Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
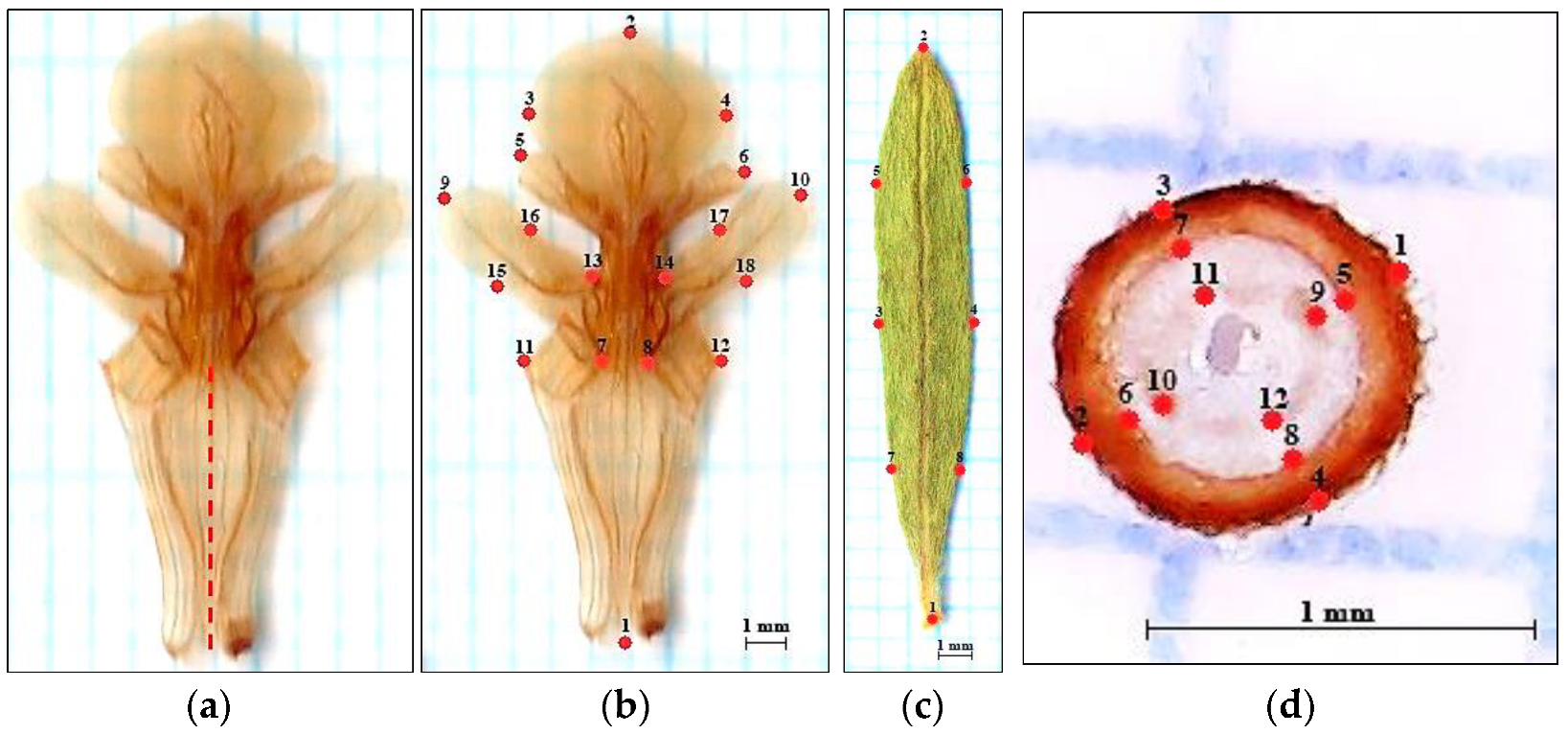
2.2. Preparation of Plant Structures for Geometric-Morphometric Analyzes
2.3. Statistical Analyses
3. Results
3.1. The Influence of the Geological Substrate on the Variation in Size and Shape in the Corolla, Leaf, and Stem of T. montanum
3.2. Variation in the Shape of the Plant Structures of T. montanum
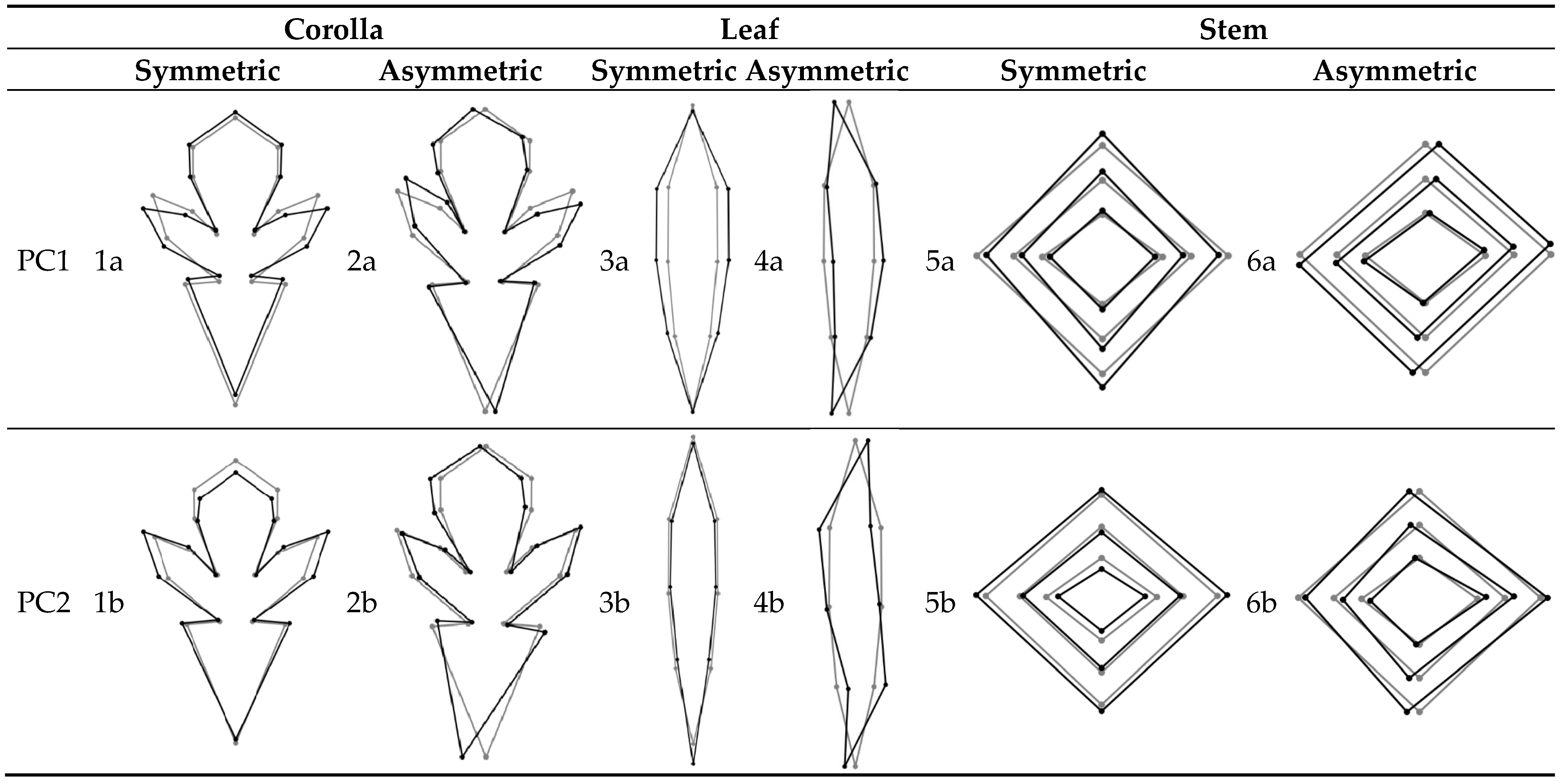
3.3. Symmetric and Asymmetric Components of Corolla Shape Variation
3.4. Symmetric and Asymmetric Components of Leaf Shape Variation
3.5. Symmetric and Asymmetric Components of Stem Shape Variation
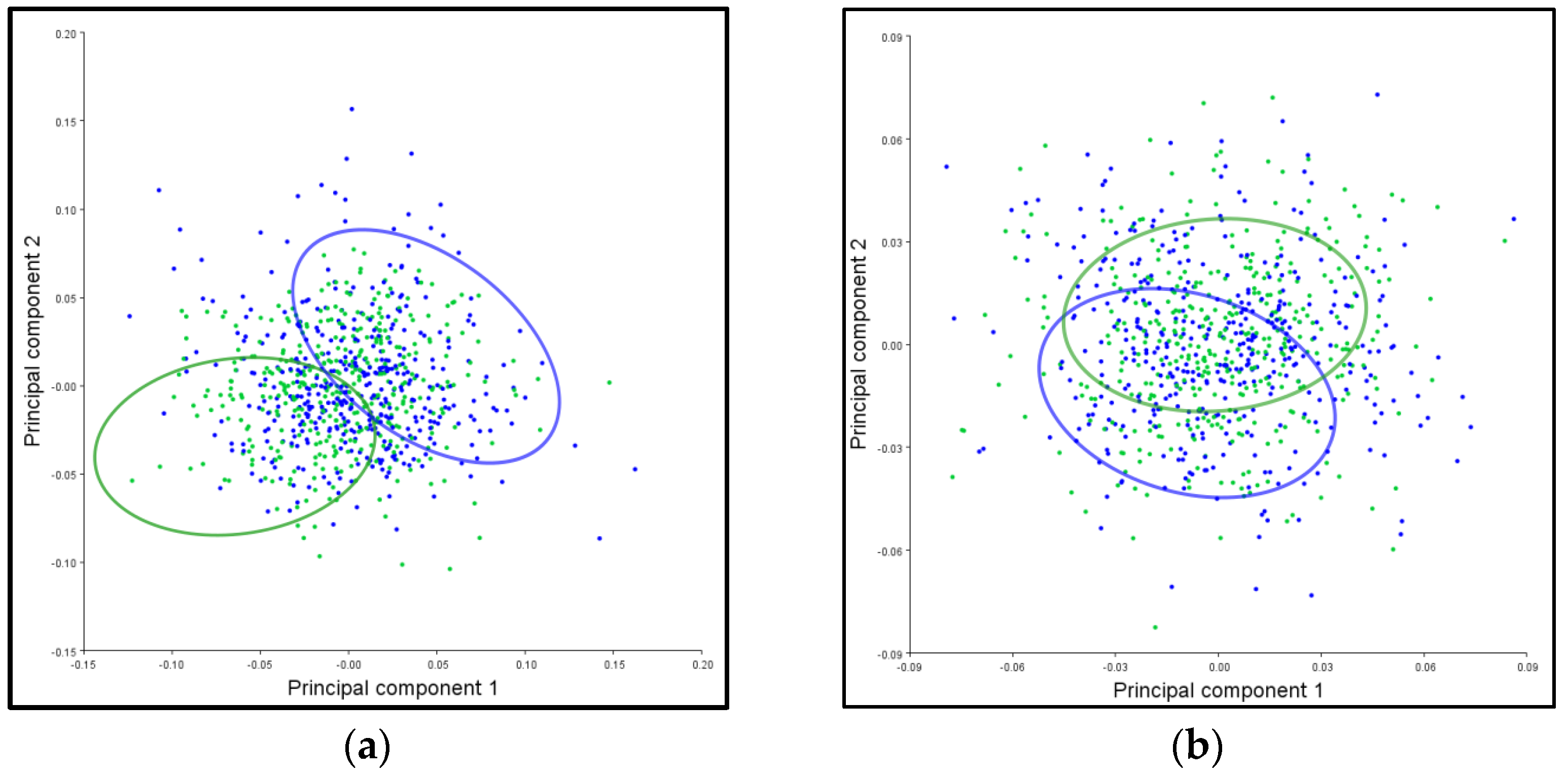
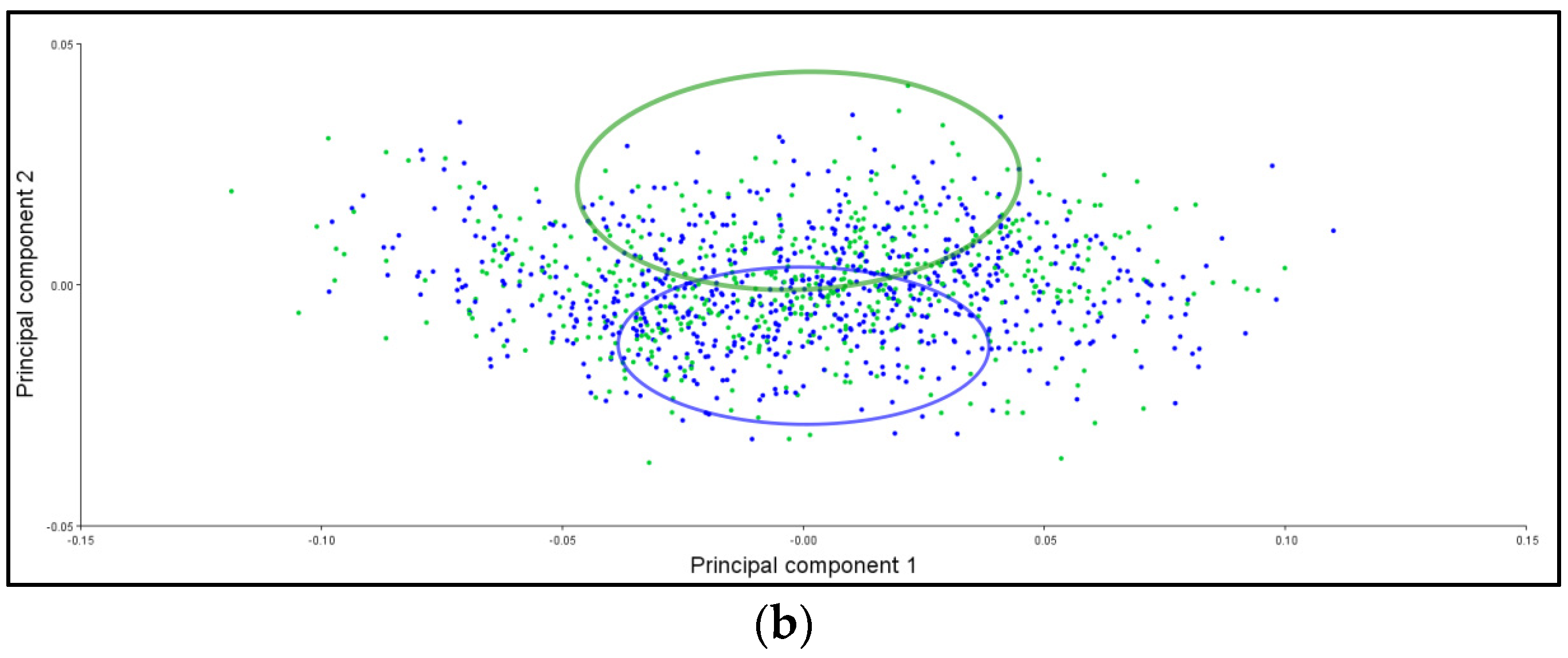
3.6. PCA Ellipses
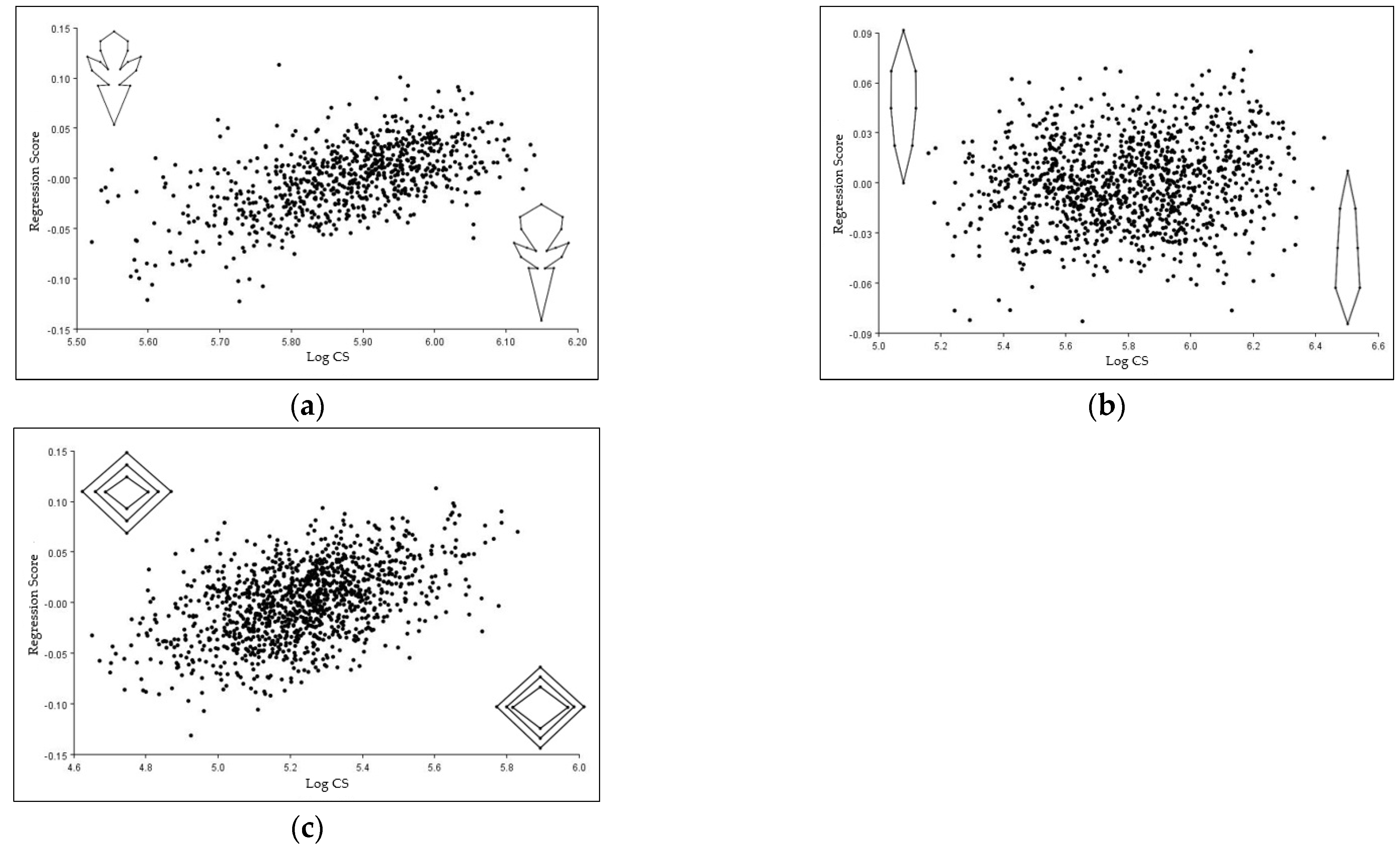
3.7. Allometric Shape Differences in the Corolla, Leaf, and Stem of T. montanum
3.8. Discriminant Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer Associates Incorporated: Sunderland, MA, USA, 1998; pp. 147–171. [Google Scholar]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; JHU Press: Baltimore, MD, USA, 2001; pp. 40–65. [Google Scholar]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 4, 208747. [Google Scholar] [CrossRef] [Green Version]
- Habuš Jerčić, I.; Bošnjak Mihovilović, A.; Matković Stanković, A.; Lazarević, B.; Goreta Ban, S.; Ban, D.; Major, N.; Tomaz, I.; Banjavčić, Z.; Kereša, S. Garlic ecotypes utilise different morphological, physiological and biochemical mechanisms to cope with drought stress. Plants 2023, 12, 1824. [Google Scholar] [CrossRef]
- Scheiner, S.M. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 1993, 24, 35–68. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity in plants: A case study in ecological development. Evol. Dev. 2003, 5, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Kruuk, E.B.L.; Nicotra, B.A. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Valladares, F.; Gianoli, E.; Gómez, M.J. Ecological limits to plant phenotypic plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Shykoff, J.A. Morphological developmental stability in plants: Patterns and causes. Int. J. Plant Sci. 1999, 160, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dongen, V.S. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef]
