Pedicularis rostratospicata subsp. marsica (P. Sect. Rostratae, Orobanchaceae), a New Subspecies from the Central Apennines (Italy)
Abstract
1. Introduction
2. Materials and Methods
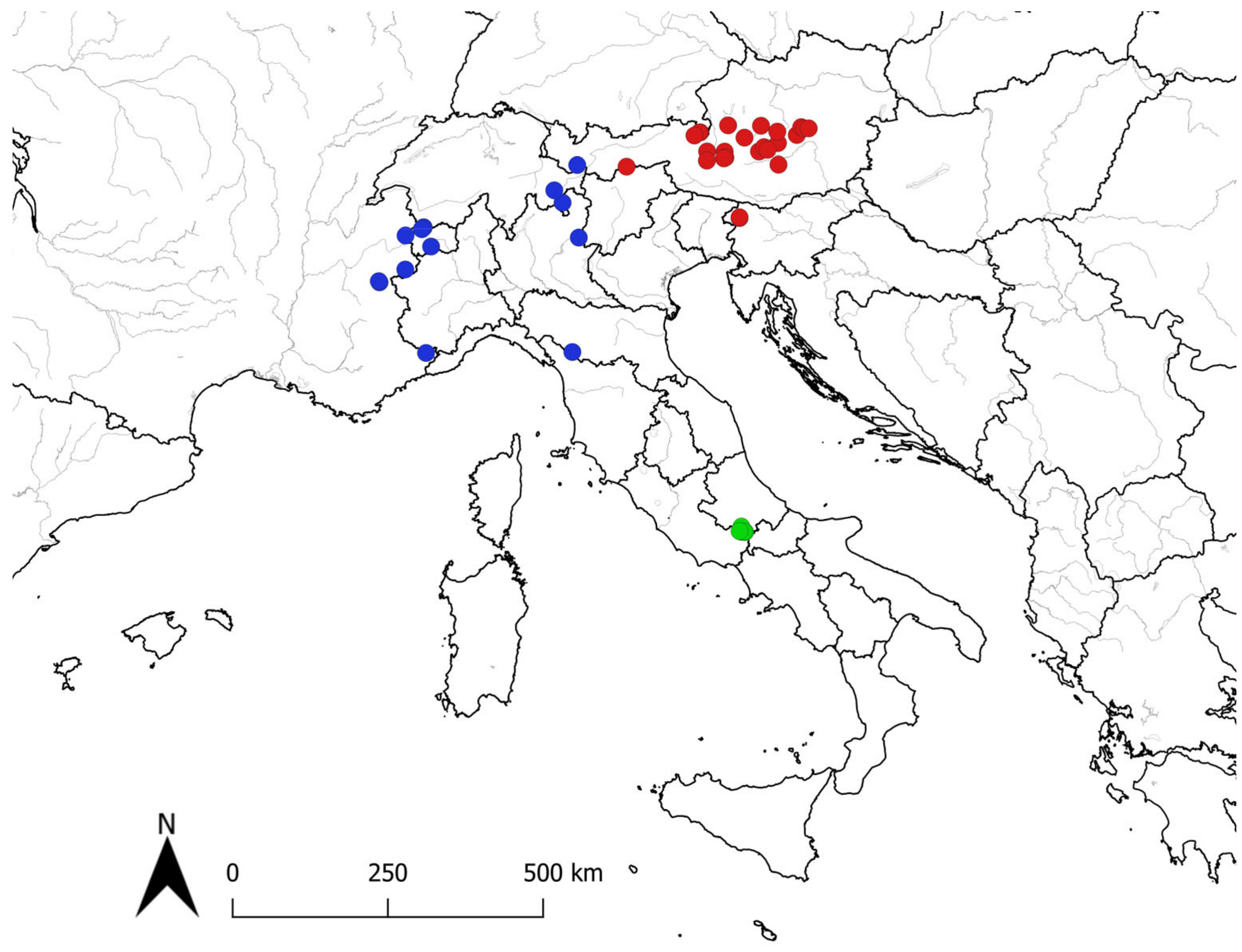
2.1. Plant Material
2.2. Morphometric Analyses
2.3. AFLPseq Fingerprinting
3. Results and Discussion
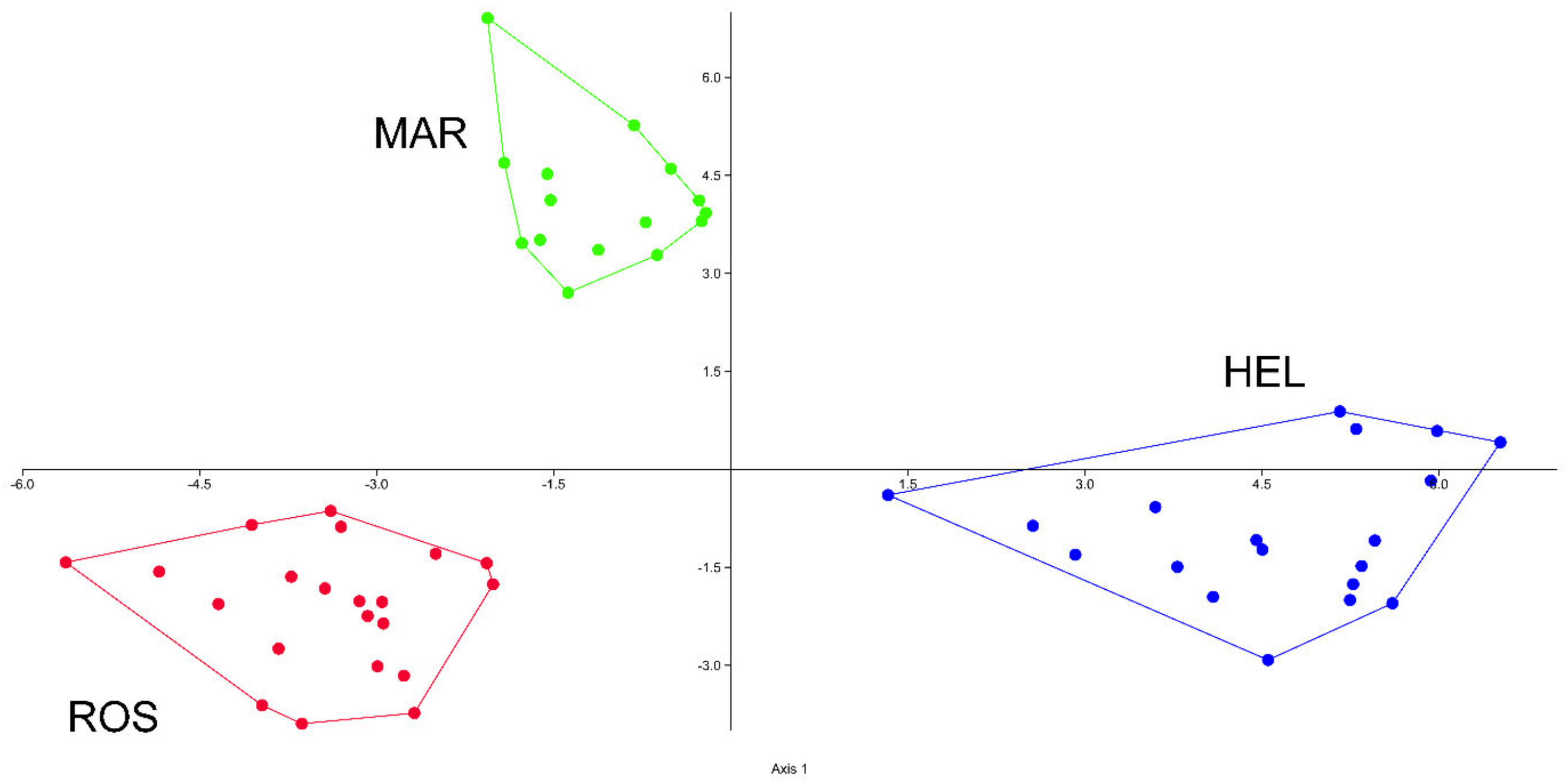
