Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review
Abstract
1. Introduction
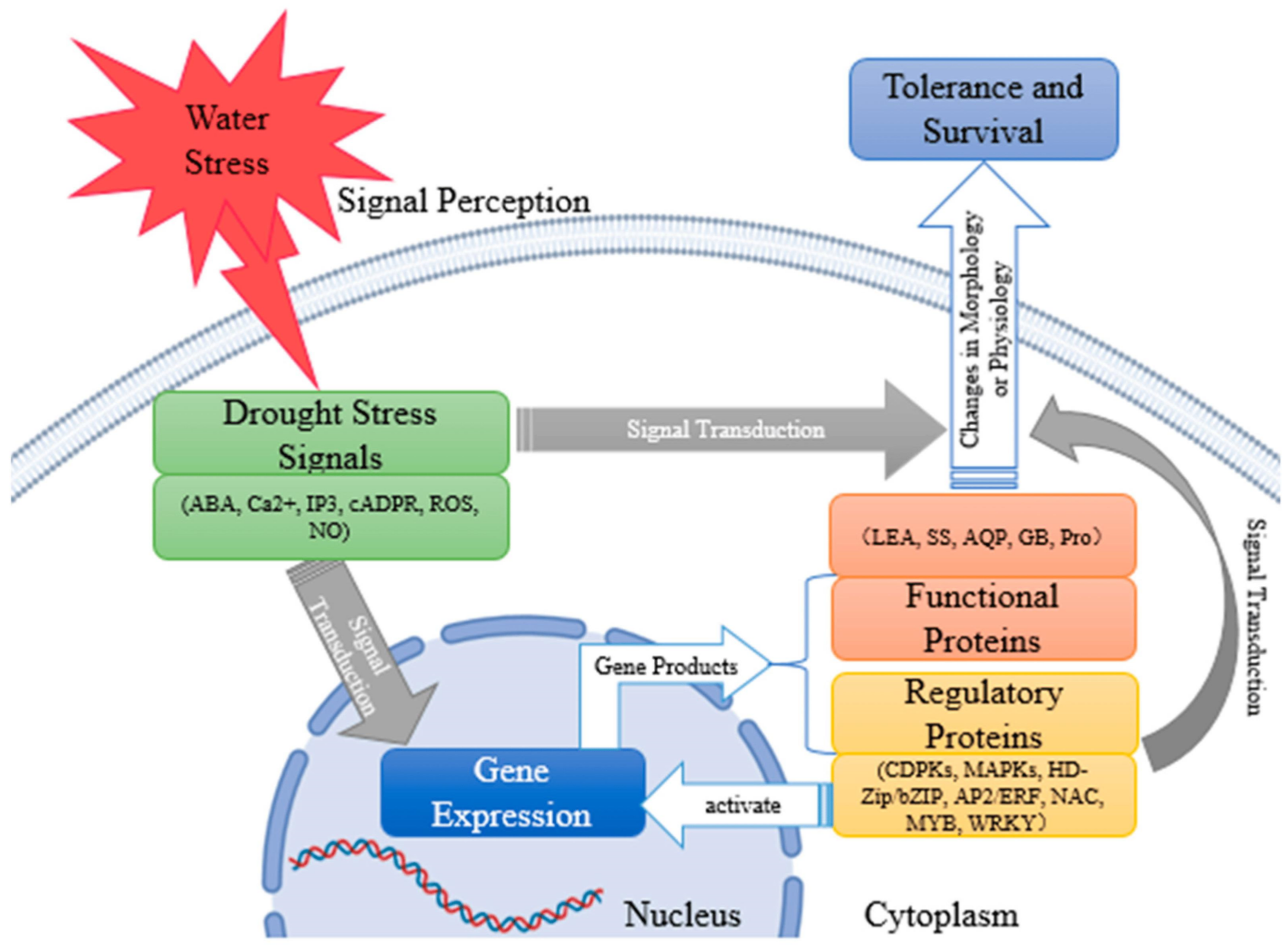
2. Drought Tolerance Mechanisms of Sweet Potato
3. Physiological Responses of Plants to Drought and Water Stress
3.1. Chlorophyll Content Index
3.2. Reactive Oxygen Species
3.3. Betaines
3.4. Trehalose
3.5. Polyamines
3.6. Sugar Alcohols
3.7. Free Proline Content
3.8. Ascorbate Peroxidase
3.9. Superoxide Dismutase
3.10. Glutathione Reductase
3.11. Nitrate Reductase
4. Effect of Drought Stress on Yield
5. Crop Management for Drought Mitigation
6. Breeding for Drought Tolerance
7. Molecular Breeding
7.1. Induction of the Expression of Genes Encoding Stress Proteins
7.2. Induction of Expression of Genes Encoding Transport Proteins
7.3. Preventing Oxidative Stress
7.4. Activation of Phytohormone Signaling Pathways
7.5. Expression of Genes
8. Response of the Sweet Potato to Transformation
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elameen, A.; Fjellheim, S.; Larsen, A.; Rognli, O.A.; Sundheim, L.; Msolla, S.; Masumba, E.; Mtunda, K.; Klemsdal, S.S. Analysis of genetic diversity in a sweet potato (Ipomoea batatas L.) germplasm collection from Tanzania as revealed by AFLP. Genet. Resour. Crop. Evol. 2008, 55, 397–408. [Google Scholar] [CrossRef]
- Jackson, D.M.; Harrison, H.F.; Ryan-Bohac, J.R. Insect resistance in sweetpotato plant introduction accessions. J. Econ. Entomol. 2012, 105, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kulembeka, H.P.; Rufutu, C.K.; Kanju, E.; Chirimi, B.; Rwiza, E.; Amour, R. The agronomic performance and acceptability of orange fleshed sweetpotato varieties in the lake zone of Tanzania. Afr. Crop. Sci. J. 2004, 12, 229–240. [Google Scholar] [CrossRef]
- Bradshaw, L. Root and Tuber Crops; Springer: New York, USA, 2010; p. 304. ISBN 978-0-387-92764-0. [Google Scholar] [CrossRef]
- O’Brien, P.J. The Sweet Potato: Its Origin and Dispersal. Am. Anthropol. 1972, 74, 342–365. [Google Scholar] [CrossRef]
- Ricardo, J. Screening Sweetpotato (Ipomoea batatas L.) for Drought Tolerance and High β-Carotene Content in Mozambique. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2011; p. 132. [Google Scholar]
- Kays, S. Sweetpotato production worldwide: Assessment, trends and the future. Acta Hortic. 2005, 670, 19–25. [Google Scholar] [CrossRef]
- Stathers, T. Crop Protection Programme Expansion of Sustainable Sweetpotato Production and Post-Harvest Management through FFS in East Africa and Sharing of the Lessons Learnt during the Pilot Schools R 8458 (ZA 0682) Project R8458; Final Technical Report: Natural Resources Institute: Kent, UK, 2005; p. 24. [Google Scholar]
- Loebenstein, G.; Fuentes, S.; Cohen, J.; Salazar, L.F. Sweet Potato. In Virus and Virus-like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 223–248. [Google Scholar] [CrossRef]
- Reddy, R.; Soibam, H.; Ayam, V.; Panja, P.; Mitra, S. Morphological characterization of sweet potato cultivars during growth, development and harvesting. Indian J. Agric. Res. 2017, 52, 46–50. [Google Scholar] [CrossRef]
- Srisuwan, S.; Sihachakr, D.; Siljak-Yakovlev, S. The origin and evolution of sweet potato (Ipomoea batatas Lam.) and its wild relatives through the cytogenetic approaches. Plant Sci. 2006, 171, 424–433. [Google Scholar] [CrossRef]
- Woolfe, J.A. Sweet Potato: An Untapped Food Resource, 1st ed.; Cambridge University Press: New York, USA, 1992; p. 643. ISBN 978-0521402958. [Google Scholar]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 8. [Google Scholar] [CrossRef]
- Kwak, S.S. Biotechnology of the sweetpotato: Ensuring global food and nutrition security in the face of climate change. Plant Cell Rep. 2019, 38, 1361–1363. [Google Scholar] [CrossRef]
- Rännäli, M.; Czekaj, V.; Jones, R.A.C.; Fletcher, J.D.; Davis, R.I.; Mu, L.; Dwyer, G.I.; Coutts, B.A.; Valkonen, J.P.T. Molecular genetic characterization of Sweet potato virus G (SPVG) isolates from areas of the Pacific Ocean and southern Africa. Plant Dis. 2008, 92, 1313–1320. [Google Scholar] [CrossRef]
- The State of Food and Agriculture Home Page. Available online: http://www.fao.org/catalog/inter-e.htm (accessed on 13 March 2023).