- Budečević, S. Morphological Variation, Phenotypic Plasticity and Fluctuating Asymmetry of Floral Organ Shapes in Iris pumila L. Ph.D. Thesis, University of Belgrade, Beograd, Serbia, 2018. [Google Scholar]
- Mendes, G.; Boaventura, G.M.; Cornelissen, T. Fluctuating asymmetry as a bioindicator of environmental stress caused by pollution in a pioneer plant species. Environ. Entomol. 2018, 47, 1479–1484. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Monteiro, L.R. Distances and directions in multidimensional shape spaces: Implications for morphometric applications. Syst. Biol. 2005, 54, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Fan, D. Effects of climate, biotic factors, and phylogeny on allometric relationships: Testing the metabolic scaling theory in plantations and natural forests across China. For. Ecosyst. 2020, 7, 51. [Google Scholar] [CrossRef]
- Niklas, J.K. The evolutionary-developmental origins of multicellularity. Am. J. Bot. 2014, 101, 6–25. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Fang, F.; Wu, N.; Sun, S. Stem architectural effect on leaf size, leaf number, and leaf mass fraction in plant twigs of woody species. Int. J. Plant Sci. 2009, 170, 999–1008. [Google Scholar] [CrossRef]
- Gray, F.E.; Wright, J.I.; Falster, S.D.; Eller, D.A.S.; Lehmann, R.C.E.; Bradford, G.M.; Cernusak, A.L. Leaf: Wood allometry and functional traits together explain substantial growth rate variation in rainforest trees. AoB Plants 2019, 11, plz024. [Google Scholar] [CrossRef] [Green Version]
- Hulshof, M.C.; Spasojević, J.M. The edaphic control of plant diversity. Glob. Ecol. Biogeogr. 2020, 29, 1634–1650. [Google Scholar] [CrossRef]