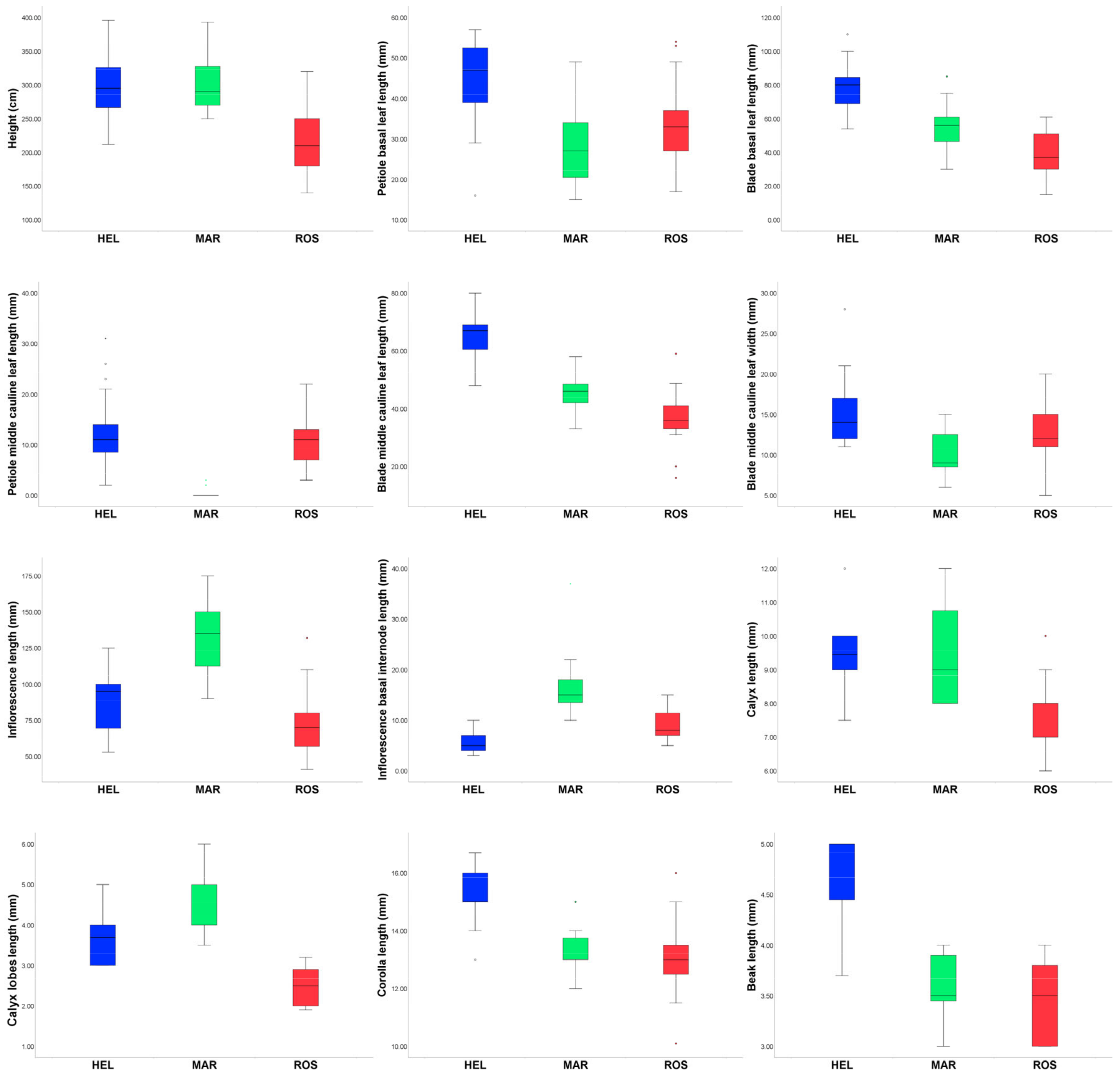
3.1. Morphometric Analyses
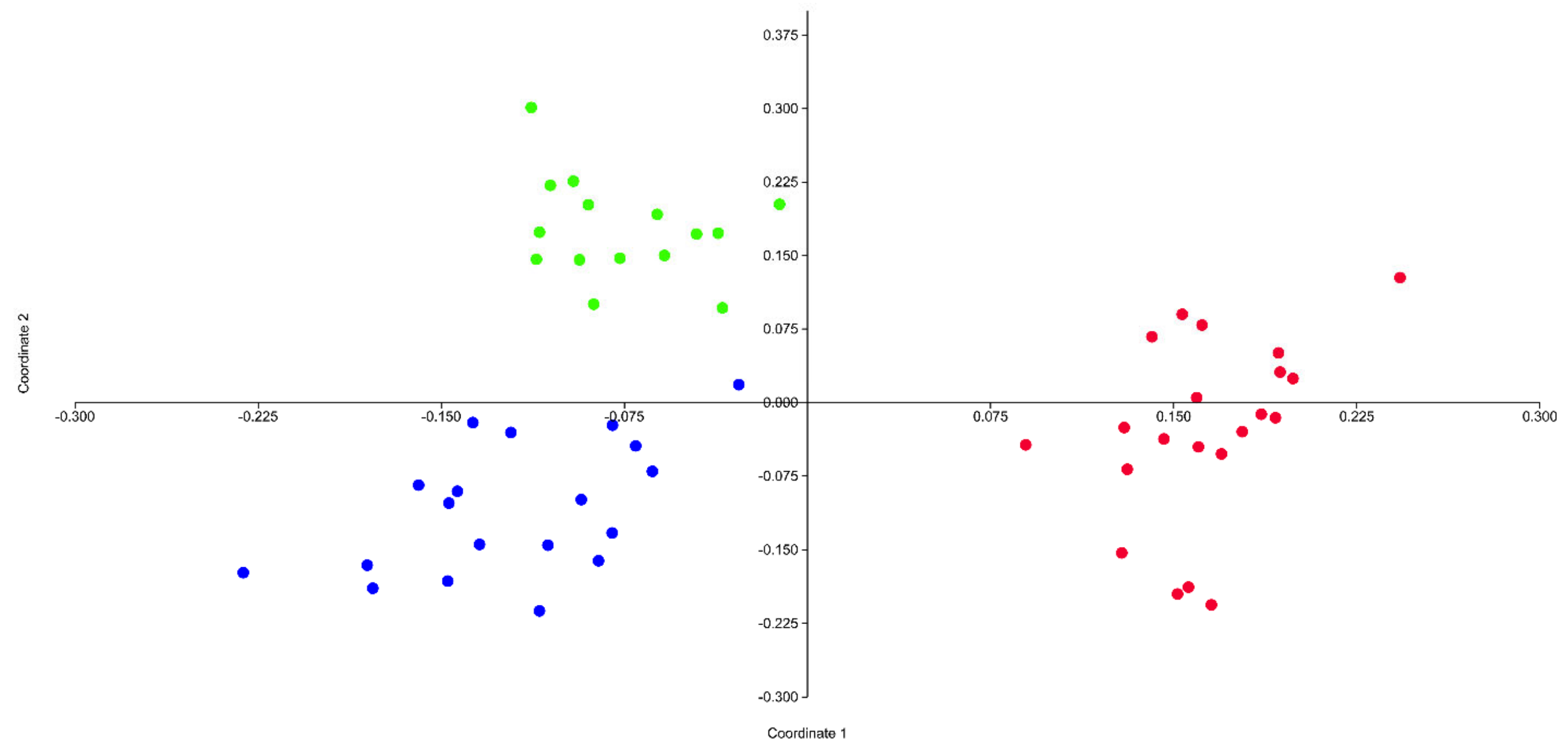
3.2. AFLPseq Fingerprinting
4. Taxonomic Treatment
4.1. List of the Specimens Examined
4.2. Key to the Subspecies Belonging to Pedicularis rostratospicata
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, E. Scrophulariaceae. In Flowering Plants·Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae); Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 333–432. [Google Scholar]
- Yu, W.-B.; Liu, M.-L.; Wang, H.; Mill, R.R.; Ree, R.H.; Yang, J.-B.; Li, D.-Z. Towards a comprehensive phylogeny of the large temperate genus Pedicularis (Orobanchaceae), with an emphasis on species from the Himalaya-Hengduan Mountains. BMC Plant Biol. 2015, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, L.; Conti, F.; Bartolucci, F. An inventory of vascular plants endemic to Italy. Phytotaxa 2014, 168, 1–75. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- PFI. Portale Della Flora d’Italia/Portal to the Flora of Italy, Version 2022.2. Available online: https://dryades.units.it/floritaly/ (accessed on 10 June 2023).
