNAs that are influenced by different climatic stresses and their effects on these developmental processes.
4. Future Prospects
Climate change is a natural process that cannot be stopped, but due to human activities the changes are faster and more severe. The observed environmental changes, mainly related to temperature increase and water scarcity, are particularly important in the case of plant crop production (Figure 4). Therefore, there is a need to focus on linking gene function to important agronomic traits. The development of high-throughput genetic techniques to study genomes and transcriptomes, together with the modern breeding technologies, provides potential for functional studies in cucumbers. The analysis of individual genes and their molecular functions is important, although the basic research is very laborious. In particular, the study of these genes in relation to important aspects that affect growth, development and yield in the context of how they are expressed under stress are of great importance. Knowledge of the function of genes can make a significant contribution to the development of modern varieties that are not only resistant to disease, but also able to withstand and adapt to the climatic conditions brought about by climate change. Plants can respond differently depending on where they are grown, the climate zone and the soil conditions. The ability to cope with adverse conditions in agriculture or horticulture is a power that will benefit humanity. Using techniques such as sequencing to learn about the regulation of the transcriptome and genome of crops will make it possible to learn not only about individual genes, but entire sets of the genes, how they are regulated and how they respond to changing climate conditions. Modern analytical technologies and the knowledge gained from gene regulation will make it possible to breed cucumbers well to produce sufficient yields to feed the world’s growing population.
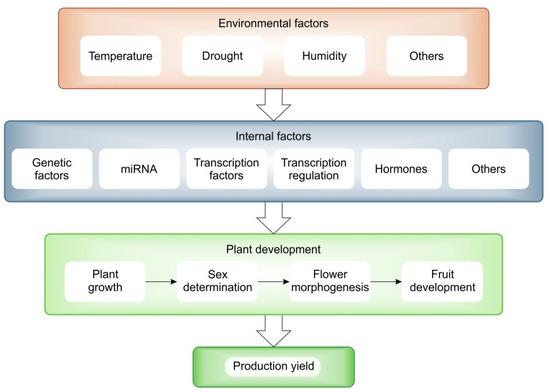
Figure 4.
General diagram of the influence of climatic factors (red box) through internal regulatory factors (blue box) on plant growth and yield (green boxes).
Author Contributions
Conceptualization, M.P.; resources, A. and M.P.; writing—original draft preparation, A., A.S. and M.P.; writing—review and editing, W.P.; visualization, A.S.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a project from the National Science Center UMO-2020/37/B/NZ9/00586.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, A.; Singh, S.; Islam, Z.; Munshi, A.D.; Behera, T.K.; Dutta, S.; Weng, Y.; Dey, S.S. Current progress in genetic and ge-nomics-aided breeding for stress resistance in cucumber (Cucumis sativus L.). Sci. Hortic. 2022, 300, 111059. [Google Scholar] [CrossRef]
- Leisner, C.P. Review: Climate change impacts on food security-focus on perennial cropping systems and nutritional value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribu-tion, etiology, epidemiology, and Management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, A.M.; Picó, B. Natural Resistances to Viruses in Cucurbits. Agronomy 2020, 11, 23. [Google Scholar] [CrossRef]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]
- Jeffrey, C. An outline classification of the cucurbitaceae. In Biology and Utilization of the Cucurbitaceae; Cornell University Press: Ithaca, NY, USA, 2019; pp. 449–464. [Google Scholar]
- Pitrat, M.; Chauvet, M.; Foury, C. Diversity, history and production of cultivated cucurbits. Int. Symp. Cucurbits 1997, 492, 21–28. [Google Scholar] [CrossRef]
- Wubs, A.M.; Ma, Y.; Heuvelink, E.; Marcelis, L.F.M. Genetic differences in fruit-set patterns are determined by differences in fruit sink strength and a source: Sink threshold for fruit set. Ann. Bot. 2009, 104, 957–964. [Google Scholar] [CrossRef]
- Kahlen, K.; Stützel, H. Estimation of Geometric Attributes and Masses of Individual Cucumber Organs Using Three-dimensional Digitizing and Allometric Relationships. J. Am. Soc. Hortic. Sci. 2007, 132, 439–446. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.S.; Yi, C.Y.; Wang, F.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide mediates abscisic acid-induced HSP 70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef]
- Singh, M.C.; Singh, J.P.; Pandey, S.K.; Mahay, D.; Srivastava, V. Factors Affecting the Performance of Greenhouse Cucumber Cultivation-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2304–2323. [Google Scholar] [CrossRef]
- Khurana, A.J.; Singh, M.B. Optimum temperature for the germination of seed. J. Appl. Ecol. 2001, 6, 71–78. [Google Scholar]
- EL-Aidy, F. Effect of plastic tunnel size on production of cucumber in Delta of egypt. Appl. Ecol. Environ. Res. 2007, 5, 11–24. [Google Scholar] [CrossRef]
