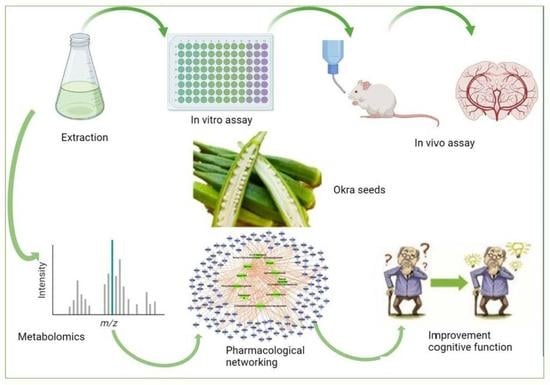
Abelmoschus eculentus Seed Extract Exhibits In Vitro and In Vivo Anti-Alzheimer’s Potential Supported by Metabolomic and Computational Investigation
Abstract
1. Introduction
2. Results
2.1. In Vitro DPPH Radical Scavenging Activity Assay of A. esculentus Seed Extract
2.2. In Vitro Evaluation of Cholinesterase Potential of the A. esculentus Seed Extract
2.3. Acute Toxicity Study
2.4. Potential Activities of A. esculentus Seed Extract in the T-Maze Test
2.5. Potential Effects of A. esculentus Seed Extract in the Beam Balance Test
2.6. Potential Effects of A. esculentus Seed Extract in Acetylcholine Esterase Levels in the Brain
2.7. Potential Effects of A. esculentus Seed Extract in Norepinephrine, Dopamine, and Serotonin Levels in Brain
2.8. Potential Effects of A. esculentus Seed Extract in the Levels of BDNF, Glycated End Product, and IL-6 in the Brain
2.9. Potential Effects of A. esculentus Seed Extract in TAC, MDA, and GSH Levels in the Brain
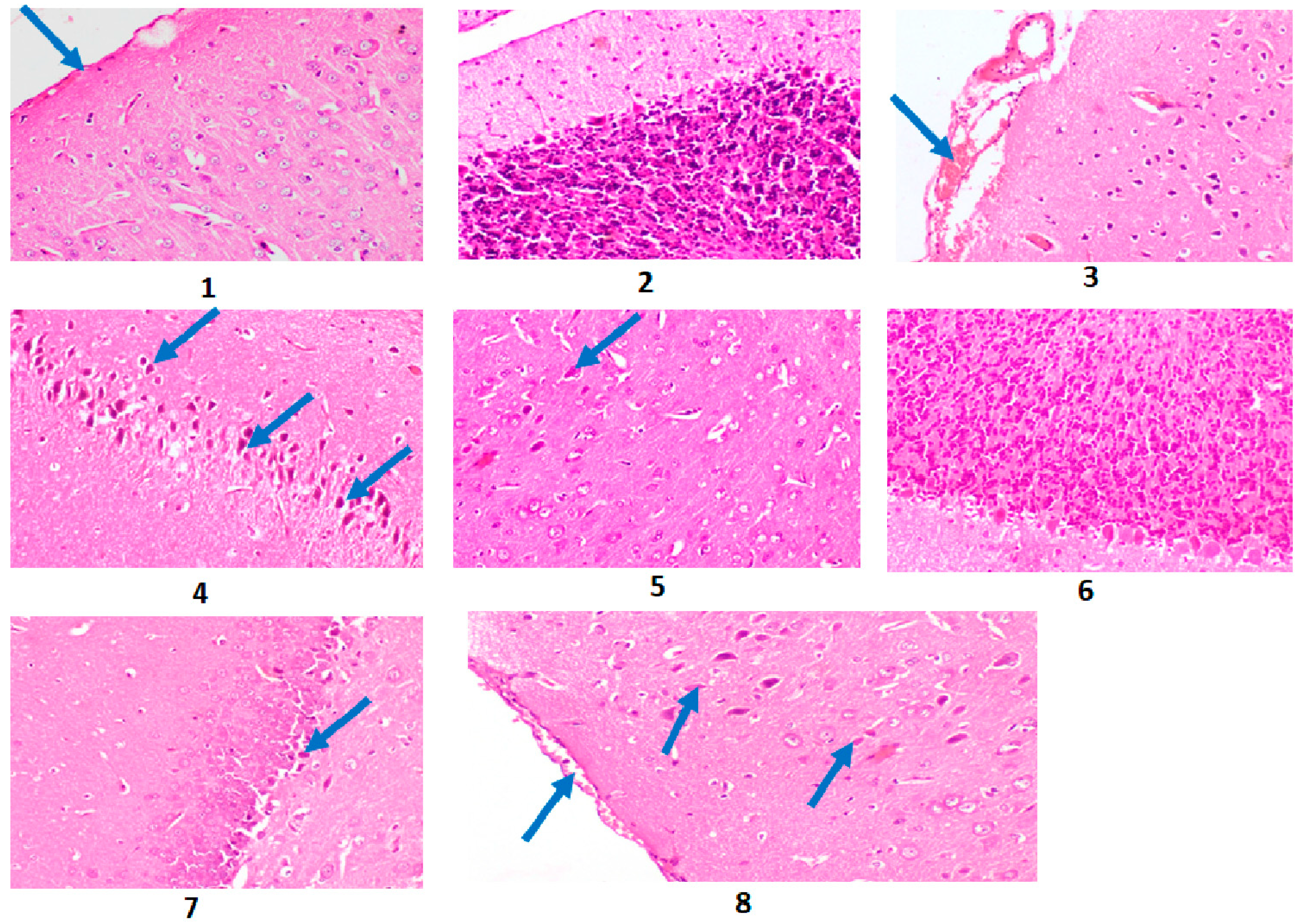
2.10. Results of the Histopathological Investigation
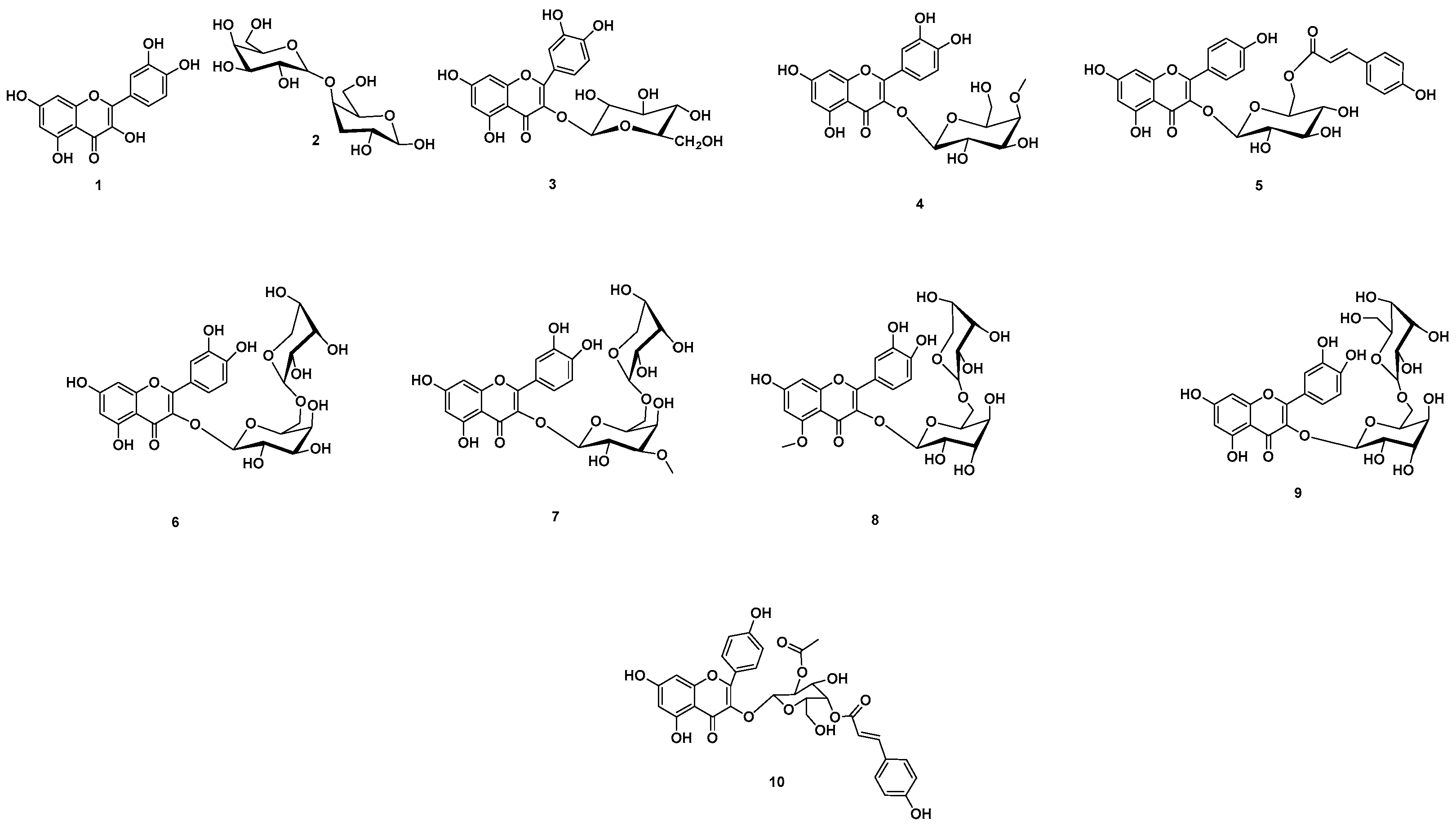
2.11. Chemical Dereplication of A. esculentus Seed Extract
3. Discussion
3.1. Evaluation of the Anti-Alzheimer’s Potential of A. esculentus Seed Extract
3.2. Metabolomics Profiling of A. esculentus Seed Extract
3.3. Pharmacology Networking
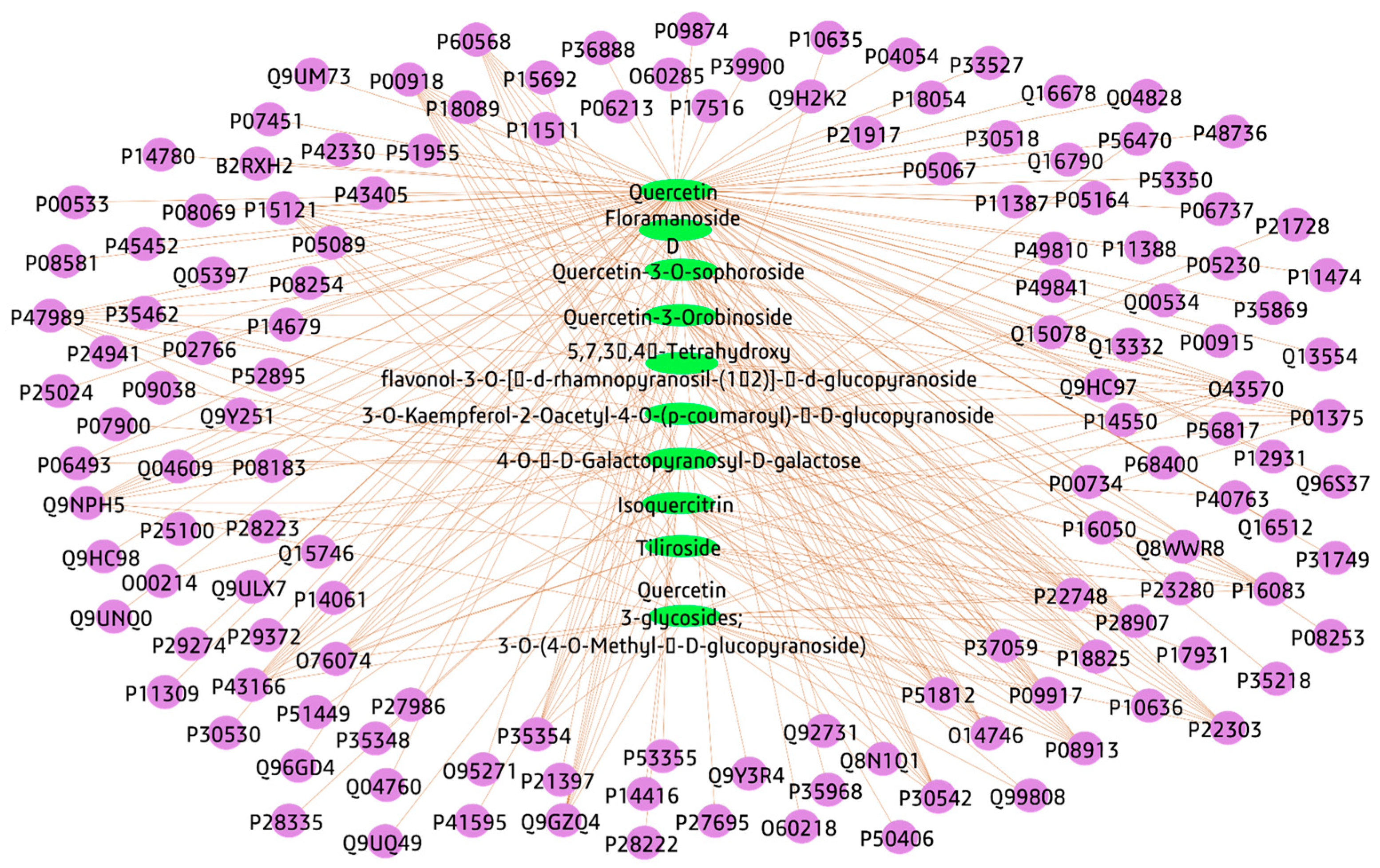
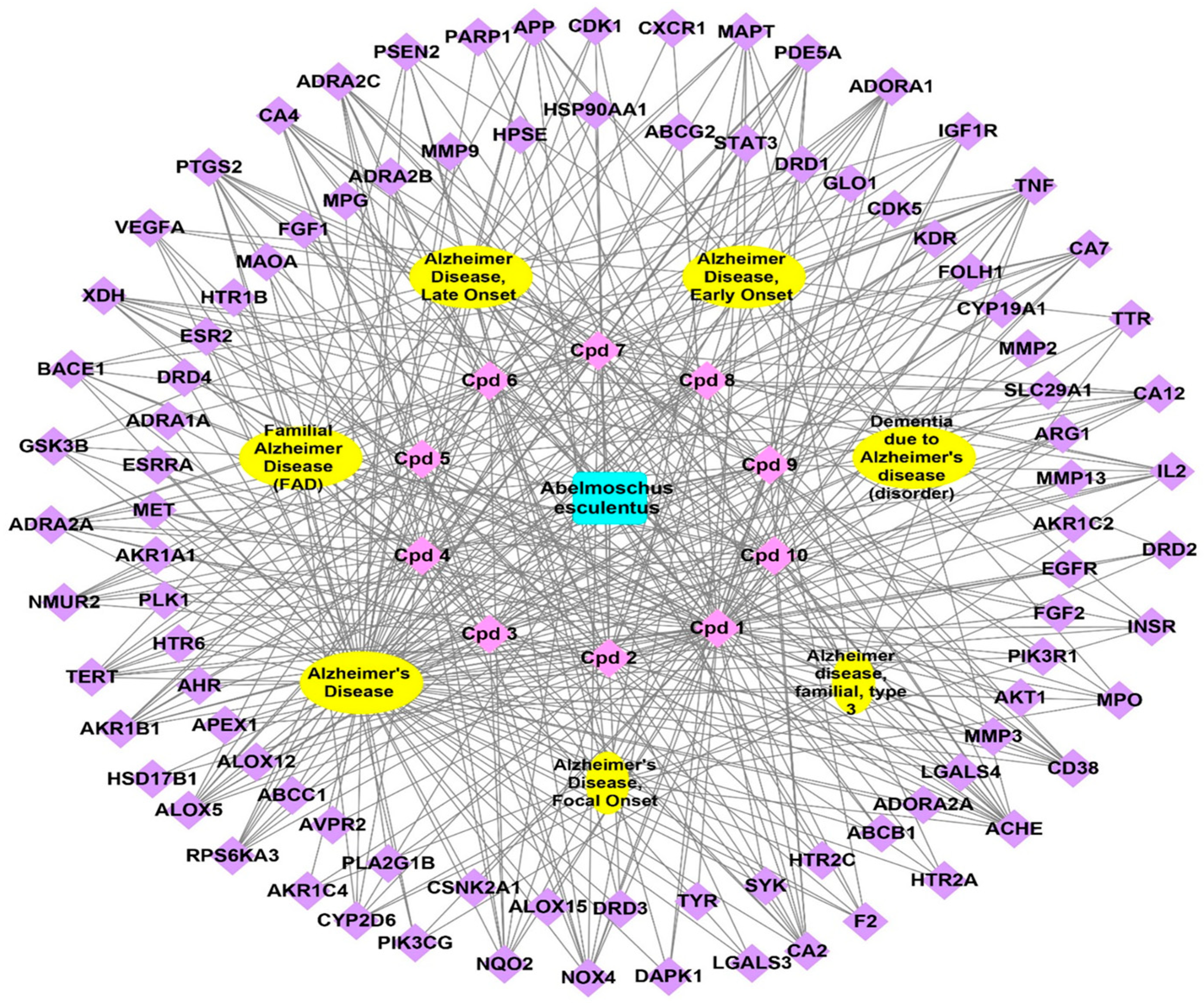
3.3.1. Plant-Compounds Network
3.3.2. Compounds-Genes Network
3.3.3. Genes–Alzheimer’s Disorders Network
3.3.4. Total Network Pharmacology
3.3.5. Protein–Protein Interactions (PPI)
3.3.6. Gene Ontology and Pathway Enrichment Analysis
4. Materials and Methods
4.1. A. esculentus Material, Chemicals, Reagents, Extraction of A. esculentus Seeds
4.2. In Vitro DPPH Free Radical Scavenging Activity Assay of A. esculentus Seed Extract
4.3. In Vitro Determination of Cholinesterase Potential of A. esculentus Seed Extract
4.4. In Vivo Anti-Alzheimer’s Potential
4.4.1. Animals
4.4.2. Animal Ethical Report
4.4.3. Induction of AlCl3-Induced Alzheimer’s Illness
4.4.4. Acute Toxicity Investigation
4.4.5. Behavioral Measurement
4.4.6. Experimental Design, and Blood Samples Preparation
4.4.7. Preparation of the Brain Tissue Samples
4.4.8. Estimation of Brain Neurotransmitters
4.4.9. Histopathological Investigations
4.5. Metabolomic Investigation Procedure
4.6. Network Pharmacology Study
4.6.1. Identified Compounds-Genes
4.6.2. Genes-Alzheimer’s Disorders
4.6.3. The Protein-Protein Interaction (PPI)
4.6.4. Network Construction and Visualization
4.6.5. Gene Enrichment Investigation
4.7. Statistical Investigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, A.; Pérez-Areales, F.J.; Martínez, P.; Arce, E.M.; Galdeano, C.; Muñoz-Torrero, D.J.P. Unveiling the multitarget anti-Alzheimer drug discovery landscape: A bibliometric analysis. Pharmaceuticals 2022, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- World Alzheimer Report 2022. Life after Diagnosis: Navigating Treatment, Care and Support. Available online: www.alzint.org/u/World-Alzheimer-Report-2022.pdf (accessed on 12 June 2023).