- Laurie, S.M.; Berg, V.D.; Magoro, M.D.; Kgonyane, M.C. Breeding of sweet potato and evaluation of advanced breeding lines and imported varieties in off-station trials in South Africa. Afr. Crop. Sci. J. 2004, 12, 189–196. Available online: https://www.researchgate.net/publication/313139370 (accessed on 13 March 2023).
- Sivparsad, B. The Development of Transgenic Sweet Potato (Ipomoea batatas L.) with Broad Virus Resistance in South Africa. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2013; p. 192. [Google Scholar]
- Chattopadhyay, A.; Chakraborty, I.; Mukhopadhyay, S.K.; Kumar, P.R.; Sen, H. Compositional changes of sweetpotato as influenced by cultivar, harvest date and cooking. Acta Hortic. 2006, 703, 211–217. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Chattopadhyay, A.; Chakraborty, I.; Bhattacharya, I. Crops that feed the world 5. Sweetpotato. Sweetpotatoes for income and food security. Food Sec. 2011, 3, 283–305. [Google Scholar] [CrossRef]
- Yamakawa, O.; Yoshimoto, M. Sweetpotato as food material with physiological functions. Acta Hort. 2002, 583, 179–185. [Google Scholar] [CrossRef]
- Duque, L.O.; Sánchez, E.; Pecota, K.; Yencho, C. A Win-Win Situation: Performance and Adaptability of Petite Sweetpotato Production in a Temperate Region. Horticulture 2022, 8, 172. [Google Scholar] [CrossRef]
- Mwanga, R.O.M.; Ghislain, M.; Kreuze, J.; Ssemakula, G.N.; Yencho, C. Exploiting the use of biotechnology in sweetpotato for improved nutrition and food security: Progress and future outlook. In Proceedings of the International Conference on Agro-Biotechnology, Biosafety and Seed Systems in Developing Countries, Kampala, Uganda, 8–11 March 2010; Nampala, P., Makara, M.A., Eds.; Science Foundation for Livelihoods and Development: Kampala, Uganda, 2011; pp. 25–31. [Google Scholar]
- Iese, V.; Holland, E.; Wairiu, M.; Havea, R.; Patolo, S.; Nishi, M.; Hoponoa, T.; Bourke, R.M.; Dean, A.; Waqainabete, L. Facing food security risks: The rise and rise of the sweet potato in the Pacific Islands. Glob. Food Sec. 2018, 18, 48–56. [Google Scholar] [CrossRef]
- Mcewan, M.A.; Almekinders, C.J.M.; Matui, M.S.; Lusheshanija, D.; Massawe, M.; Chirimi, B.; Ogero, K. Decentralised sweetpotato (Ipomoea batatas) vine multiplication in lake zone, Tanzania: Five years later. Open Agric. 2020, 5, 677–689. [Google Scholar] [CrossRef]
- Shikuku, K.M.; Okello, J.J.; Wambugu, S.; Sindi, K.; Low, J.W.; McEwan, M. Nutrition and food security impacts of quality seeds of biofortified orange-fleshed sweetpotato: Quasi-experimental evidence from Tanzania. World Dev. 2019, 124, 104646. [Google Scholar] [CrossRef]
- Tairo, F.; Jones, R.A.C.; Valkonen, J.P.T. Phytoplasma from little leaf disease affected sweetpotato in Western Australia: Detection and phylogeny. Ann. Appl. Biol. 2006, 149, 9–14. [Google Scholar] [CrossRef]
- Agili, S.; Nyende, B.; Ngamau, K.; Masinde, P. Selection, yield evaluation, drought tolerance indices of orange-flesh sweet potato (Ipomoea batatas Lam) hybrid clone. J. Nutr. Sci. 2012, 2, 1000138. [Google Scholar] [CrossRef]
- Xiong, X.; Kaluwasha, W. Sweet Potato (Ipomoea batatas) biology and importance in U.S. agriculture. Agri. Res. Tech. Open Access J. 2022, 26, 556346. [Google Scholar] [CrossRef]
- Zhapar, K.; Daurov, D.; Volkov, D.; Daurova, A.; Tolegenova, D.; Abay, Z.; Argynbaeva, A.; Kim, H.S.; Kwak, S.S.; Shamekova, M.; et al. Selection of sweetpotato cultivars with high yields in Almaty region, Kazakhstan. Exp. Biol. 2021, 88, 45–52. [Google Scholar] [CrossRef]
- Shao, H.H.; Chen, S.D.; Zhang, K.; Cao, Q.H.; Zhou, H.; Ma, Q.Q.; He, B.; Yuan, X.H.; Wang, Y.; Chen, Y.H.; et al. Isolation and expression studies of the ERD15 gene involved in drought-stressed responses. Genet. Mol. Res. 2014, 13, 10852–10862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Liu, D.; Guo, F.; Yang, Y.; Dong, T.; Zhang, Y.; Ma, C.; Tang, Z.; Li, F.; et al. Genome-wide survey and expression analysis of GRAS transcription factor family in sweet potato provides insights into their potential roles in stress response. BMC Plant Biol. 2022, 22, 232. [Google Scholar] [CrossRef]
- Laurie, S.M.; Bairu, M.W.; Laurie, R.N. Analysis of the nutritional composition and drought tolerance traits of sweet potato: Selection criteria for breeding lines. Plants 2022, 11, 1804. [Google Scholar] [CrossRef]