- Olechowska, E.; Słomnicka, R.; Kaźmińska, K.; Olczak-Woltman, H.; Bartoszewski, G. The genetic basis of cold tolerance in cucumber (Cucumis sativus L.)—The latest developments and perspectives. J. Appl. Genet. 2022, 63, 597–608. [Google Scholar] [CrossRef]
- Onovo, J.A. Survey of Disease Incidence and Severity of Cucurbitaceous Crops in the Southeast, Annual Cropping Scheme Report; Vegetable Research Programme National Horticultural Research Institute: Mbato, Ivory Coast, 1992; 47p. [Google Scholar]
- Umeh, O.A.; Ojiako, F.O. Limitations of cucumber (Cucumis sativus L.) production for nutrition security in Southeast Nigeria. Int. J. Agric. Rural Dev. 2018, 21, 3437–3443. [Google Scholar]
- Martínez, C.; Jamilena, M. To be a male or a female flower, a question of ethylene in cucurbits. Curr. Opin. Plant Biol. 2021, 59, 101981. [Google Scholar] [CrossRef]
- Malepszy, S.; Niemirowicz-Szczytt, K. Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci. 1991, 80, 39–47. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Chen, X.-J.; Shi, K.; Xia, X.-J.; Considine, M.J.; Yu, J.-Q.; Zhou, Y.-H. The sub/supra-optimal temperature-induced inhibition of photosynthesis and oxidative damage in cucumber leaves are alleviated by grafting onto figleaf gourd/luffa rootstocks. Physiol. Plant. 2014, 152, 571–584. [Google Scholar] [CrossRef]
- Pawełkowicz, M.E.; Skarzyńska, A.; Pląder, W.; Przybecki, Z. Genetic and molecular bases of cucumber (Cucumis sativus L.) sex determination. Mol. Breed. 2019, 39, 50. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Averyt, K.; Marquis, M.; Tignor, M.M. Climate Change 2007-The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Apata, T.G.; Samuel, K.; Adeola, A. Analysis of climate change perception and adaptation among arable food crop farmers in South Western Nigeria. In Proceedings of the International Association of Agricultural Economists’ 2009 Conference, Beijing, China, 16–22 August 2009; pp. 16–22. [Google Scholar]
- Ozor, N. Understanding climate change: Implications for Nigeria Agriculture, Policy and Extension. In Proceedings of the National Conference on Climate Change and the Nigeria Environment, Enugu, Nigeria, 29 June–2 July 2009. [Google Scholar]
- Backlund, P. Effects of Climate Change on Agriculture, Land Resources, Water Resources, and Biodiversity in the United States; DIANE Publishing: New York, NY, USA, 2009. [Google Scholar]
- Henson, R. The Rough Guide to Climate Change, 2nd ed.; The Science, The Solutions; Rough Guides Ltd.: London, UK, 2008; p. 384. [Google Scholar]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Farquhar, G.D. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Lawlor, D.W. Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ. 1999, 22, 659–682. [Google Scholar] [CrossRef]
- Naraghi, M.; Lotfi, M. Effect of different levels of shading on yield and fruit quality of cucumber (Cucumis sativus). IV Int. Symp. Cucurbits 2009, 871, 385–388. [Google Scholar] [CrossRef]
- Meng, L.; Qin, Z.; Li, S. Effect of high temperature on yield and quality of different cucumber cultivars. J. Am. Soc. Hortic. Sci. 2004, 6, 4–6. [Google Scholar]
- Kumar, S.N.; Aggarwal, P.K.; Rani, S.; Jain, S.; Saxena, R.; Chauhan, N. Impact of climate change on crop productivity in Western Ghats, coastal and northern regions of India. Curr. Sci. 2011, 101, 332–341. [Google Scholar]
- Ertan, S.K. Modelling the effect of temperature on seed germination in some cucurbits. Afr. J. Biotechnol. 2010, 9, 1343–1353. [Google Scholar] [CrossRef]
- Chen, L.; Yun, M.; Cao, Z.; Liang, Z.; Liu, W.; Wang, M.; Yan, J.; Yang, S.; He, X.; Jiang, B.; et al. Phenotypic Characteristics and Transcriptome of Cucumber Male Flower Development Under Heat Stress. Front. Plant Sci. 2021, 12, 758976. [Google Scholar] [CrossRef]
- Miao, M.M.; Lis, J. Effect of high temperature treatment at seedling stage on senescence, sexual differentiation and hormone contents of cucumber. Plant Physiol. Comm. Chin. Acad. Agric. Sci. 2001, 37, 195–198. [Google Scholar]
- Miao, M.M.; Cao, B.S. The relationship between heat injury and polyamines or proline contents during anther development and pollen germination in cucumber. Acta Hort. Sin. Chin. 2002, 29, 233–237. [Google Scholar]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—Drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Thuenprom, N.; Tisarum, R.; Cha-Um, S.; Datta, A. Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Eid, M.A.M.; El-Hady, M.A.A.; Abdelkader, M.A.; Abd-Elkrem, Y.M.; El-Gabry, Y.A.; El-Temsah, M.E.; El-Areed, S.R.M.; Rady, M.M.; Alamer, K.H.; Alqubaie, A.I.; et al. Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations. Agronomy 2022, 12, 803. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind. Crop. Prod. 2020, 158, 113034. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Ghamdi, A.A.M. Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis and enhancing osmotic adjustment capacity. Arab. J. Geosci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Anjum, F.; Yaseen, M.; Rasul, E.; Wahid, A.; Anjum, S. Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pak. J. Agric. Sci. 2003, 40, 45–49. [Google Scholar]