- Limpricht, W. Studien über die Gattung Pedicularis. Feddes Repert. Specierum Nov. Regni Veg. 1924, 20, 161–265. [Google Scholar] [CrossRef]
- Mayer, E. Pedicularis L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 269–276. [Google Scholar]
- Soriano, I. Pedicularis L. In Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Benedí, C., Rico, E., Güemes, J., Herrero, A., Eds.; CSIC: Madrid, Spain, 2009; Volume XIII, pp. 512–530. [Google Scholar]
- Marhold, K. Pedicularis. In Euro + Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: https://europlusmed.org/ (accessed on 14 June 2023).
- Alessandrini, A.; Branchetti, G. Flora Reggiana; Cierre Edizioni: Verona, Italy, 1997. [Google Scholar]
- Anzalone, B.; Bazzichelli, G. La flora del Parco Nazionale d’Abruzzo. Ann. Bot. 1960, 26, 1–182. [Google Scholar]
- Conti, F.; Bartolucci, F. La flora vascolare del Parco Nazionale d’Abruzzo, Lazio e Molise; Fast Edit: Acquaviva Picena, Italy, 2022. [Google Scholar]
- Bruno, F.; Bazzichelli, G. Note illustrative alla carta della vegetazione del Parco Nazionale d’Abruzzo (scala 1: 25000). Progetto conservazione geobotanico. Ann. Bot. 1966, 28, 739–778. [Google Scholar]
- Bazzichelli, G.; Furnari, F. Ricerche Sulla Flora e Sulla Vegetazione di Altitudine nel Parco Nazionale d’Abruzzo: I. Ambiente e Flora II. La Vegetazione; Tip. Coniglione: Catania, Italy, 1970; pp. 1–41. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 1. [Google Scholar]
- Conti, F.; Bartolucci, F. The Vascular Flora of the National Park of Abruzzo, Lazio and Molise (Central Italy); An Annotated Checklist; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 10 June 2023).
- QGIS. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: https://qgis.org/it/site/ (accessed on 3 July 2023).
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–874. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows; Version 25.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Doyle, J.; Dickson, E. Preservation of plant samples for DNA restriction endonuclease analysis. Taxon 1987, 36, 715–722. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Dorfner, M.; Ott, T.; Ott, P.; Oberprieler, C. Long-read genotyping with SLANG (Simple Long-read loci Assembly of Nanopore data for Genotyping). Appl. Plant Sci. 2022, 10, e11484. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Lee, T.V.D.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Li, H. MINIMAP2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Foggi, B.; Rossi, G.; Tomaselli, M. La Flora di Altitudine dell’Appennino Tosco-Emiliano; Regione Emilia-Romagna: Bologna, Italy, 2003. [Google Scholar]
- Conti, F.; Abbate, G.; Alessandrini, A.; Blasi, C. An Annotated Checklist of the Italian Vascular Flora; Palombi & Partner: Roma, Italy, 2005. [Google Scholar]
- Oberprieler, C. The Wettstein tesseract: A tool for conceptualising species-rank decisions and illustrating speciation trajectories. Taxon 2023, 72, 1–7. [Google Scholar] [CrossRef]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.-P. Flora Alpina; Zanichelli: Bologna, Italy, 2004; Volume 2. [Google Scholar]
- Rovelli, E.; Conti, F. Note floristiche per l’Appennino Centrale. Arch. Geobot. 1995, 1, 185–188. [Google Scholar]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- IUCN. IUCN Guidelines for Using the IUCN Red List Categories and Criteria. Version 15.1. Available online: https://www.iucnredlist.org/resources/redlistguidelines (accessed on 23 September 2022).