- Lounds, J.; Seltzer, M.M.; Greenberg, J.S.; Shattuck, P.T. Transition and change in adolescents and young adults with autism: Longitudinal effects on maternal well-being. Am. J. Ment. Retard. 2007, 112, 401–417. [Google Scholar] [CrossRef]
- Ko, S.-Y.; Lin, Y.-P.; Lin, Y.-S.; Chang, S.-S. Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic. Biol. Med. 2010, 49, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Elwood, E.; Lim, Z.; Naveed, H.; Galea, I. The effect of systemic inflammation on human brain barrier function. Brain Behav. Immun. 2017, 62, 35–40. [Google Scholar] [CrossRef]
- e Silva, N.d.M.L.; Gonçalves, R.A.; Boehnke, S.E.; Forny-Germano, L.; Munoz, D.P.; De Felice, F.G. Understanding the link between insulin resistance and Alzheimer’s disease: Insights from animal models. Exp. Neurol. 2019, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Ko, H.-A.; Chu, K.-H.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chang, W.-C.; Chang, S.-S. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer’s disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef]
- Olivieri, G.; Baysang, G.; Meier, F.; Müller-Spahn, F.; Stähelin, H.; Brockhaus, M.; Brack, C. N-Acetyl-l-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: Effects on β-amyloid secretion and tau phosphorylation. J. Neurochem. 2001, 76, 224–233. [Google Scholar] [CrossRef]
- Münch, G.; Mayer, S.; Michaelis, J.; Hipkiss, A.R.; Riederer, P.; Müller, R.; Neumann, A.; Schinzel, R.; Cunningham, A.M. Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of β-amyloid peptide. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 1997, 1360, 17–29. [Google Scholar] [CrossRef]
- Klose, J.; Griehl, C.; Roßner, S.; Schilling, S.J.B. Natural products from plants and algae for treatment of Alzheimer’s disease: A review. Biomolecules 2022, 12, 694. [Google Scholar] [CrossRef]
- Ding, H.; Reiss, A.B.; Pinkhasov, A.; Kasselman, L.J.J.M. Plants, Plants, and More Plants: Plant-Derived Nutrients and Their Protective Roles in Cognitive Function, Alzheimer’s Disease, and Other Dementias. Medicina 2022, 58, 1025. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, İ.J.L. Determination of Antioxidant, Anti-Alzheimer, Antidiabetic, Antiglaucoma and Antimicrobial Effects of Zivzik Pomegranate (Punica granatum)—A Chemical Profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Benchikha, N.; Messaoudi, M.; Larkem, I.; Ouakouak, H.; Rebiai, A.; Boubekeur, S.; Ferhat, M.A.; Benarfa, A.; Begaa, S.; Benmohamed, M.J.L. Evaluation of possible antioxidant, anti-hyperglycaemic, anti-alzheimer and anti-inflammatory effects of Teucrium polium aerial parts (Lamiaceae). Life 2022, 12, 1579. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alshehri, O.M.J.B. Isolation, In Vitro and In Silico Anti-Alzheimer and Anti-Inflammatory Studies on Phytosteroids from Aerial Parts of Fragaria× ananassa Duch. Biomolecules 2022, 12, 1430. [Google Scholar] [CrossRef]