- O’Dell, E.R.; Rajakaruna, N. Intraspecific variation, adaptation, and evolution. In Serpentine: Evolution and Ecology in a Model System; Harrison, S.P., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 97–137. [Google Scholar]
- Zlatić, M.N.; Stanković, S.M. Effects of calcareous and serpentinite parent material on the mineral characteristics of soils and plant material of Teucrium montanum L. (Lamiaceae). Environ. Monit. Assess. 2019, 191, 564. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I. How soil nutrient availability influences plant biomass and how biomass stimulation alleviates heavy metal toxicity in soils: The cases of nutrient use efficient genotypes and phytoremediators, respectively. In Biomass Now—Cultivation and Utilization; Matović, D.M., Ed.; Intech Open: London, UK, 2013; pp. 427–448. [Google Scholar]
- da Silva, C.E.; de Albuquerque, B.M.; de Azevedo Neto, D.A.; da Silva Junior, D.C. Drought and its consequences to plants—From individual to ecosystem. In Responses of Organisms to Water Stress; Akinci, S., Ed.; IntechOpen: London, UK, 2013; pp. 18–47. [Google Scholar]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of soil pH on the growth, reproductive finvestment and pollen allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Galey, M.L.; van der Ent, A.; Iqbal, M.C.M.; Rajakaruna, N. Ultramafic geoecology of South and Southeast Asia. Bot. Stud. 2017, 58, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Dioscorides Press: Portland, OR, USA, 1987; pp. 86–96. [Google Scholar]
- Kruckenberg, R.A. Californian Serpentines: Flora, Vegetation, Geology, Soils and Management Problems; University of California Press: Berkeley, CA, USA, 1984; pp. 32–54. [Google Scholar]
- Proctor, J.; Woodell, K. The ecology of serpentine soils. Adv. Ecol. Res. 1975, 9, 255–365. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D. Evolutionary ecology of plant adaptation to ultramafic soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Alexander, E.B.; Coleman, R.G.; Keeler-Wolf, T.; Harrison, S.P. Serpentine Geoecology of Western North America: Geology, Soil, and Vegetation; Oxford University Press: New York, NY, USA, 2008; pp. 215–287. [Google Scholar]