- Ashraf, M.; Shahbaz, M.; Ali, Q. Drought-induced modulation in growth and mineral nutrients in canola (Brassica napus L.). Pak. J. Bot. 2013, 45, 93–98. [Google Scholar]
- Bañon, S.; Ochoa, J.; Franco, J.A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot. 2006, 56, 36–43. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2016, 40, 4–10. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- González-Villagra, J.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Cohen, J.D.; Reyes-Díaz, M.M. Age-related mechanism and its relationship with secondary metabolism and abscisic acid in Aristotelia chilensis plants subjected to drought stress. Plant Physiol. Biochem. 2018, 124, 136–145. [Google Scholar] [CrossRef]
- Liu, B.B.; Li, M.; Li, Q.M.; Cui, Q.Q.; Zhang, W.D.; Ai, X.Z.; Bi, H.G. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- Farag, M.I.; Behera, T.K.; Munshi, A.D.; Bharadwaj, C.; Jat, G.S.; Khanna, M.; Chinnusamy, V. Physiological analysis of drought tolerance of cucumber (Cucumis sativus) genotypes. Indian J. Agric. Sci. 2019, 89, 1445–1450. [Google Scholar] [CrossRef]
- Metwaly, E.-S.E.; Al-Yasi, H.M.; Ali, E.F.; Farouk, H.A.; Farouk, S. Deteriorating Harmful Effects of Drought in Cucumber by Spraying Glycinebetaine. Agriculture 2022, 12, 2166. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Zhang, J.; Xiao, Y.; Yue, Y.; Duan, L.; Zhang, M.; Li, Z. Overexpression of Arabidopsis Molybdenum Cofactor Sulfurase Gene Confers Drought Tolerance in Maize (Zea mays L.). PLoS ONE 2013, 8, e52126. [Google Scholar] [CrossRef]
- He, J.-D.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Takahashi, H.; Saito, T.; Suge, H. Intergeneric Translocation of Floral Stimulus across a Graft in Monoecious Cucurbitaceae with Special Reference to the Sex Expression of Flowers. Plant Cell Physiol. 1982, 23, 1–9. [Google Scholar] [CrossRef]
- Rudich, J.; Halevy, A.H.; Kedar, N. Increase in femaleness of three cucurbits by treatment with Ethrel, an ethylene releasing compound. Planta 1969, 86, 69–76. [Google Scholar] [CrossRef]
- Yin, T.; Quinn, J.A. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am. J. Bot. 1995, 82, 1537–1546. [Google Scholar] [CrossRef]
- Trebitsh, T.; Rudich, J.; Riov, J. Auxin, biosynthesis of ethylene and sex expression in cucumber (Cucumis sativus). Plant Growth Regul. 1987, 5, 105–113. [Google Scholar] [CrossRef]
- Manzano, S.; Martínez, C.; Megías, Z.; Gómez, P.; Garrido, D.; Jamilena, M. The role of ethylene and brassinosteroids in the control of sex expression and flower development in Cucurbita pepo. Plant Growth Regul. 2011, 65, 213–221. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Ethylene Biosynthesis and Regulation in Plants. In Ethylene in Plants; Wen, C.-K., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–25. ISBN 978-94-017-9483-1. [Google Scholar]
- Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016, 14, 7. [Google Scholar]
- Trebitsh, T.; Staub, J.E.; O’Neill, S.D. Identification of a 1-Aminocyclopropane-1-Carboxylic Acid Synthase Gene Linked to the Female (F) Locus That Enhances Female Sex Expression in Cucumber. Plant Physiol. 1997, 113, 987–995. [Google Scholar] [CrossRef]
- Boualem, A.; Troadec, C.; Kovalski, I.; Sari, M.-A.; Perl-Treves, R.; Bendahmane, A. A Conserved Ethylene Biosynthesis Enzyme Leads to Andromonoecy in Two Cucumis Species. PLoS ONE 2009, 4, e6144. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, S.; Liu, S.; Pan, J.; Zhang, Z.; Tao, Q.; Shi, Q.; Jia, Z.; Zhang, W.; Chen, H.; et al. Molecular Isolation of the M Gene Suggests That a Conserved-Residue Conversion Induces the Formation of Bisexual Flowers in Cucumber Plants. Genetics 2009, 182, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, B. Investigations of sex determination in cucumber (Cucumis sativus L.). VI. Androecism. Genet. Pol. 1969, 10, 88–99. [Google Scholar]
- Kubicki, B. Investigations on sex determination in cucumber (Cucumis sativus L.). VII. Andromonoecism and hermaphroditism. Genet. Pol. 1969, 10, 101–120. [Google Scholar]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.-A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R.; et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Li, S.; Cui, Q.; Zhang, H.; Xin, F.; Wang, H.; Lin, T.; Gao, D.; Wang, S.; et al. An ACC Oxidase Gene Essential for Cucumber Carpel Development. Mol. Plant 2016, 9, 1315–1327. [Google Scholar] [CrossRef]