- Oyeleke, M.B.; Owoyele, B.V.J.J.o.E. Saponins and flavonoids from Bacopa floribunda plant extract exhibit antioxidant and anti-inflammatory effects on amyloid beta 1-42-induced Alzheimer’s disease in BALB/c mice. J. Ethnopharmacol. 2022, 288, 114997. [Google Scholar] [CrossRef]
- Zieneldien, T.; Kim, J.; Cao, C.J.G. The multifaceted role of neuroprotective plants in Alzheimer’s Disease treatment. Geriatrics 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties—Focused on Antidiabetic Role—For Sustainable Health Applications. Molecules 2019, 24, 38. [Google Scholar] [CrossRef]
- Yan, T.; Liu, B.; Wang, N.; Liao, Z.; Wu, B.; He, B.; Jia, Y. The flavonoids of okra insulates against oxidative stress, neuroinflammation and restores bdnf levels in aβ1–42 induced mouse model of alzheimer’s disease. Exp. Gerontol. 2021, 147, 111263. [Google Scholar] [CrossRef]
- Yan, T.; Nian, T.; Wu, B.; Xiao, F.; He, B.; Bi, K.; Jia, Y. Okra polysaccharides can reverse the metabolic disorder induced by high-fat diet and cognitive function injury in Aβ1–42 mice. Exp. Gerontol. 2020, 130, 110802. [Google Scholar] [CrossRef]
- Mairuae, N.; Connor, J.R.; Lee, S.Y.; Cheepsunthorn, P.; Tongjaroenbuangam, W. The effects of okra (Abelmoschus esculentus Linn.) on the cellular events associated with Alzheimer’s disease in a stably expressed HFE neuroblastoma SH-SY5Y cell line. Neurosci. Lett. 2015, 603, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Doreddula, S.K.; Bonam, S.R.; Gaddam, D.P.; Desu, B.S.R.; Ramarao, N.; Pandy, V. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci. World J. 2014, 2014, 519848. [Google Scholar] [CrossRef]
- Arapitsas, P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. Food Process. Technol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Islam, M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; de Los Ríos, C.; Egea, J.; Del Pino, J.; Reiter, R.J. A review of metal-catalyzed molecular damage: Protection by melatonin. J. Pineal Res. 2014, 56, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Dogra, S. Neuroprotective effect of carvedilol against aluminium induced toxicity: Possible behavioral and biochemical alterations in rats. Pharmacol. Rep. 2011, 63, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-Y.; Lee, Y.-J.; Hsu, G.-S.W. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 2012, 19, 51. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohammed, R.; Owis, A.I.; Hetta, M.H.; AboulMagd, A.M.; Siddique, A.B.; Abdelmohsen, U.R.; Rateb, M.E.; El Sayed, K.A.; Hassan, H.M. Triple-negative breast cancer suppressive activities, antioxidants and pharmacophore model of new acylated rhamnopyranoses from Premna odorata. RSC Adv. 2020, 10, 10584–10598. [Google Scholar] [CrossRef]