- Huang, C.; Liao, J.; Huang, W.; Qin, N. Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. Int. J. Mol. Sci. 2022, 23, 14819. [Google Scholar] [CrossRef]
- Rahmawati, N.; Lahay, R.R.; Irmansyah, T.; Mawarni, L. Yield and tuber quality of orange-fleshed sweet potato cultivars under drought stress in greenhouse. IOP Conf. Ser.: Earth Environ. Sci. 2020, 454, 012159. [Google Scholar] [CrossRef]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J. Plant Res. 2009, 122, 235–243. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Siamhan, D.; Wamala, M.H.; Risimeri, J.B.; Chinyamakobvu, E.; Henderson, S.A.; Fukai, S. The use of seedling leaf death score for evaluation of drought resistance of rice. Field Crops Res. 1998, 55, 129–139. [Google Scholar] [CrossRef]
- Naawe, E.K.; İbrahim İbrahim, S.; Köken, İ.; Demirel, U.; Çaliskan, E. A review: Morphological, physiological and molecular responses of sweetpotato to drought. Eurasian J. Sci. Eng. Technol. 2021, 2, 43–53. [Google Scholar]
- Mgcibelo, M.N. Agronomic and Physiological Approaches to Improving Productivity of Selected Sweet Potato (Ipomoea batatas L.) Cultivars in Kwazulu-Natal: A Focus on Drought Tolerance. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2014; p. 142. [Google Scholar]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, S.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Liu, E.; Xu, L.; Luo, Z.; Li, Z.; Zhou, G.; Gao, H.; Fang, F.; Tang, J.; Zhao, Y.; Zhou, Z.; et al. Transcriptomic analysis reveals mechanisms for the different drought tolerance of sweet potatoes. Front. Plant Sci. 2023, 14, 1136709. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress inbarley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Laurie, R.N. Biochemical, Physiological and Agronomic Response of Various Sweet Potato Cultivars/Varieties to Drought Stress in Rainout Shelters and Field Conditions. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2014; p. 209. [Google Scholar]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Delazari, F.T.; Assis, I.R.; Cabrera, D.F.V.; Ferreira, M.G.; Dias, L.E.; Rueda, A.; Zanuncio, J.C.; Silva, D.J.H. Morpho-physiological characteristics by sweet potato cultivars as function of irrigation depth. An. Acad. Bras. Cienc. 2018, 90, 3541–3549. [Google Scholar] [CrossRef]
- Martin, F.W.; Jones, A. Breeding Sweet Potatoes. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons: New York, USA, 2011; pp. 313–345. [Google Scholar] [CrossRef]
- Gouveia, C.S.S.; Ganança, J.F.T.; de Nóbrega, H.G.M.; de Freitas, J.G.R.; Lebot, V.; de Carvalho, M.A.A.P. Drought avoidance and phenotypic flexibility of sweet potato (Ipomoea batatas (L.) Lam.) under water scarcity conditions. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 1036–1046. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Q.; Jin, R.; Zhao, P.; Zhu, X.; Wang, J.; Yu, Y.; Tang, Z. The Role of IAA in regulating root architecture of sweetpotato (Ipomoea batatas [L.] Lam) in response to potassium deficiency Stress. Plants 2023, 12, 1779. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, W.; Xie, B.; Wang, B.; Hou, F.; Li, A.; Dong, S.; Qin, Z.; Wang, Q.; Zhang, L. Root yield, antioxidant capacities, and hormone contents in different drought-tolerant sweet potato cultivars treated with ABA under early drought stress. Acta Physiol. Plant 2020, 42, 132. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Heider, B.; Struelens, Q.; Faye, É.; Flores, C.; Palacios, J.E.; Eyzaguirre, R.; de Haan, S.; Dangles, O. Intraspecific diversity as a reservoir for heat-stress tolerance in sweet potato. Nat. Clim. Chang. 2021, 11, 64–69. [Google Scholar] [CrossRef]
- Imbo, M.C.; Budambula, N.L.M.; Mweu, C.M.; Muli, J.K.; Anami, S.E. Genetic transformation of sweet potato for improved tolerance to stress: A review. Adv. Life Sci. Technol. 2016, 49, 67–76. [Google Scholar]