- Li, D.; Sheng, Y.; Niu, H.; Li, Z. Gene Interactions Regulating Sex Determination in Cucurbits. Front. Plant Sci. 2019, 10, 1231. [Google Scholar] [CrossRef]
- Robinson, R.W.; Munger, H.M.; Whitaker, T.W.; Bohn, G.W. Genes of the Cucurbitaceae1. Hortscience 1976, 11, 554–568. [Google Scholar] [CrossRef]
- Mibus, H.; Tatlioglu, T. Molecular characterization and isolation of the F/f gene for femaleness in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2004, 109, 1669–1676. [Google Scholar] [CrossRef]
- More, T.A.; Munger, H.M. Gynoecious sex expression and stability in cucumber (Cucumis sativus L.). Euphytica 1986, 35, 899–903. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Yang, L.; Cai, G.; Chen, H.; Gao, D.; Lin, T.; Cui, Q.; Wang, D.; Li, Z.; et al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene ACS1G induces female flower development in cucumber gynoecy. Plant Cell 2020, 33, 306–321. [Google Scholar] [CrossRef]
- Martin, A.; Troadec, C.; Boualem, A.; Rajab, M.; Fernandez, R.; Morin, H.; Pitrat, M.; Dogimont, C.; Bendahmane, A. A transposon-induced epigenetic change leads to sex determination in melon. Nature 2009, 461, 1135–1138. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef]
- Yamasaki, S.; Yamakuchi, R.; Yamanaka, S.; Manabe, K. Potential Involvement of Cell Cycle-Related Genes in the Arrest of Stamen Development of Female Flowers During Sex Expression in Cucumber (Cucumis sativus L.). Environ. Control. Biol. 2017, 55, 105–112. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Y.; Jin, Q.; Yao, C.; Zhong, Y.; Wu, T. RNA-Seq analysis of gynoecious and weak female cucumber revealing the cell cycle pathway may regulate sex determination in cucumber. Gene 2019, 687, 289–297. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, M.; Shang, J.; Liu, Z.; Weng, Y.; Yue, H.; Li, Y.; Chen, P. A 5.5-KB LTR-retrotransposon insertion inside phy-tochrome B gene (csphyb) results in long hypocotyl and early flowering in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2023, 136, 68. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Quan, R.; Wang, X.-C.; Huang, R. Transcriptional Regulation of the Ethylene Response Factor LeERF2 in the Expression of Ethylene Biosynthesis Genes Controls Ethylene Production in Tomato and Tobacco. Plant Physiol. 2009, 150, 365–377. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Tao, Q.; Niu, H.; Wang, Z.; Zhang, W.; Wang, H.; Wang, S.; Zhang, X.; Li, Z. Ethylene responsive factor ERF110 mediates ethylene-regulated transcription of a sex determination-related orthologous gene in two Cucumis species. J. Exp. Bot. 2018, 69, 2953–2965. [Google Scholar] [CrossRef]
- Pan, J.; Wang, G.; Wen, H.; Du, H.; Lian, H.; He, H.; Pan, J.; Cai, R. Differential Gene Expression Caused by the F and M Loci Provides Insight Into Ethylene-Mediated Female Flower Differentiation in Cucumber. Front. Plant Sci. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Li, F.; Duan, Q.-H.; Han, T.; Xu, Z.-H.; Bai, S.-N. Ethylene perception is involved in female cucumber flower development. Plant J. 2010, 61, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-N.; Xu, Z.-H. Unisexual cucumber flowers, sex and sex differentiation. Int. Rev. Cell Mol. Biol. 2013, 304, 1–55. [Google Scholar] [PubMed]
- García, A.; Aguado, E.; Parra, G.; Manzano, S.; Martínez, C.; Megías, Z.; Cebrián, G.; Romero, J.; Beltrán, S.; Garrido, D.; et al. Phenomic and Genomic Characterization of a Mutant Platform in Cucurbita pepo. Front. Plant Sci. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Aguado, E.; Martínez, C.; Loska, D.; Beltrán, S.; Valenzuela, J.L.; Garrido, D.; Jamilena, M. The ethylene receptors CpETR1A and CpETR2B cooperate in the control of sex determination in Cucurbita pepo. J. Exp. Bot. 2019, 71, 154–167. [Google Scholar] [CrossRef]
- Gu, H.T.; Wang, D.H.; Li, X.; He, C.X.; Xu, Z.H.; Bai, S.N. Characterization of an ethylene-inducible, calcium-dependent nu-clease that is differentially expressed in cucumber flower development. New Phytol. 2011, 192, 590–600. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Zhou, X.; Feng, Z.; Du, Y. Transcriptome profile analysis of floral sex determination in cucumber. J. Plant Physiol. 2010, 167, 905–913. [Google Scholar] [CrossRef]
- Niu, H.; Wang, H.; Zhao, B.; He, J.; Yang, L.; Ma, X.; Cao, J.; Li, Z.; Shen, J. Exogenous auxin-induced enhancer of shoot re-generation 2 (ESR2) enhances femaleness of cucumber by activating the csacs2 gene. Hortic. Res. 2022, 9, uhab085. [Google Scholar] [CrossRef]
- Gu, R.; Liu, X.; Zhao, W.; Yan, S.; Sun, L.; Wu, B.; Zhang, X. Functional Characterization of the Promoter and Second Intron of CUM1 During Flower Development in Cucumber (Cucumis sativus L.). Hortic. Plant J. 2018, 4, 103–110. [Google Scholar] [CrossRef]