- Komakech, R.; Kim, Y.-g.; Matsabisa, G.M.; Kang, Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn. (Fabaceae): A narrative review. Integr. Med. Res. 2019, 8, 181–186. [Google Scholar] [CrossRef]
- Luan, F.; Wu, Q.; Yang, Y.; Lv, H.; Liu, D.; Gan, Z.; Zeng, N. Traditional uses, chemical constituents, biological properties, clinical settings, and toxicities of Abelmoschus manihot L.: A comprehensive review. Front. Pharmacol. 2020, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-M.; Xu, R.; Wang, H.; Chen, J.-Y.; Tu, Z.-C. Structural properties, bioactivities, and applications of polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, Q.; Yu, J.; Wang, M.; Chen, M.; Wang, R.; He, X.; Gao, M.; Chen, X. Separation of α-amylase inhibitors from Abelmoschus esculentus (L). Moench by on-line two-dimensional high-speed counter-current chromatography target-guided by ultrafiltration-HPLC. J. Sep. Sci. 2015, 38, 3897–3904. [Google Scholar] [CrossRef] [PubMed]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Abdelmohsen, U.R.; Alsenani, F.; Aly, H.F.; Shams, S.G.E.; Younis, E.A.; Ahmed, K.A.; Sayed, A.M.; Owis, A.I.; Afifi, N. The anti-Alzheimer potential of Tamarindus indica: An in vivo investigation supported by in vitro and in silico approaches. RSC Adv. 2022, 12, 11769–11785. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.F.; Younis, E.A.; Gaafar, A.A.; Shams, S.G.E.; Ahmed, K.A.; Hashish, H.M.A.; Salama, Z.A. The Efficacy of Egyptian Clementine oil identified by GC/MS analysis on Alzheimer’s disease–induced rats. Egypt. J. Chem. 2022, 65, 465–477. [Google Scholar]
- Aly, H.F.; Metwally, F.M.; Ahmed, H.H. Neuroprotective effects of dehydroepiandrosterone (DHEA) in rat model of Alzheimer’s disease. Acta Biochim. Pol. 2011, 58, 513–520. [Google Scholar] [CrossRef]
- Kaur, S.; Singla, N.; Dhawan, D. Neuro-protective potential of quercetin during chlorpyrifos induced neurotoxicity in rats. Drug Chem. Toxicol. 2019, 42, 220–230. [Google Scholar] [CrossRef]
- Crockett, M.J.; Clark, L.; Tabibnia, G.; Lieberman, M.D.; Robbins, T.W. Serotonin modulates behavioral reactions to unfairness. Science 2008, 320, 1739. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Chung, H.D.; Ruggiero, D.A.; Kristal, B.S.; Johnson, E.M.; Lampe, P.; Kumar, V.B.; Franko, M.; Williams, E.A. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: Role in neurodegenerative diseases. Neurotoxicology 2004, 25, 101–115. [Google Scholar] [CrossRef]
- Kaur, G.; Verma, N. Nature curing cancer–review on structural modification studies with natural active compounds having anti-tumor efficiency. Biotechnol. Rep. 2015, 6, 64–78. [Google Scholar] [CrossRef]
- Gokul, K. Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: Relevance to Parkinson’s disease. Neurochem. Res. 2014, 39, 1382–1394. [Google Scholar] [CrossRef]