- Alexander, E.B. Soil and vegetation differences from peridotite to serpentinite. Northeast. Nat. 2009, 16, 178–192. [Google Scholar] [CrossRef]
- Shao, B.H.; Chu, Y.L.; Jaleel, A.C.; Znao, X.C. Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Covelo, F.; Sales, F.; Silva, M.M.V.G.; Garcia, C.A. Vegetation on limestone versus phyllite soils: A case study in the west Iberian Peninsula. Flora Mediterr. 2017, 27, 159–173. [Google Scholar]
- Haling, E.R.; Simpson, J.R.; Delhaize, E.; Hocking, J.P.; Richardson, E.A. Effect of lime on root growth, morphology and the rhizosheath of cereal seedlings growing in an acid soil. Plant Soil 2010, 327, 199–212. [Google Scholar] [CrossRef]
- Tyler, G.; Zohlen, A. Plant seeds as mineral nutrient resource for seedlings–A comparison of plants from calcareous and silicate soils. Ann. Bot. 1998, 81, 455–459. [Google Scholar] [CrossRef]
- Zlatić, M.N.; Krstić, Ž.D.; Stanković, S.M. Radioactivity level in relation to geological substrate: Dynamics of natural and artificial radionuclides on Teucrium montanum L. (Lamiaceae) habitats. Environ. Monit. Assess. 2021, 193, 749. [Google Scholar] [CrossRef]
- Zlatić, N.; Mihailović, V.; Lješević, M.; Beškoski, V.; Stanković, M. Geological substrate-related variability of Teucrium montanum L. (Lamiaceae) essential oil. Biochem. Syst. Ecol. 2022, 100, 104372. [Google Scholar] [CrossRef]
- Salmaki, Y.S.; Kattari Heub, G.; Bräuchler, C. Phylogeny of non-monophyletic Teucrium (Lamiaceae: Ajugoideae): Implications for character evolution and taxonomy. Taxon 2016, 65, 805–822. [Google Scholar] [CrossRef]
- Rohlf, F.J. tpsDig. Digitize Landmarks and Outlines, Version 2.10. Stony Brook, NY: Department of Ecology and Evolution; State University of New York: Albany, NY, USA, 2006. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Elobeid, M.; Polle, A. Interference of heavy metal toxicity with auxin physiology. In Metal Toxicity in Plants: Percetion, Signaling adn Remediation; Gupta, D., Sandalio, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 249–259. [Google Scholar]