- Yan, S.; Che, G.; Ding, L.; Chen, Z.; Liu, X.; Wang, H.; Zhao, W.; Ning, K.; Zhao, J.; Tesfamichael, K.; et al. Different cucumber CsYUC genes regulate response to abiotic stresses and flower development. Sci. Rep. 2016, 6, 20760. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Liu, B.; Wang, W.; Liu, X.; Chen, C.; Liu, X.; Yang, S.; Ren, H. A GAMYB homologue CsGAMYB1 regulates sex expression of cucumber via an ethylene-independent pathway. J. Exp. Bot. 2014, 65, 3201–3213. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Yang, S.; An, J.; Chen, C.; Zhang, X.; Ren, H. A cucumber della homolog csgaip may inhibit staminate de-velopment through transcriptional repression of B class floral homeotic genes. PLoS ONE 2014, 9, e91804. [Google Scholar]
- Zhang, Y.; Zhao, G.; Li, Y.; Mo, N.; Zhang, J.; Liang, Y. Transcriptomic Analysis Implies That GA Regulates Sex Expression via Ethylene-Dependent and Ethylene-Independent Pathways in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 10. [Google Scholar] [CrossRef]
- Ferrero, L.V.; Viola, I.L.; Ariel, F.D.; Gonzalez, D.H. Class I TCP Transcription Factors Target the Gibberellin Biosynthesis Gene GA20ox1 and the Growth-Promoting Genes HBI1 and PRE6 during Thermomorphogenic Growth in Arabidopsis. Plant Cell Physiol. 2019, 60, 1633–1645. [Google Scholar] [CrossRef]
- Fukuda, M.; Matsuo, S.; Kikuchi, K.; Mitsuhashi, W.; Toyomasu, T.; Honda, I. The endogenous level of GA1 is upregulated by high temperature during stem elongation in lettuce through LsGA3ox1 expression. J. Plant Physiol. 2009, 166, 2077–2084. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Grumet, R. Brassinosteriod-induced Femaleness in Cucumber and Relationship to Ethylene Production. Hortscience 2005, 40, 1763–1767. [Google Scholar] [CrossRef]
- Pawełkowicz, M.; Osipowski, P.; Wojcieszek, M.; Woycicki, R.; Witkowicz, J.; Hincha, D.; Przybecki, Z. Corrected version Identification and characterization of genes connected with flower morphogenesis in cucumber. BioTechnologia 2012, 3, 123–134. [Google Scholar] [CrossRef]
- Pawełkowicz, M.E.; Wojcieszek, M.; Osipowski, P.; Krzywkowski, T.; Pląder, W.; Przybecki, Z. Identification and bioinformatics comparison of two novel phosphatases in monoecious and gynoecious cucumber lines. Photonics Appl. Astron Commun. Ind. High-Energy Phys. Exp. 2016, 10031, 756–766. [Google Scholar]
- León, P. Sugar and hormone connections. Trends Plant Sci. 2003, 8, 110–116. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Turek, S.; Aparna; Skarzyńska, A.; Pląder, W.; Pawełkowicz, M. Understanding transcription factors and how they affect processes in cucumber sex determination. Metabolites 2023, 13, 740. [Google Scholar] [CrossRef]
- Cai, Y.; Bartholomew, E.S.; Dong, M.; Zhai, X.; Yin, S.; Zhang, Y.; Feng, Z.; Wu, L.; Liu, W.; Shan, N.; et al. The HD-ZIP IV transcription factor GL2-LIKE regulates male flowering time and fertility in cucumber. J. Exp. Bot. 2020, 71, 5425–5437. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ning, K.; Che, G.; Yan, S.; Han, L.; Gu, R.; Li, Z.; Weng, Y.; Zhang, X. Csspl functions as an adaptor between HD-ZIP III and CsWUS transcription factors regulating anther and ovule development in Cucumis sativus (cucumber). Plant J. 2018, 94, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Kaneko, F.; Yano, K.; Fujioka, T.; Masuko, H.; Park, J.-I.; Kikuchi, S.; Hamada, K.; Endo, M.; Nagano, K.; et al. Mor-phological and gene expression analysis under cool temperature conditions in rice anther development. Genes Genet. Syst. 2010, 85, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; De Souza, L.P.; Yoshida, T.; Fernie, A.R. Flowers and climate change: A metabolic perspective. New Phytol. 2019, 224, 1425–1441. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High temperature susceptibility of sexual reproduction in crop plants. J. Exp. Bot. 2019, 71, 555–568. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Sfakiotakis, E.M. Effect of high temperature stress on ethylene biosynthesis, respiration and ripening of ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2000, 20, 251–259. [Google Scholar] [CrossRef]
- Biggs, M.S.; Woodson, W.R.; Handa, A.K. Biochemical basis of high-temperature inhibition of ethylene biosynthesis in ripening tomato fruits. Physiol. Plant. 1988, 72, 572–578. [Google Scholar] [CrossRef]
- Zhao, X.-C.; Schaller, G.E. Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS Lett. 2004, 562, 189–192. [Google Scholar] [CrossRef]
- Young, T.E.; Meeley, R.B.; Gallie, D.R. ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J. 2004, 40, 813–825. [Google Scholar] [CrossRef]
- Jegadeesan, S.; Chaturvedi, P.; Ghatak, A.; Pressman, E.; Meir, S.; Faigenboim, A.; Rutley, N.; Beery, A.; Harel, A.; Weckwerth, W.; et al. Proteomics of Heat-Stress and Ethylene-Mediated Thermotolerance Mechanisms in Tomato Pollen Grains. Front. Plant Sci. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Apelbaum, A.; Yang, S.F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981, 68, 594–596. [Google Scholar] [CrossRef]