- Rautenbach, F.; Faber, M.; Laurie, S.; Laurie, R. Antioxidant capacity and antioxidant content in roots of 4 sweetpotato varieties. J. Food Sci. 2010, 75, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Deng, X.P.; Kwak, S.S. Over expression of CuZn superoxide dismutase (CuZn SOD) and ascorbate peroxidase (APX) in transgenic sweet potato enhances tolerance and recovery from drought stress. Afr. J. Biotechnol. 2010, 9, 8378–8391. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Comparative characterization of sweetpotato antioxidant genes from expressed sequence tags of dehydration-treated fibrous roots under different abiotic stress conditions. Mol. Biol. Rep. 2013, 40, 2887–2896. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. J. Food Compost. Anal. 2009, 22, 546–550. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Murata, N.; Mohanty, P.S.; Hayashi, H.; Papageorgiou, G.C. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett. 1992, 296, 187–189. [Google Scholar] [CrossRef]
- McNeil, S.D.; Nuccio, M.L.; Hanson, A.D. Betaines and related osmoprotectants. targets for metabolic engineering of stress resistance. Plant Physiol. 1999, 120, 945–949. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Fan, W.; Deng, G.; Wang, H.; Zhang, H.; Zhang, P. Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol. Plant 2015, 154, 560–571. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, M.; Zhang, H.; Zhang, P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS ONE 2012, 7, e37344. [Google Scholar] [CrossRef]
- MacIntyre, A.M.; Meline, V.; Gorman, Z.; Augustine, S.P.; Dye, C.J.; Hamilton, C.D.; Iyer-Pascuzzi, A.S.; Kolomiets, M.V.; McCulloh, K.A.; Allen, C. Trehalose increases tomato drought tolerance, induces defenses, and increases resistance to bacterial wilt disease. PLoS ONE 2022, 17, e0266254. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Jiang, T.; Zhai, H.; Wang, F.; Zhou, H.; Si, Z.; He, S.; Liu, Q. Cloning and characterization of a salt tolerance-associated gene encoding trehalose-6-phosphate synthase in sweetpotato. J. Integr. Agric. 2014, 13, 1651–1661. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Pan, X.; Jiang, Q.; Xi, Z. Exogenous putrescine alleviates drought stress by altering reactive oxygen species scavenging and biosynthesis of polyamines in the seedlings of cabernet sauvignon. Front. Plant Sci. 2021, 12, 767992. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [PubMed]
- Kasukabe, Y.; He, L.; Watakabe, Y.; Otani, M.; Shimada, T.; Tachibana, S. Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 2006, 23, 75–83. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, F.; Si, Z.; Huo, J.; Xing, L.; An, Y.; He, S.; Liu, Q. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2016, 14, 592–602. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Kishor, K.; Hong, Z.; Miao, C.H.; Hu, C.A.A.; Verma, D.P.S. Overexpression of A1-Pyrroline-5-CarboxylateS ynthetase lncreases Proline Production and Confers Osmotolerance in Transgenic Plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef]
- Nanjo, T.; Kobayashi, M.; Yoshiba, Y.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999, 461, 205–210. [Google Scholar] [CrossRef]
- Liu, D.; He, S.; Zhai, H.; Wang, L.; Zhao, Y.; Wang, B.; Li, R.; Liu, Q. Overexpression of IbP5CR enhances salt tolerance in transgenic sweetpotato. Plant Cell Tiss Organ Cult. 2014, 117, 1–16. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Liu, C.; Song, X.; He, S.; Zhai, H.; Liu, Q. An Ipomoea batatas iron-sulfur cluster scaffold protein gene, IbNFU1, is involved in salt tolerance. PLoS ONE 2014, 9, e93935. [Google Scholar] [CrossRef] [PubMed]
- Mbinda, W.; Dixelius, C.; Oduor, R. Induced Expression of Xerophyta viscosa XvSap1 Gene Enhances Drought Tolerance in Transgenic Sweet Potato. Front. Plant Sci. 2019, 10, 1119. [Google Scholar] [CrossRef]
- Armengaud, P.; Thiery, L.; Buhot, N.; Grenier-De March, G.; Savoure, A.S. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol. Plant. 2004, 120, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Dalton, A.D.; Russel, S.A.; Hanus, F.J.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815. [Google Scholar] [CrossRef]
- Masoumi, H.; Darvish, F.; Daneshian, J.; Normohammadi, G.; Habibi, D. Effects of water deficit stress on seed yield and antioxidants content in soybean (Glycine max L.) cultivars. Afr. J. Agric. Res. 2011, 6, 1209–1218. Available online: https://www.academicjournals.org/ajar/ (accessed on 15 March 2023).