- Tarasjev, A.; Avramov, S.; Miljković, D. Evolutionary biology studies on the Iris pumila clonal plant: Advantages of a good model system, main findings and directions for further research. Arch. Biol. Sci. 2012, 64, 159–174. [Google Scholar] [CrossRef]
- Stevanović, B.; Stevanović, V. Morpho-anatomical characteristics of the species Teucrium montanum L. from different habitats. Glasn. Bot. Zavoda Bašte Univ. Beograd. 1985, 19, 73–89. (In Serbian) [Google Scholar]
- Vujić, V.; Avramov, S.; Tarasjev, A.; Barišić Klisaric, N.; Živković, U.; Miljković, D. The effects of traffic-related air pollution on the flower morphology of Iris pumila—Comparison of a polluted city area and the unpolluted Deliblato Sands (nature reserve). Appl. Ecol. Environ. Res. 2015, 13, 405–415. [Google Scholar]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef] [Green Version]
- Viscosi, V. Geometric morphometrics and leaf phenotypic plasticity: Assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot. J. Linn. Soc. 2015, 179, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Spencer, V.; Kim, M. Re“CYC”ling molecular regulators in the evolution and development of flower symmetry. Semin. Cell Dev. Biol. 2018, 79, 16–26. [Google Scholar] [CrossRef]
- Bresson, C.C.; Vitasse, Y.; Kremer, A.; Delzon, S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011, 31, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.M. Multiplicity in Unity: Plant Subindividual Variation and Interactions with Animals; University of Chicago Press: Chicago, IL, USA, 2009; pp. 174–201. [Google Scholar]
- Klingenberg, C.P.; Duttke, S.; Whelan, S.; Kim, M. Developmental plasticity, morphological variation and evolvability: A multilevel analysis of morphometric integration in the shape of compound leaves. J. Evol. Biol. 2012, 25, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Lempa, K.; Haukioja, E. Effects of stress and rapid growth on fluctuating asymmetry and insect damage in birch leaves. Oikos 1999, 86, 208–216. [Google Scholar] [CrossRef]