| Abbreviation | Description of the Character | Type |
|---|---|---|
| H | Height (cm) | quantitative continuous |
| PBLL | Petiole length of basal leaf (mm) | quantitative continuous |
| BBLL | Blade length of basal leaf (mm) | quantitative continuous |
| BBLW | Blade width of basal leaf (mm) | quantitative continuous |
| PMLL | Petiole length of middle cauline leaf (mm) | quantitative continuous |
| BMLL | Blade length of middle cauline leaf (mm) | quantitative continuous |
| BMLW | Blade width of middle cauline leaf (mm) | quantitative continuous |
| PULL | Petiole length of upper cauline leaf (mm) | quantitative continuous |
| BULL | Blade length of upper cauline leaf (mm) | quantitative continuous |
| BULW | Blade width of upper cauline leaf (mm) | quantitative continuous |
| IL | Inflorescence length (mm) | quantitative continuous |
| IBIL | Length of basal internode of inflorescence (mm) | quantitative continuous |
| NF | Number of flowers | quantitative discrete |
| BL | Bract length (mm) | quantitative continuous |
| CL | Calyx length (mm) | quantitative continuous |
| CLL | Calyx lobe length (mm) | quantitative continuous |
| COL | Corolla length (mm) | quantitative continuous |
| NL | Beak length (mm) | quantitative continuous |
| SS | Shape of calyx lobe (0: entire; 1: toothed) | qualitative |
| IH | Hairiness of inflorescence (0: sparsely hairy; 1: densely hairy) | qualitative |
| Sample | Taxon | Locality | Collectors | Voucher Specimens |
|---|---|---|---|---|
| A1285 | P. rostratospicata subsp. rostratospicata | I, Trentino-Alto Adige, Colle Isarco, Val di Fleres. | Conti & Bartolucci s.n. | APP 66183 |
| A1286 | P. rostratospicata subsp. rostratospicata | I, Trentino-Alto Adige, Colle Isarco, Val di Fleres. | Conti & Bartolucci s.n. | APP 66185 |
| A1287 | P. rostratospicata subsp. rostratospicata | I, Trentino-Alto Adige, Colle Isarco, Val di Fleres. | Conti & Bartolucci s.n. | APP 66186 |
| A1288 | P. rostratospicata subsp. helvetica | I, Valle d’Aosta, Courmayeur, Val Veny. | Conti & Bartolucci s.n. | APP 65463 |
| A1289 | P. rostratospicata subsp. helvetica | I, Valle d’Aosta, Courmayeur, Val Veny. | Conti & Bartolucci s.n. | APP 65465 |
| A1290 | P. rostratospicata subsp. helvetica | I, Valle d’Aosta, Courmayeur, Val Veny. | Conti & Bartolucci s.n. | APP 65466 |
| A1291 | P. rostratospicata subsp. helvetica | I, Valle d’Aosta, Gran San Bernardo. | Bovio, Broglio & Ganz s.n. | APP 66182 |
| A1292 | P. rostratospicata subsp. marsica | I, Abruzzo, Serra delle Gravare (Opi), M. Irto—S. Nicola. | Conti & Serafini s.n. | APP 48238 |
| A1293 | P. rostratospicata subsp. marsica | I. Abruzzo, Opi, Mt. Marsicano. | Conti & Stinca s.n. | APP 66177 |
| A1294 | P. rostratospicata subsp. marsica | I. Abruzzo, Opi, Mt. Marsicano. | Conti & Stinca s.n. | APP 66179 |
| A1295 | P. rostratospicata subsp. marsica | I. Abruzzo, Opi, Mt. Marsicano. | Conti & Stinca s.n. | APP 66180 |
| Abbreviation of Characters | ROS | HEL | MAR |
|---|---|---|---|