- Malan, C.; Greyling, M.M.; Gressel, J. Correlation between cuzn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990, 69, 157–166. [Google Scholar] [CrossRef]
- Xia, H.; Xu, T.; Zhang, J.; Shen, K.; Li, Z.; Liu, J. Drought-induced responses of nitrogen metabolism in Ipomoea batatas. Plants 2020, 9, 1341. [Google Scholar] [CrossRef]
- Pandey, H.C.; Baig, M.J.; Ahmed, S.; Kumar, V.; Singh, P. Studies on morpho-physiological characters of different Avena species under stress conditions. Afr. J. Biotechnol. 2013, 12, 6170–6175. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ne Valadier, M.H.; Migge, A.; Becker, T.W. Drought-Induced Effects on Nitrate Reductase Activity and mRNA and on the Coordination of Nitrogen and Carbon Metabolism in Maize Leaves. Plant Physiol. 1998, 117, 283–292. Available online: https://academic.oup.com/plphys/article/117/1/283/6098554 (accessed on 15 March 2023). [CrossRef]
- Ekanayake, I.J. Evaluation of potato and sweetpotato genotypes for drought resistance. In Sweet Potato Germplasm Management; Ekanayake, I.J., Ed.; CIP: Lima, Peru, 1990; p. 16. [Google Scholar]
- Atung, C.; Guaf, E.; Komolong, B. Screening sweetpotato (Ipomoea batatas) genotypes under soil moisture deficit condition using stress tolerance indices. Arch. Appl. Sci. 2015, 7, 23–29. [Google Scholar]
- Rimsaite, R.; Gibson, J.; Brozović, N. Informing drought mitigation policy by estimating the value of water for crop production. Environ. Res. Commun. 2021, 3, 041004. [Google Scholar] [CrossRef]
- Ekanayake, I.J.; Collins, W. Effect of irrigation on sweet potato root carbohydrates and nitrogenous compounds. J. Food Agric. Environ. 2004, 2, 243–248. [Google Scholar]
- Laurie, R.N.; Laurie, S.M.; Du Plooy, C.P.; Finnie, J.F.; Staden, J.V. Yield of drought-stressed sweet potato in relation to canopy cover, stem length and stomatal conductance. J. Agric. Sci. 2015, 7, 201–214. [Google Scholar] [CrossRef]
- Andrade, M.I.; Naico, A.; Ricardo, J.; Eyzaguirre, R.; Makunde, G.S.; Ortiz, R.; Grüneberg, W.J. Genotype × environment interaction and selection for drought adaptation in sweetpotato (Ipomoea batatas [L.] Lam.) in Mozambique. Euphytica 2016, 209, 261–280. [Google Scholar] [CrossRef]
- Monti, A.; Amaducci, M.T.; Pritoni, G.; Venturi, G. Variation in carbon isotope discrimination during growth and at different organs in sugar beet (Beta vulgaris L.). Field Crops Res. 2006, 98, 157–163. [Google Scholar] [CrossRef]
- Deblonde, P.M.K.; Ledent, J.F. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 2001, 14, 31–41. [Google Scholar] [CrossRef]
- de Brito, G.G.; Sofiatti, V.; de Andrade Lima, M.M.; de Carvalho, L.P.; da Silva Filho, J.L. Physiological traits for drought phenotyping in cotton. Acta Sci. Agron. 2011, 33, 117–125. [Google Scholar] [CrossRef]
- Leidi, E.O.; Lopez, M.; Gorham, J.; Gutierrez, J.C. Variation in carbon isotope discrimination and other traits related to drought tolerance in upland cotton cultivars under dryland conditions. Field Crops Res. 1999, 61, 109–123. [Google Scholar] [CrossRef]
- Akhter, J.; Sabir, S.A.; Lateef, Z.; Yaseen, M.; Haq, M.A. Relationships between carbon isotope discrimination and grain yield, water-use efficiency and growth parameters in wheat (Triticum aestivum L.) under different water regimes. Pak. J. Bot. 2008, 40, 1441–1454. [Google Scholar]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Kesmala, T.; Nageswara Rao, R.C.; Wright, G.C.; Patanothai, A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop. Sci. 2008, 194, 113–125. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Kunz, K.; Hu, Y.; Schmidhalter, U. Carbon isotope discrimination as a key physiological trait to phenotype drought/heat resistance of future climate-resilient German winter wheat compared with relative leaf water content and canopy temperature. Front. Plant Sci. 2022, 13, 1043458. [Google Scholar] [CrossRef] [PubMed]
- Gitore, S.A.; Danga, B.; Henga, S.; Gurmu, F. Evaluating Drought tolerance indices for selection of drought tolerant Orange Fleshed Sweet Potato (OFSP) genotypes in Ethiopia. J Agric. Sci. Food Technol. 2021, 7, 249–254. [Google Scholar] [CrossRef]
- Turk, K.J.; Hall, A.E. Drought Adaptation of Cowpea. II. Influence of Drought on Plant Water Status and Relations with Seed Yield. Agron. J. 1980, 72, 421–427. [Google Scholar] [CrossRef]
- Lawan, R.J. Responses of four grain legumes to water stress in higher plants. Annu. Rev. Plant Physiol. 1983, 35, 299–319. [Google Scholar]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. Int J Mol Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Kubota, F. The effects of drought stress and leaf ageing on leaf photosynthesis and electron transport in photosystem 2 in sweet potato (Ipomoea batatas Lam.) cultivars. Photosynthetica 2003, 41, 253–258. [Google Scholar] [CrossRef]
- Sung, F.J.M. The effect of leaf water status on stomatal activity, transpiration and nitrate reductase of sweet potato. Agric. Water Manag. 1981, 4, 465–470. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zou, H.; ·Chen, J.; Wang, Z.; ·Luo, Z.; Yao, Z.; Fang, B.; ·Huang, L. Exploration of molecular mechanism of intraspecifc cross-incompatibility in sweetpotato by transcriptome and metabolome analysis. Plant Mol. Biol. 2022, 109, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Simion, T. Breeding Sweet potato [Ipomoeae batatas (L.) Lam] for low moisture stress tolerance. Ann. Rev. Res. 2018, 3, 555601. [Google Scholar] [CrossRef]
- Kivuva, B.M.; Githiri, S.M.; Yencho, G.C.; Sibiya, J. Screening sweetpotato genotypes for tolerance to drought stress. Field Crops Res. 2015, 171, 11–22. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and genomic research on sweet potato for sustainable food and nutritional security. Genes 2022, 13, 1833. [Google Scholar] [CrossRef]