- Møller, A.P.; Eriksson, M. Patterns of fluctuating asymmetry in flowers: Implications for sexual selection in plants. J Evol. Biol. 1994, 7, 97–113. [Google Scholar] [CrossRef]
- Sandner, T.M.; Matthies, D. Fluctuating asymmetry of leaves is a poor indicator of environmental stress and genetic stress by inbreeding in Silene vulgaris. Ecol. Indic. 2017, 79, 247–253. [Google Scholar] [CrossRef]
- Tucić, B.; Tarasjev, A.; Vujčić, S.; Milojković, S.; Tucić, N. Phenotypic plasticity and character differentiation in a subdivided population of Iris pumila (Iridaceae). Plant Syst. Evol. 1990, 170, 1–9. [Google Scholar] [CrossRef]
- Savriama, Y.; Gómez, J.M.; Perfectti, F.; Klingenberg, C.P. Geometric morphometrics of corolla shape: Dissecting components of symmetric and asymmetric variation in Erysimum mediohispanicum (Brassicaceae). New Phytol. 2012, 196, 945–954. [Google Scholar] [CrossRef]
- Nagy, L.; Proctor, J. Plant growth and reproduction on a toxic alpine ultramafic soil: Adaptation to nutrient limitation. New Phytol. 1997, 137, 267–274. [Google Scholar] [CrossRef]
- Murren, J.C.; Douglass, L.; Gibson, A.; Dudas, R.M. Individual and combined effects of ca/mg ratio and water on trait expression in Mimulus guttatus. Ecology 2006, 87, 2591–2602. [Google Scholar] [CrossRef]
- Burnett, E.S.; Zhang, D.; Stack, B.L.; He, Z. Effects of phosphorus on morphology and foliar nutrient concentrations of hydroponically grown Scaevola aemula R. br. ‘Whirlwind Blue’. J. Am. Soc. Hortic. Sci. 2008, 43, 902–905. [Google Scholar] [CrossRef] [Green Version]
- Hladun, R.K.; Parker, R.D.; Trumble, T.J. Selenium accumulation in the floral tissues of two Brassicaceae species and its impact on floral traits and plant performance. Environ. Exp. Bot. 2011, 74, 90–97. [Google Scholar] [CrossRef]