| H | 21.64 ± 4.48 (14–)18–25(–32) | 29.58 ± 4.85 (21.2–)26.47–32.65(–39.6) | 30.39 ± 4.55 (25–)27–32.75(–39.3) |
| PBLL | 33.1 ± 10.91 (17–)25.75–37.25(–54) | 44.42 ± 11.31 (16–)39–52.5(–57) | 28.2 ± 9.95 (15–)20.5–34(–49) |
| BBLL | 38.5 ± 13.9 (15–)29.25–51.5(–61) | 78.61 ± 15.08 (54–)67.5–85.25(–110) | 55.27 ± 14.24 (30–)46.5–61(–85) |
| BBLW | 15.12 ± 4.7 (7–)12–19(–22) | 17.67 ± 5.9 (10–)14–20(–32) | 16.73 ± 4.3 (10–)14–20(–25) |
| PMLL | 10.48 ± 5.32 (7–)12–19(–22) | 12.88 ± 7.97 (2–)8–15(–31) | 0.47 ± 0.99 0(–3) |
| BMLL | 36.27 ± 9.8 (16–)33–41(–59) | 65.47 ± 8.78 (48–)60.5–69(–80) | 45.13 ± 6.61 (33–)42–48.5(–58) |
| BMLW | 12.57 ± 3.8 (5–)11–15(–20) | 15.05 ± 4.17 (11–)12–17(–28) | 10.53 ± 2.95 (6–)8.5–12.5(–15) |
| PULL | 1.33 ± 1.88 0–2(–6) | 0.1 ± 0.46 0(–2) | 0.13 ± 0.52 0(–2) |
| BULL | 12.33 ± 5.03 (14–)19–25(–38) | 28.63 ± 10.3 (13–)22.5–34.5(–53) | 25.6 ± 8.71 (9–)20–31(–41) |
| BULW | 5.67 ± 2.26 (1–)4–7(–10) | 6.67 ± 2.07 (3.8–)5–8(–11) | 5.2 ± 2.21 (2–)3.5–6.5(–9) |
| IL | 71.48 ± 22.4 (41–)57–80(–132) | 87.05 ± 21.23 (53–)69.5–100(–125) | 131.6 ± 27.04 (90–)112.5–150(–175) |
| IBIL | 9.23 ± 3.29 (5–)7–11.4(–15) | 6 ± 2.54 (3–)4–7(–10) | 16.73 ± 6.53 (10–)13.5–18(–37) |
| NF | (7–)11–15(–17) | (11–)16–25(–32) | (16–)20–25(–29) |
| BL | 16.52 ± 4.59 (9–)13–19(–28) | 15.55 ± 3.9 (10–)13–17.5(–26) | 17.67 ± 7.11 (8–)14–20(–39) |
| CL | 7.46 ± 1 (6–)7–8(–10) | 9.44 ± 1.01 (7–5–)9–10(–12) | 9.47 ± 1.39 8–10.75(–12) |
| CLL | 2.51 ± 0.43 (1.9–)2–2.9(–3.2) | 3.69 ± 0.69 3–4(–5) | 4.49 ± 0.74 (3.5–)4–5(–6) |
| COL | 12.97 ± 1.23 (10.1–)12.5–13.5(–16) | 15.19 ± 0.95 (13–)15–16(–16.7) | 13.18 ± 0.87 (12–)13–13.87(–15) |
| NL | 3.48 ± 0.37 3–3.8(–4) | 4.65 ± 0.47 (3.7–)4.45–5 | 3.55 ± 0.36 (3–)3.45–3.9(–4) |
| SS | entire | toothed | toothed |
| IH | sparsely hairy | sparsely hairy | densely hairy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, F.; Oberprieler, C.; Dorfner, M.; Schabel, E.; Bartolucci, F. Pedicularis rostratospicata subsp. marsica (P. Sect. Rostratae, Orobanchaceae), a New Subspecies from the Central Apennines (Italy). Plants 2023, 12, 2614. https://doi.org/10.3390/plants12142614
Conti F, Oberprieler C, Dorfner M, Schabel E, Bartolucci F. Pedicularis rostratospicata subsp. marsica (P. Sect. Rostratae, Orobanchaceae), a New Subspecies from the Central Apennines (Italy). Plants. 2023; 12(14):2614. https://doi.org/10.3390/plants12142614
Chicago/Turabian StyleConti, Fabio, Christoph Oberprieler, Marco Dorfner, Erik Schabel, and Fabrizio Bartolucci. 2023. "Pedicularis rostratospicata subsp. marsica (P. Sect. Rostratae, Orobanchaceae), a New Subspecies from the Central Apennines (Italy)" Plants 12, no. 14: 2614. https://doi.org/10.3390/plants12142614
APA StyleConti, F., Oberprieler, C., Dorfner, M., Schabel, E., & Bartolucci, F. (2023). Pedicularis rostratospicata subsp. marsica (P. Sect. Rostratae, Orobanchaceae), a New Subspecies from the Central Apennines (Italy). Plants, 12(14), 2614. https://doi.org/10.3390/plants12142614