- Nedunchezhiyan, M.; Gangadharan, B.; Jata, S.K. Sweet potato agronomy. In Fruit, Vegetable and Cereal Science and Biotechnology; Global Science Books: Bhubaneswar, India, 2012; Volume 6, pp. 1–10. [Google Scholar]
- Abdallah, B.; Githiri, S.M.; Kariuki, W.; Saha, H.M. Evaluation of the Performance of Sweet Potato (Ipomoea batatas) Clones under Water Stress in the Coastal Lowlands of Kenya. World J. Agric. Res. 2020, 8, 114–120. [Google Scholar] [CrossRef]
- Omotobara, B.O.; Adebola, P.O.; Modise, D.M.; Laurie, S.M.; Gerrano, A.S. Greenhouse and field evaluation of selected sweetpotato (Ipomoea batatas (L.) LAM) accessions for drought tolerance in South Africa. Am. J. Plant Sci. 2014, 5, 3328–3339. [Google Scholar] [CrossRef]
- Nhanala, S.E.C.; Yencho, G.C. Assessment of the potential of wild Ipomoea spp. for the improvement of drought tolerance in cultivated sweetpotato Ipomoea batatas (L.) Lam. Crop. Sci. 2021, 61, 234–249. [Google Scholar] [CrossRef]
- Akomeah, B.; Quain, M.D.; Ramesh, S.A.; Anand, L.; Rodríguez Loópez, C.M. Common garden experiment reveals altered nutritional values and DNA methylation profiles in micropropagated three elite Ghanaian sweet potatogenotypes. PLoS ONE 2019, 14, e0208214. [Google Scholar] [CrossRef]
- Yang, J.; Bi, H.P.; Fan, W.J.; Zhang, M.; Wang, H.X.; Zhang, P. Efficient embryogenic suspension culturing and rapid transformation of a range of elite genotypes of sweet potato (Ipomoea batatas [L.] Lam.). Plant Sci. 2011, 181, 701–711. [Google Scholar] [CrossRef]
- Luo, H.R.; Maria, M.S.; Benavides, J.; Zhang, D.P.; Zhang, Y.Z.; Ghislain, M. Rapid genetic transformation of sweetpotato (Ipomoea batatas (L.) Lam) via organogenesis. Afr. J. Biotechnol. 2006, 5, 1851–1857. [Google Scholar]
- Okada, Y.; Saito, A.; Nishiguchi, M.; Kimura, T.; Mori, M.; Hanada, K.; Sakai, J.; Miyazaki, C.; Matsuda, Y.; Murata, T. Virus resistance in transgenic sweetpotato [Ipomoea batatas L. (Lam)] expressing the coat protein gene of sweet potato feathery mottle virus. Theor. Appl. Genet. 2001, 103, 743–751. [Google Scholar] [CrossRef]
- Ji, C.Y.; Jin, R.; Xu, Z.; Kim, H.S.; Lee, C.J.; Kang, L.; Kim, S.E.; Lee, H.U.; Lee, J.S.; Kang, C.H.; et al. Overexpression of Arabidopsis P3B increases heat and low temperature stress tolerance in transgenic sweetpotato. BMC Plant Biol. 2017, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Mertenz, J.; Aliyu, H.; Cowan, D.A. LEA proteins and the evolution of the Why domain. Appl. Environ. Microbiol. 2018, 84, e00539-18. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.H.; Jeong, J.C.; Kim, C.Y.; Lee, H.S.; Bang, J.W.; Kwak, S.S. Sweetpotato late embryogenesis abundant 14 (IbLEA14) gene influences lignification and increases osmotic- and salt stress-tolerance of transgenic calli. Planta 2011, 233, 621–634. [Google Scholar] [CrossRef]
- Adabnejad, H.; Kavousi, H.R.; Hamidi, H.; Tavassolian, I. Assessment of the vacuolar Na+/H+ antiporter (NHX1) transcriptional changes in Leptochloa fusca L. in response to salt and cadmium stresses. Mol. Biol. Res. Commun. 2015, 4, 133–142. [Google Scholar]
- Wang, B.; Zhai, H.; He, S.; Zhang, H.; Ren, Z.; Zhang, D.; Liu, Q. A vacuolar Na+/H+antiporter gene, IbNHX2, enhances salt and droughttolerance in transgenic sweetpotato. Sci. Hortic. 2016, 201, 153–166. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Gao, S.; Yuan, L.; Zhai, H.; Liu, C.; He, S.; Liu, Q. Transgenic sweetpotato plants expressing an LOS5 gene are tolerant to salt stress. Plant Cell Tiss. Organ Cult. 2011, 107, 205–213. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Zhai, H.; Song, X.; He, S.; Liu, Q. A novel a/b-hydrolase gene IbMas enhances salt tolerance in transgenic sweetpotato. PLoS ONE 2014, 9, e115128. [Google Scholar] [CrossRef]
- Jia, X.; Zhen, Z.; Lyu, Y.; Zhao, S. Drought-Responsive NAC Transcription Factor RcNAC72 Is Recognized by RcABF4, Interacts with RcDREB2A to enhance drought tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1755. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic leucine zipper transcription factor SlbZIP1 mediates salt and drought stress tolerance in tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, D.; Shen, J.; Deng, S.; Xuan, H.; Wang, C.; Xu, J.; Zhou, L.; Guo, N.; Zhao, J.; et al. Genome-Wide Identification of the AP2/ERF Gene Family and Functional Analysis of GmAP2/ERF144 for Drought Tolerance in Soybean. Front. Plant Sci. 2022, 13, 848766. [Google Scholar] [CrossRef] [PubMed]
- Lindomose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci. 2013, 14, 5842–5878. [Google Scholar] [CrossRef]
- Dai, Z.; Yan, P.; He, S.; Jia, L.; Wang, Y.; Liu, Q.; Zhai, H.; Zhao, N.; Gao, S.; Zhang, H. Genome-Wide Identification and Expression Analysis of SWEET Family Genes in Sweet Potato and Its Two Diploid Relatives. Int. J. Mol. Sci. 2022, 23, 15848. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, Y.; Dong, F.; Zhang, Y.; Huang, Y.; Zhou, Y.; Zhao, Z.; Yin, Q.; Xie, X.; Gao, X.; et al. The Expression of IbMYB1 Is essential to maintain the purple color of leaf and storage root in sweet potato [Ipomoea batatas (L.) Lam]. Front. Plant Sci. 2021, 12, 688707. [Google Scholar] [CrossRef]
- Bian, X.; Kim, H.S.; Kwak, S.S.; Zhang, Q.; Liu, S.; Ma, P.; Jia, Z.; Xie, Y.; Zhang, P.; Yu, Y. Different functions of IbRAP2.4, a drought-responsive AP2/ERF transcription factor, in regulating root development between arabidopsis and sweetpotato. Front. Plant Sci. 2022, 13, 820450. [Google Scholar] [CrossRef]