- Garzon, D.; Yu, G.; Fahnestock, M. A new brain-derived neurotrophic factor transcript and decrease inbrain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J. Neurochem. 2002, 82, 1058–1064. [Google Scholar] [CrossRef]
- Khan, R.S.; Dine, K.; Das Sarma, J.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol. Commun. 2014, 2, 3. [Google Scholar] [CrossRef]
- Rosa, E.; Mahendram, S.; Ke, Y.D.; Ittner, L.M.; Ginsberg, S.D.; Fahnestock, M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging 2016, 48, 135–142. [Google Scholar] [CrossRef]
- Aso, E.; Semakova, J.; Joda, L.; Semak, V.; Halbaut, L.; Calpena, A.; Escolano, C.; C Perales, J.; Ferrer, I. Triheptanoin supplementation to ketogenic diet curbs cognitive impairment in APP/PS1 mice used as a model of familial Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.; Resende, E.d.P.F.; Vieira, E.L.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Im, Y.B.; Jung, J.S.; Cho, J.; Suh, H.W.; Kim, Y.H. Central β-amyloid peptide-induced peripheral interleukin-6 responses in mice. J. Neurochem. 2001, 76, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Sumathi, T.; Shobana, C.; Mahalakshmi, V.; Sureka, R.; Subathra, M.; Vishali, A.; Rekha, K. Oxidative stress in brains of male rats intoxicated with aluminium and neuromodulating effect of Celastrus paniculatus alcoholic seed extract. Asian J. Pharm. Clin. Res. 2013, 6, 80–90. [Google Scholar]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sa, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Singh, S. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef] [PubMed]
- Erdemci, F.; Fırat, A.; Fatih, T. Etiology and Histopathology of Alzheimer’s Disease and Current Approaches. Black Sea J. Health Sci. 2022, 5, 322–327. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs of Today 2003, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- O’brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Rozpędek-Kamińska, W.; Siwecka, N.; Wawrzynkiewicz, A.; Wojtczak, R.; Pytel, D.; Diehl, J.A.; Majsterek, I. The PERK-dependent molecular mechanisms as a novel therapeutic target for neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 2108. [Google Scholar] [CrossRef]
- Alzarea, S.I.; Elmaidomy, A.H.; Saber, H.; Musa, A.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Youssif, K.A.; Alanazi, A.S.; Alharbi, M. Potential anticancer lipoxygenase inhibitors from the red sea-derived brown algae Sargassum cinereum: An in-silico-supported In-Vitro Study. Antibiotics 2021, 10, 416. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S. Antioxidant and wound healing potential of Vitis vinifera seeds supported by phytochemical characterization and docking studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Elmaidomy, A.H.; Zahran, E.M.; Soltane, R.; Alasiri, A.; Saber, H.; Ngwa, C.J.; Pradel, G.; Alsenani, F.; Sayed, A.M.; Abdelmohsen, U.R. New Halogenated Compounds from Halimeda macroloba Seaweed with Potential Inhibitory Activity against Malaria. Molecules 2022, 27, 5617. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Alhadrami, H.A.; Amin, E.; Aly, H.F.; Othman, A.M.; Rateb, M.E.; Hetta, M.H.; Abdelmohsen, U.R.; M. Hassan, H. Anti-inflammatory and antioxidant activities of terpene-and polyphenol-rich Premna odorata leaves on alcohol-inflamed female wistar albino rat liver. Molecules 2020, 25, 3116. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Elmaidomy, A.H.; Maher, S.A.; Abu-Baih, D.H.; Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Ghoneim, M.M.; Mostafa, E.M. The Wound-Healing Potential of Olea europaea L. Cv. Arbequina Leaves Extract: An Integrated In Vitro, In Silico, and In Vivo Investigation. Metabolites 2022, 12, 791. [Google Scholar] [CrossRef]
- Ahmed, W.M.; Ibrahim, M.A.; Helmy, N.A.; ElKashlan, A.M.; Elmaidomy, A.H.; Zaki, A.R. Amelioration of aluminum-induced hepatic and nephrotoxicity by Premna odorata extract is mediated by lowering MMP9 and TGF-β gene alterations in Wistar rat. Environ. Sci. Pollut. Res. 2022, 29, 72827–72838. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Elmaidomy, A.H.; Selim, S.; Al-Sanea, M.M.; Albqmi, M.; Mostafa, E.M.; Ibrahim, S.; Ghoneim, M.M.; Sayed, A.M.; Abdelmohsen, U.R. Bioactive Phytochemicals of Citrus reticulata Seeds—An Example of Waste Product Rich in Healthy Skin Promoting Agents. Antioxidants 2022, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Bagalagel, A.A.; El-Hawary, S.S.; Alaaeldin, R.; Elmaidomy, A.H.; Altemani, F.H.; Waggas, D.S.; Algehainy, N.A.; Saeedi, N.H.; Alsenani, F.; Mokhtar, F.A. The Protective and Therapeutic Anti-Alzheimer Potential of Olea europaea L. cv. Picual: An In Silico and In Vivo Study. Metabolites 2022, 12, 1178. [Google Scholar] [CrossRef]
- El-Sharawy, D.M.; Khater, S.; Essam, H.; Sherif, N.H.; Hassan, H.M.; Elmaidomy, A.H. 99mTc-Luteolin: Radiolabeling, in silico ADMET and biological evaluation as a natural tracer tumor imaging. J. Radiat. Res. Appl. Sci. 2021, 14, 125–132. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Hassan, H.M.; Amin, E.; Mohamed, W.; Hetta, M.H. Premna odorata volatile oil as a new mycobacterium tuberculosis growth inhibitor for the control of tuberculosis disease. Eur. J. Med. Plants 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohamed, E.M.; Aly, H.F.; Younis, E.A.; Shams, S.G.E.; Altemani, F.H.; Alzubaidi, M.A.; Almaghrabi, M.; Harbi, A.A.; Alsenani, F. Anti-Inflammatory and Antioxidant Properties of Malapterurus electricus Skin Fish Methanolic Extract in Arthritic Rats: Therapeutic and Protective Effects. Mar. Drugs 2022, 20, 639. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, W.A.; Abbas, S.S.; Schaalan, M.F.; Elmaidomy, A.H.; Hassan, H.M.; Amin, E.; Hetta, M.H. Immunomodulatory effect of Premna odorata volatile oils in Mycobacterium tuberculosis by inhibiting TLR4/NF-κB pathway. J. Herbmed Pharmacol. 2019, 8, 1–7. [Google Scholar]