- Jin, Y.; Pan, W.; Zheng, X.; Cheng, X.; Liu, M.; Ma, H.; Ge, X. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol. Biol. 2018, 98, 51–65. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional Activation of OsDERF1 in OsERF3 and OsAP2-39 Negatively Modulates Ethylene Synthesis and Drought Tolerance in Rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, D.; Zhou, S.; Gu, J.; Wang, F.; Dong, J.; Huang, R. The ethylene response factor OSERF109 negatively affects ethylene biosynthesis and drought tolerance in Rice. Protoplasma 2016, 254, 401–408. [Google Scholar] [CrossRef]
- Trujillo, L.E.; Sotolongo, M.; Menéndez, C.; Ochogavía, M.E.; Coll, Y.; Hernández, I.; Borrás-Hidalgo, O.; Thomma, B.P.; Vera, P.; Hernández, L. Soderf3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol. 2008, 49, 512–525. [Google Scholar] [CrossRef]
- Liang, S.; Xiong, W.; Yin, C.; Xie, X.; Jin, Y.-J.; Zhang, S.; Yang, B.; Ye, G.; Chen, S.; Luan, W.-J. Overexpression of OsARD1 Improves Submergence, Drought, and Salt Tolerances of Seedling Through the Enhancement of Ethylene Synthesis in Rice. Front. Plant Sci. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Linsley-Noakes, G.C.; Allan, P. Effects of winter temperatures on flower development in two clones of kiwifruit (Actinidia deliciosa (A. Chev.) C.F. Liang et A.R. Ferguson). Sci. Hortic. 1987, 33, 249–260. [Google Scholar] [CrossRef]
- Ding, X.; Guo, Q.; Li, Q.; Gai, J.; Yang, S. Comparative Transcriptomics Analysis and Functional Study Reveal Important Role of High-Temperature Stress Response Gene GmHSFA2 During Flower Bud Development of CMS-Based F1 in Soybean. Front. Plant Sci. 2020, 11, 600217. [Google Scholar] [CrossRef]
- Dias, A.S.; Barreiro, M.G.; Campos, P.S.; Ramalho, J.C.; Lidon, F.C. Wheat Cellular Membrane Thermotolerance Under Heat Stress. J. Agron. Crop. Sci. 2010, 196, 100–108. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V. Ethylene production under high temperature stress causes premature leaf senescence in soybean. Funct. Plant Biol. 2010, 37, 1071–1084. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Alfalfa leaf senescence induced by drought stress: Photosynthesis, hydrogen peroxide metabolism, lipid peroxidation and ethylene evolution. Physiol. Plant. 1992, 84, 67–72. [Google Scholar] [CrossRef]
- McMichael, B.L.; Jordan, W.R.; Powell, R.D. An Effect of Water Stress on Ethylene Production by Intact Cotton Petioles. Plant Physiol. 1972, 49, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Arraes, F.B.M.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.; Albuquerque, E.V.; Marin, S.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Spikman, G. Development and ethylene production of buds and florets of cut freesia inflorescences as influenced by silver thiosulphate, aminoethoxyvinylglycine and sucrose. Sci. Hortic. 1989, 39, 73–81. [Google Scholar] [CrossRef]
- Dar, R.A.; Nisar, S.; Tahir, I. Ethylene: A key player in ethylene sensitive flower senescence: A review. Sci. Hortic. 2021, 290, 110491. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wang, S.; Lin, T.; Yang, L.; Zhao, Z.; Zhang, Z.; Huang, S.; Yang, X. Genome-wide Target Mapping Shows Histone Deacetylase Complex1 Regulates Cell Proliferation in Cucumber Fruit. Plant Physiol. 2020, 182, 167–184. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.-H.; Huang, S.; Yang, X. Genetic Regulation of Ethylene Dosage for Cucumber Fruit Elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Che, G.; Pan, Y.; Li, Y.; Hou, Y.; Zhao, W.; Zhong, Y.; Ding, L.; Yan, S.; et al. A Functional Allele of CsFUL1 Regulates Fruit Length through Repressing CsSUP and Inhibiting Auxin Transport in Cucumber. Plant Cell 2019, 31, 1289–1307. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, C.; Liu, X.; Yang, K.; Wang, C.; Lu, X.; Tian, Y.; Chen, H. The formation of hollow trait in cucumber (Cucumis sativus L.) fruit is controlled by CS ALMT2. Int. J. Mol. Sci. 2022, 23, 6173. [Google Scholar] [CrossRef]
- Martínez, C.; Manzano, S.; Megías, Z.; Garrido, D.; Picó, B.; Jamilena, M. Involvement of ethylene biosynthesis and signalling in fruit set and early fruit development in zucchini squash (Cucurbita pepo L.). BMC Plant Biol. 2013, 13, 139. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef]