- Saikkonen, K.; Koivunen, S.; Vuorisalo, T.; Mutikainen, P. Interactive effects of pollination and heavy metals on resource allocation in Potentilla anserina L. Ecology 1998, 79, 1620–1629. [Google Scholar] [CrossRef]
- Maruthi Sridhar, B.B.; Diehl, V.S.; Han, X.F.; Monts, L.D.; Su, Y. Anatomical changes due to uptake and accumulation of Zn and Cd in Indian mustard (Brassica juncea). Environ. Exp. Bot. 2005, 54, 131–141. [Google Scholar] [CrossRef]
- Maruthi Sridhar, B.B.; Han, X.F.; Diehl, V.S.; Monts, L.D.; Su, Y. Effects of Zn and Cd accumulation on structural and physiological characteristics of barley plants. Braz. J. Plant Physiol. 2007, 19, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Syed, K.; Tiwari, S.; Sikka, J.; Panwar, K. Impact of air pollution on floral morphology and characteristics of Cassia glauca Lamk. In Indore (India). J. Environ. Res. Dev. 2008, 3, 91–96. [Google Scholar]
- Meindl, G.A.; Bain, J.D.; Ashman, L.T. Edaphic factors and plant–insect interactions: Direct and indirect effects of serpentine soil on florivores and pollinators. Oecologia 2013, 173, 1355–1366. [Google Scholar] [CrossRef]
- Breygina, M.; Matveyeva, N.; Polevova, S.; Meychik, N.; Nikolaeva, Y.; Mamaeva, A.; Yermakov, I. Ni2+ effects on Nicotiana tabacum L. pollen germination and pollen tube growth. BioMetals 2012, 25, 1221–1233. [Google Scholar] [CrossRef]
- Alados, L.; Navarro, T.; Cabezuro, B.; Emlen, M.J.; Freeman, C. Developmental instability in gynodioecious Teucrium lusitanicum. Evol. Ecol. 1998, 12, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Dudić, B.; Rakić, T.; Šinžar-Sekulić, J.; Atanacković, V.; Stevanović, B. Differences of metal concentrations and morpho-anatomical adaptations between obligate and facultative serpentinophytes from Western Serbia. Arch. Biol. Sci. 2007, 59, 341–349. [Google Scholar] [CrossRef]