- Zahran, E.M.; Abdel-Maqsoud, N.M.R.; Tammam, O.Y.; Abdel-Rahman, I.M.; Elrehany, M.A.; Bakhsh, H.T.; Altemani, F.H.; Algehainy, N.A.; Alzubaidi, M.A.; Abdelmohsen, U.R. Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics 2022, 12, 43. [Google Scholar] [CrossRef]
- Young, D.S. Effects of Drugs on Clinical Laboratory Tests; AACC Press: Washington, DC, USA, 2000. [Google Scholar]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimer’s Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Pioli, E.Y.; Gaskill, B.N.; Gilmour, G.; Tricklebank, M.D.; Dix, S.L.; Bannerman, D.; Garner, J.P. An automated maze task for assessing hippocampus-sensitive memory in mice. Behav. Brain Res. 2014, 261, 249–257. [Google Scholar] [CrossRef]
- Altun, M.; Bergman, E.; Edström, E.; Johnson, H.; Ulfhake, B. Behavioral impairments of the aging rat. Physiol. Behav. 2007, 92, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-M.; Kwon, O.-D.; Kim, H.S.; Park, S.-J.; Song, C.-H.; Ku, S.-K. Pharmacokinetic Profiles of Donepezil in Combination with Gwibi-Chongmyungtang in Rats. Int. J. Pharmacol. 2015, 11, 343–350. [Google Scholar] [CrossRef]
- Aune, H.; Hals, P.-A.; Hansen, B.; Aarbakke, J. Effect of diethylether on the formation of paracetamol sulphate and glucuronide in isolated rat hepatocytes. Pharmacology 1984, 28, 67–73. [Google Scholar] [CrossRef]
- Awad, H.M.; Abd-Alla, H.I.; Ibrahim, M.A.; El-Sawy, E.R.; Abdalla, M.M. Flavones from Heavenly Blue as modulators of Alzheimer’s amyloid-beta peptide (Aβ) production. Med. Chem. Res. 2018, 27, 768–776. [Google Scholar] [CrossRef]
- Engvall, E.; Perlman, P. Enzyme-linked immunosorbent assay (EL1SA). Quantitative assay of characterization G. J. Immunochem. 1971, 8, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Giday, M.; Asfaw, Z.; Woldu, Z. Medicinal plants of the Meinit ethnic group of Ethiopia: An ethnobotanical study. J. Ethnopharmacol. 2009, 124, 513–521. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Farag, O.M.; Abd-Elsalam, R.M.; Ogaly, H.A.; Ali, S.E.; El Badawy, S.A.; Alsherbiny, M.A.; Li, C.G.; Ahmed, K.A. Metabolomic profiling and neuroprotective effects of purslane seeds extract against acrylamide toxicity in rat’s brain. Neurochem. Res. 2021, 46, 819–842. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.; Elmaidomy, A.H.; Sayed, A.M.; Alzarea, S.I.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Abdelgawad, M.A.; Youssif, K.A.; Refaat, H. Cytotoxic potential, metabolic profiling, and liposomes of Coscinoderma sp. crude extract supported by in silico analysis. Int. J. Nanomed. 2021, 16, 3861. [Google Scholar] [CrossRef] [PubMed]