- Shnaider, Y.; Mitra, D.; Miller, G.; Baniel, A.; Doniger, T.; Kuhalskaya, A.; Scossa, F.; Fernie, A.R.; Brotman, Y.; Perl-Treves, R. Cucumber ovaries inhibited by dominant fruit express a dynamic developmental program, distinct from either senes-cence-determined or fruit-setting ovaries. Plant J. 2018, 96, 651–669. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yang, S.; Chen, C.; Xue, S.; Cai, Y.; Wang, D.; Yin, S.; Gai, X.; Ren, H. Silencing of the gibberellin receptor homolog, csgid1a, affects locule formation in cucumber (Cucumis sativus) fruit. New Phytol. 2016, 210, 551–563. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Zhou, X.; Liu, D.; Liu, C.; Luan, J.; Qin, Z.; Xin, M. The curvature of cucumber fruits is associated with spatial variation in auxin accumulation and expression of a YUCCA biosynthesis gene. Hortic. Res. 2020, 7, 135. [Google Scholar] [CrossRef]
- Wei, K.; Chen, J.; Wang, Y.; Chen, Y.; Chen, S.; Lin, Y.; Pan, S.; Zhong, X.; Xie, D. Genome-wide analysis of Bzip-encoding genes in maize. DNA Res. 2012, 19, 463–476. [Google Scholar] [CrossRef]
- Baloglu, M.C.; Eldem, V.; Hajyzadeh, M.; Unver, T. Genome-Wide Analysis of the bZIP Transcription Factors in Cucumber. PLoS ONE 2014, 9, e96014. [Google Scholar] [CrossRef]
- Li, D.; Fu, F.; Zhang, H.; Song, F. Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L.). BMC Genom. 2015, 16, 771. [Google Scholar] [CrossRef]
- Yang, S.; Cai, Y.; Liu, X.; Dong, M.; Zhang, Y.; Chen, S.; Zhang, W.; Li, Y.; Tang, M.; Zhai, X.; et al. A CsMYB6-CsTRY module regulates fruit trichome initiation in cucumber. J. Exp. Bot. 2018, 69, 1887–1902. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Duan, L.; Luan, Q.; Li, J.; Yang, A.; Qi, X.; Ren, Z. csmyb60 is a key regulator of flavonols and proantho-cyanidans that determine the colour of fruit spines in cucumber. J. Exp. Bot. 2018, 70, 69–84. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Yang, Y.; Luo, J.; Zheng, X.; Wen, C.; Xu, Y. Transcription Factor CsWIN1 Regulates Pericarp Wax Biosynthesis in Cucumber Grafted on Pumpkin. Front. Plant Sci. 2019, 10, 1564. [Google Scholar] [CrossRef]
- Che, G.; Pan, Y.; Liu, X.; Li, M.; Zhao, J.; Yan, S.; He, Y.; Wang, Z.; Cheng, Z.; Song, W.; et al. Natural variation in CRABS CLAW contributes to fruit length divergence in cucumber. Plant Cell 2022, 35, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, H.E.; Song, H.; Staub, J.E.; Simon, P.W. Inheritance of beta-carotene-associated flesh color in cucumber (Cucumis sativus L.) fruit. Euphytica 2010, 171, 301–311. [Google Scholar] [CrossRef]
- Lu, H.W.; Miao, H.; Tian, G.L.; Wehner, T.C.; Gu, X.F.; Zhang, S.P. Molecular mapping and candidate gene analysis for yellow fruit flesh in cucumber. Mol. Breed. 2015, 35, 64. [Google Scholar] [CrossRef]
- Bo, K.; Wei, S.; Wang, W.; Miao, H.; Dong, S.; Zhang, S.; Gu, X. QTL mapping and genome-wide association study reveal two novel loci associated with green flesh color in cucumber. BMC Plant Biol. 2019, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Rymal, K.S.; Chambliss, O.L.; Johnson, F.A. Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J. Agric. Food Chem. 1981, 29, 194–196. [Google Scholar] [CrossRef]
- Balkema-Boomstra, A.G.; Zijlstra, S.; Verstappen, F.W.A.; Inggamer, H.; Mercke, P.E.; Jongsma, M.A.; Bouwmeester, H.J. Role of Cucurbitacin C in Resistance to Spider Mite (Tetranychus urticae) in Cucumber (Cucumis sativus L.). J. Chem. Ecol. 2003, 29, 225–235. [Google Scholar] [CrossRef]
- Shang, Y.; Ma, Y.; Zhou, Y.; Zhang, H.; Duan, L.; Chen, H.; Zeng, J.; Zhou, Q.; Wang, S.; Gu, W.; et al. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 2014, 346, 1084–1088. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cao, C.; Liang, S.; Ma, Y.; Liu, X.; Pei, Y. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 2019, 99, 535–544. [Google Scholar] [CrossRef]
- Xin, M.; Wang, L.; Liu, Y.; Feng, Z.; Zhou, X.; Qin, Z. Transcriptome profiling of cucumber genome expression in response to long-term low nitrogen stress. Acta Physiol. Plant. 2017, 39, 130. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, B.; Peng, Q.; Liu, W.; He, X.; Liang, Z.; Lin, Y. Transcriptome Analyses in Different Cucumber Cultivars Provide Novel Insights into Drought Stress Responses. Int. J. Mol. Sci. 2018, 19, 2067. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Liang, Z.; He, X.; Liu, W.; Jiang, B.; Yan, J.; Sun, P.; Cao, Z.; Peng, Q.; et al. Metabolome and transcriptome analyses reveal chlorophyll and anthocyanin metabolism pathway associated with cucumber fruit skin color. BMC Plant Biol. 2020, 20, 386. [Google Scholar] [CrossRef]
- Liu, H.-C.; Liao, H.-T.; Charng, Y.-Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef]
- He, X.; Guo, S.; Wang, Y.; Wang, L.; Shu, S.; Sun, J. Systematic identification and analysis of heat-stress-responsive lncrnas, circrnas and mirnas with associated co-expression and Cerna networks in cucumber (Cucumis sativus L.). Physiol. Plant. 2019, 168, 736–754. [Google Scholar] [CrossRef]
- Ara, N.; Nakkanong, K.; Lv, W.; Yang, J.; Hu, Z.; Zhang, M. Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int. J. Mol. Sci. 2013, 14, 24008–24028. [Google Scholar] [CrossRef]