- Lau, K.H.; del Rosario Herrera, M.; Crisovan, E.; Wu, S.; Fei, Z.; Khan, M.A.; Buell, C.R.; Gemenet, D.C. Transcriptomic analysis of sweet potato under dehydration stress identifies candidate genes for drought tolerance. Plant Direct. 2018, 2, e00092. [Google Scholar] [CrossRef]
- Ferreira, J.A.B.; Silva-Mann, R.; de Oliveira Júnior, L.F.G. Genes to monitor ecophysiological parameters for plants under abiotic stress conditions. ASRJETS 2021, 82, 78–98. [Google Scholar]
- Zhou, Z.; Tang, J.; Cao, Q.; Li, Z.; Ma, D. Differential response of physiology and metabolic response to drought stress in different sweetpotato cultivars. PLoS ONE 2022, 17, e0264847. [Google Scholar] [CrossRef]
- Kim, S.E.; Bian, X.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Kim, B.H.; Park, W.S.; Ahn, M.J.; Ji, C.Y.; Yu, Y.; et al. Overexpression of 4-hydroxyphenylpyruvate dioxygenase (IbHPPD) increases abiotic stress tolerance in transgenic sweetpotato plants. Plant Physiol. Biochem. 2021, 167, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Arisha, M.H.; Zhang, Z.; Yan, H.; Kou, M.; Song, W.; Li, C.; Gao, R.; Ma, M.; Wang, X.; et al. Comparative transcriptomic and proteomic analysis reveals common molecular factors responsive to heat and drought stresses in sweetpotaoto (Ipomoea batatas). Front. Plant Sci. 2023, 13, 1081948. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT Prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef]
- Liu, E.; Li, Z.; Luo, Z.; Xu, L.; Jin, P.; Ji, S.; Zhou, G.; Wang, Z.; Zhou, Z.; Zhang, H. Genome-wide identification of duf668 gene family and expression analysis under drought and salt stresses in sweet potato [Ipomoea batatas (L.) Lam]. Genes 2023, 14, 217. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Satish, L.; Adarshan, S.; Lakshmi, M.A.; Pandian, S.K.; Chen, J.T.; Ahmar, S.; Wang, X.; Mora-Poblete, F.; et al. The Role of OsWRKY Genes in rice when faced with single and multiple abiotic stresses. Agronomy 2021, 11, 1301. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Guo, F.; Sun, Q.; Yu, J.; Dong, T.; Wang, X.; Song, W.; Li, Z.; Meng, X.; et al. A systematical genome-wide analysis and screening of WRKY transcription factor family engaged in abiotic stress response in sweetpotato. BMC Plant Biol. 2022, 22, 616. [Google Scholar] [CrossRef] [PubMed]
- Nie, N.; Huo, J.; Sun, S.; Zuo, Z.; Chen, Y.; Liu, Q.; He, S.; Gao, S.; Zhang, H.; Zhao, N.; et al. Genome-wide characterization of the PIFs family in sweet potato and functional identification of IbPIF3.1 under drought and fusarium wilt stresses. Int. J. Mol. Sci. 2023, 24, 4092. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.S.; Wang, Z.; Ke, Q.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Park, W.S.; Ahn, M.J.; Kwak, S.S. A single amino acid change at position 96 (Arg to His) of the sweetpotato Orange protein leads to carotenoid overaccumulation. Plant Cell Rep. 2019, 38, 1393–1402. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Park, W.S.; Kim, H.J.; Ahn, M.J.; Kwak, S.S.; Kim, H.S. Overexpression of the golden snp-carrying orange gene enhances carotenoid accumulation and heat stress tolerance in sweetpotato plants. Antioxidants 2021, 10, 51. [Google Scholar] [CrossRef]
- Nawiri, S.O.; Oduor, R.O.; Jalemba, A.M. Genetic engineering of sweet potatoes (Ipomoea batatas) using isopentenyl transferase gene for enhanced drought tolerance. Asian J. Agric. 2017, 1, 85–99. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of Plant Cytokinin Biosynthetic Enzymes as Dimethylallyl Diphosphate:ATP/ADP Isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. Available online: http://www.ddbj.nig.ac.jp/E-mail/clustalw-e.html (accessed on 15 March 2023). [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Zhang, H.; Liu, Q.; Zhai, H.; Zhao, N.; Gao, S.; He, S. Genome-Wide Identification and Characterization of CDPK Family Reveal Their Involvements in Growth and Development and Abiotic Stress in Sweet Potato and Its Two Diploid Relatives. Int. J. Mol. Sci. 2022, 23, 3088. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, H.H.; Jeng, S.T. Calcium influxes and mitogen-activated protein kinase kinase activation mediate ethylene inducing ipomoelin gene expression in sweet potato. Plant Cell Environ. 2008, 31, 62–72. [Google Scholar] [CrossRef]
- Arisha, M.H.; Ahmad, M.Q.; Tang, W.; Liu, Y.; Yan, H.; Kou, M.; Wang, X.; Zhang, Y.; Li, Q. RNA-sequencing analysis revealed genes associated drought stress responses of different durations in hexaploid sweet potato. Sci Rep. 2020, 10, 12573. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Anwar, N.; Watanabe, K.N.; Watanabe, J.A. Transgenic sweet potato expressing mammalian cytochrome P450. Plant Cell Tissue Organ. Cult. 2011, 105, 219–231. [Google Scholar] [CrossRef]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Pal, D.; Verma, S. Removal of Feedback Inhibition of 1-Pyrroline-5-Carboxylate Synthetase Results in Increased Proline Accumulation and Protection of Plants from Osmotic Stress. Plant Physiol. 2000, 122, 1129–1136. Available online: https://academic.oup.com/plphys/article/122/4/1129/6098815 (accessed on 16 March 2023). [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.R.; Wani, S.H.; Challa, S. Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 221–254. [Google Scholar] [CrossRef]
- Arentson, B.W.; Sanyal, N.; Becker, D.F. Substrate channeling in proline metabolism. Front Biosci. 2013, 17, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encodinq mitochondrial proline dehydrogenase, an enzyme lnvolved in proline metabolism, is upregulated by proline but downregulated by dehydration in AÍrbidopsis. Plant Cell. 1996, 8, 1323–1335. Available online: https://academic.oup.com/plcell/article/8/8/1323/5985231 (accessed on 16 March 2023).