- Shamikh, Y.I.; El Shamy, A.A.; Gaber, Y.; Abdelmohsen, U.R.; Madkour, H.A.; Horn, H.; Hassan, H.M.; Elmaidomy, A.H.; Alkhalifah, D.H.M.; Hozzein, W.N. Actinomycetes from the Red Sea sponge Coscinoderma mathewsi: Isolation, diversity, and potential for bioactive compounds discovery. Microorganisms 2020, 8, 783. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Tang, D.; Qin, B.; Feng, X.; Liu, T. Effective LSTMs for target-dependent sentiment classification. arXiv 2015, arXiv:1512.01100. [Google Scholar]
- Franz, M.; Lopes, C.T.; Huck, G.; Dong, Y.; Sumer, O.; Bader, G.D. Cytoscape. js: A graph theory library for visualisation and analysis. Bioinformatics 2016, 32, 309–311. [Google Scholar] [CrossRef]
| 0.01 µg/mL | 0.05 µg/mL | |
|---|---|---|
| Crude extract | 77.2 ± 3.6 c | 93.2 ± 7.5 d |
| Ascorbic acid | 82.3 ± 4.7 b | 91.0 ± 6.1 d |
| Parameters | Control | AlCl3-AD | Extract | Donepezil Drug |
|---|---|---|---|---|
| AChE (serum) (U\L) % Change % Improvement | 215.0 ± 11.0 a | 419.0 ± 10.0 b +94.8 | 255.0 ± 8.0 c 76.2 | 240.0 ± 9.7 c 83.2 |
| BDNF (pg/g Tissue) | Glycated End Product (μg/g Tissue) | IL-6 (pg/mL) | |
|---|---|---|---|
| Control | 343.0 ± 10.2 a | 12.7 ± 1.2 a | 39.6 ± 3.0 a |
| AlCl3-AD % Change | 167.0 ± 10.9 b −50.3% | 40.8 ± 3.1 b 221.2% | 130.0 ± 8.0 b 227.7% |
| AD crude extract % Improvement | 258.0 ± 6.0 d 26.5 | 20.0 ± 2.0 d 163.7 | 45.3 ± 3.0 d 213.5 |
| Donepezil drug % Improvement | 288.0 ± 16.0 e 35.2 | 12.9 ± 1.1 a 219.6 | 39.0 ± 2.8 a 229.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhsh, H.T.; Mokhtar, F.A.; Elmaidomy, A.H.; Aly, H.F.; Younis, E.A.; Alzubaidi, M.A.; Altemani, F.H.; Algehainy, N.A.; Majrashi, M.A.A.; Alsenani, F.; et al. Abelmoschus eculentus Seed Extract Exhibits In Vitro and In Vivo Anti-Alzheimer’s Potential Supported by Metabolomic and Computational Investigation. Plants 2023, 12, 2382. https://doi.org/10.3390/plants12122382
Bakhsh HT, Mokhtar FA, Elmaidomy AH, Aly HF, Younis EA, Alzubaidi MA, Altemani FH, Algehainy NA, Majrashi MAA, Alsenani F, et al. Abelmoschus eculentus Seed Extract Exhibits In Vitro and In Vivo Anti-Alzheimer’s Potential Supported by Metabolomic and Computational Investigation. Plants. 2023; 12(12):2382. https://doi.org/10.3390/plants12122382
Chicago/Turabian StyleBakhsh, Hussain T., Fatma A. Mokhtar, Abeer H. Elmaidomy, Hanan F. Aly, Eman A. Younis, Mubarak A. Alzubaidi, Faisal H. Altemani, Naseh A. Algehainy, Mohammed Ali A. Majrashi, Faisal Alsenani, and et al. 2023. "Abelmoschus eculentus Seed Extract Exhibits In Vitro and In Vivo Anti-Alzheimer’s Potential Supported by Metabolomic and Computational Investigation" Plants 12, no. 12: 2382. https://doi.org/10.3390/plants12122382
APA StyleBakhsh, H. T., Mokhtar, F. A., Elmaidomy, A. H., Aly, H. F., Younis, E. A., Alzubaidi, M. A., Altemani, F. H., Algehainy, N. A., Majrashi, M. A. A., Alsenani, F., Bringmann, G., Abdelmohsen, U. R., & Abdelhafez, O. H. (2023). Abelmoschus eculentus Seed Extract Exhibits In Vitro and In Vivo Anti-Alzheimer’s Potential Supported by Metabolomic and Computational Investigation. Plants, 12(12), 2382. https://doi.org/10.3390/plants12122382