- Rehman, S.; Rashid, A.; Manzoor, M.A.; Li, L.; Sun, W.; Riaz, M.W.; Li, D.; Zhuge, Q. Genome-Wide Evolution and Comparative Analysis of Superoxide Dismutase Gene Family in Cucurbitaceae and Expression Analysis of Lagenaria siceraria Under Multiple Abiotic Stresses. Front. Genet. 2022, 12, 784878. [Google Scholar] [CrossRef]
- Yu, B.; Yan, S.; Zhou, H.; Dong, R.; Lei, J.; Chen, C.; Cao, B. Overexpression of CsCaM3 Improves High Temperature Tolerance in Cucumber. Front. Plant Sci. 2018, 9, 797. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Liu, Y.; Li, Y.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Zhang, W.; et al. Pumpkin (Cucurbita moschata) HSP20 gene family identification and expression under heat stress. Front. Genet. 2021, 12, 753953. [Google Scholar] [CrossRef]
- He, Y.; Fan, M.; Sun, Y.; Li, L. Genome-wide analysis of watermelon Hsp20s and their expression profiles and subcellular lo-cations under stresses. Int. J. Mol. Sci. 2018, 20, 12. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, S.; Lu, J.; Xu, A.; Huang, Y.; Bie, Z.; Cheng, F. CmRCC1 Gene From Pumpkin Confers Cold Tolerance in Tobacco by Modulating Root Architecture and Photosynthetic Activity. Front. Plant Sci. 2021, 12, 2828. [Google Scholar] [CrossRef]
- Huang, J.; Hai, Z.; Wang, R.; Yu, Y.; Chen, X.; Liang, W.; Wang, H. Genome-wide analysis of HSP20 gene family and expression patterns under heat stress in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 968418. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, S.; Wei, S.; Liu, Y.; Li, C.; Bo, K.; Miao, H.; Gu, X.; Zhang, S. Identification of Quantitative Trait Loci Controlling High-Temperature Tolerance in Cucumber (Cucumis sativus L.) Seedlings. Plants 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, S.; Wei, S.; Wang, W.; Miao, H.; Bo, K.; Gu, X.; Zhang, S. QTL mapping of heat tolerance in cucumber (Cucumis sativus L.) at adult stage. Plants 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, L.; Jiang, Q.; Zhu, C. MicroRNAs as Important Regulators of Heat Stress Responses in Plants. J. Agric. Food Chem. 2020, 68, 11320–11326. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, M.; Zhang, H.-Y.; Liu, J.-H. The MIR396B of poncirus trifoliate functions in cold tolerance by regulating ACC oxidase gene expression and modulating ethylene–polyamine homeostasis. Plant Cell Physiol. 2016, 57, 1865–1878. [Google Scholar] [CrossRef]
- Pan, C.; Ye, L.; Zheng, Y.; Wang, Y.; Yang, D.; Liu, X.; Chen, L.; Zhang, Y.; Fei, Z.; Lu, G. Identification and expression profiling of microRNAs involved in the stigma exsertion under high-temperature stress in tomato. BMC Genom. 2017, 18, 843. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.; Chen, F.; Liu, B.; Wu, L.; Li, F.; Zhang, J.; Bao, M.; Liu, G. Genome-wide identification and characterization of the SBP-box gene family in Petunia. BMC Genom. 2018, 19, 193. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, J.W.; Bade, R.; Men, Z.H.; Hasi, A. Genome-wide identification and phylogenetic analysis of the SBP-box gene family in melons. Genet. Mol. Res. 2014, 13, 8794–8806. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2015, 67, 47–60. [Google Scholar] [CrossRef]
- May, P.; Liao, W.; Wu, Y.; Shuai, B.; Richard McCombie, W.; Zhang, M.Q.; Liu, Q.A. The effects of carbon dioxide and tem-perature on microrna expression in Arabidopsis development. Nat. Commun. 2013, 4, 2145. [Google Scholar] [CrossRef]
- Huo, H.; Wei, S.; Bradford, K.J. DELAY OF GERMINATION1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways. Proc. Natl. Acad. Sci. USA 2016, 113, E2199–E2206. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, F.; Cao, H.; Peng, H.; Ni, Z.; Sun, Q.; Yao, Y. TamiR159 Directed Wheat TaGAMYB Cleavage and Its Involvement in Anther Development and Heat Response. PLoS ONE 2012, 7, e48445. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Ge, L.; Chen, X.; Zhang, L.; Song, X. Transcription factor TaGAMYB from wheat (Triticum aestivum L.) regulates flowering time and fertility in transgenic Arabidopsis thaliana. Planta 2023, 257, 16. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wang, Z.; Guo, X.; Wang, F.; Xia, X.-J.; Zhou, J.; Shi, K.; Yu, J.-Q.; Zhou, Y.-H. Microarray and genetic analysis reveals that Csa-miR159b plays a critical role in abscisic acid-mediated heat tolerance in grafted cucumber plants. Plant Cell Environ. 2016, 39, 1790–1804. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Y.; Zhang, W.; Ahmad, J.; Qiu, Y.; Zhang, X.; Duan, M.; Liu, T.; Song, J.; Wang, H.; et al. MicroRNAs and their targets in cucumber shoot apices in response to temperature and photoperiod. BMC Genom. 2018, 19, 819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).