- Li, J.; Hsia, A.P.; Schnable, P.S. Recent advances in plant recombination. Curr. Opin. Plant Biol. 2007, 10, 131–135. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)-A valuable medicinal food: A review. J. Med. Food. 2014, 17, 733–741. [Google Scholar] [CrossRef]
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically engineered crops: Importance of diversified integrated pest management for agricultural sustainability. Front. Bioeng. Biotechnol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Mollinari, M.; Olukolu, B.A.; da S Pereira, G.; Khan, A.; Gemenet, D.; Yencho, G.C.; Zeng, Z.B. Unraveling the Hexaploid Sweetpotato Inheritance Using Ultra-Dense Multilocus Mapping. G3 2020, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Moeinzadeh, M.H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.; Zheng, J.; Sun, Z.; Fan, W.; et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 2017, 3, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nie, H.; Wang, Y.; Wang, X.; Jarret, R.; Zhao, J.; Wang, H.; Yang, J. Exploring and exploiting genetics and genomics for sweetpotato improvement: Status and perspectives. Plant Commun. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, Y.; Xiao, S.; Wang, Y.; Deng, Y.; Zhao, L.; Wang, Y.; Zhao, D.; Dai, X.; Zhou, Z.; et al. Isolation, characterization, and functional verification of salt stress response genes of NAC transcription factors in Ipomoea pes-caprae. Front. Plant Sci. 2023, 14, 1119282. [Google Scholar] [CrossRef]
- Kou, M.; Li, C.; Song, W.; Shen, Y.; Tang, W.; Zhang, Y.; Wang, X.; Yan, H.; Gao, R.; Ahmad, M.Q.; et al. Identification and functional characterization of a flavonol synthase gene from sweet potato [Ipomoea batatas (L.) Lam.]. Front. Plant Sci. 2023, 4, 1181173. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Tabuchi, H.; Monden, Y.; Suematsu, K.; Shirasawa, K.; Isobe, S.; Tanaka, M. QTL analysis and GWAS of agronomic traits in sweetpotato (Ipomoea batatas L.) using genome wide SNPs. Breed. Sci. 2020, 70, 283–291. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Gao, Y.; Pan, D.; Sun, J.; Liu, M.; Tang, Z.; Li, Z. Potassium deficiency causes more nitrate nitrogen to be stored in leaves for low-K sensitive sweet potato genotypes. Front. Plant Sci. 2022, 13, 1069181. [Google Scholar] [CrossRef]
- Daurov, D.; Zhapar, K.; Daurova, A.; Volkov, D.; Bakbergenova, M.; Tolegenova, D.; Shamekova, M.; Zhambakin, K. Production of virus-free sweet potato planting material for the southeast of Kazakhstan. Int. J. Agric. Biol. 2018, 20, 851–856. [Google Scholar] [CrossRef]
- Slonecki, T.J.; Rutter, W.B.; Olukolu, B.A.; Yencho, G.C.; Jackson, D.M.; Wadl, P.A. Genetic diversity, population structure, and selection of breeder germplasm subsets from the USDA sweetpotato (Ipomoea batatas) collection. Front. Plant Sci. 2023, 13, 1022555. [Google Scholar] [CrossRef]
- Huo, R.; Zhao, Y.; Liu, T.; Xu, M.; Wang, X.; Xu, P.; Dai, S.; Cui, X.; Han, Y.; Liu, Z.; et al. Genome-wide identification and expression analysis of two-component system genes in sweet potato (Ipomoea batatas L.). Front. Plant Sci. 2023, 13, 1091620. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapakhova, Z.; Raissova, N.; Daurov, D.; Zhapar, K.; Daurova, A.; Zhigailov, A.; Zhambakin, K.; Shamekova, M. Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review. Plants 2023, 12, 2516. https://doi.org/10.3390/plants12132516
Sapakhova Z, Raissova N, Daurov D, Zhapar K, Daurova A, Zhigailov A, Zhambakin K, Shamekova M. Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review. Plants. 2023; 12(13):2516. https://doi.org/10.3390/plants12132516
Chicago/Turabian StyleSapakhova, Zagipa, Nurgul Raissova, Dias Daurov, Kuanysh Zhapar, Ainash Daurova, Andrey Zhigailov, Kabyl Zhambakin, and Malika Shamekova. 2023. "Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review" Plants 12, no. 13: 2516. https://doi.org/10.3390/plants12132516
APA StyleSapakhova, Z., Raissova, N., Daurov, D., Zhapar, K., Daurova, A., Zhigailov, A., Zhambakin, K., & Shamekova, M. (2023). Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review. Plants, 12(13), 2516. https://doi.org/10.3390/plants12132516