- Vieira, M.; Mayo, S.J.; Andrade, I.M. Geometric morphometrics of leaves of Anacardium microcarpum Ducke and A. occidentale L. (Anacardiaceae) from the coastal region of Piauí, Brazil. Braz. J. Bot. 2014, 37, 315–327. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Kazakou, E.; Fyllas, N.M.; Dimitrakopoulos, P.G. Species adaptive strategies and leaf economic relationships across serpentine and non-serpentine habitats on Lesbos, Eastern Mediterranean. PLoS ONE 2014, 9, e96034. [Google Scholar] [CrossRef] [Green Version]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, B.B.; Lamont, C.H. Utilizable water in leaves of 8 arid species as derived from pressure-volume curves and chlorophyll fluorescence. Physiol. Plant 2000, 110, 64–71. [Google Scholar] [CrossRef]
- Kruckeberg, R.A. Geology and Plant Life: The Effects of Landforms and Rock Types on Plants; University of Washington Press: Seattle, DC, USA, 2004; pp. 37–56. [Google Scholar]
- Veličković, V.M. Reduced developmental stability in Tilia cordata leaves: Effects of disturbed environment. Period. Biol. 2010, 112, 273–281. [Google Scholar]
- Pollicelli, D.L.P.M.; Idaszkin, Y.; Gonzalez-Jose, R.; Márquez, F. Leaf shape variation as a potential biomarker of soil pollution. Ecotoxicol. Environ. Saf. 2018, 164, 69–74. [Google Scholar] [CrossRef]
- Miljković, D.; Avramov, S.; Vujić, V.; Rubinjoni, L.; Barišić Klisarić, N.; Živković, U.; Tarasjev, A. Lead and nickel accumulation in Iris pumila: Consideration of its usefulness as a potential bioindicator in the natural protected area of Deliblato sands, Serbia. Arch. Biol. Sci. 2014, 66, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Y.; Song, J.; Zhang, R.; Yan, Y.; Wang, Y.; Du, F.K. Geometric morphometric analyses of leaf shapes in two sympatric Chinese oaks: Quercus dentata Thunberg and Quercus aliena Blume (Fagaceae). Ann. For. Sci. 2018, 75, 90. [Google Scholar] [CrossRef] [Green Version]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Hopkins, G.W.; Huner, P.A.N. Introduction to Plant Physiology, 4th ed.; Wiley, John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 127–145. [Google Scholar]
- Stanković, M. Teucrium Species: Biology and Applications; Springer: Cham, Switzerland, 2020. [Google Scholar]
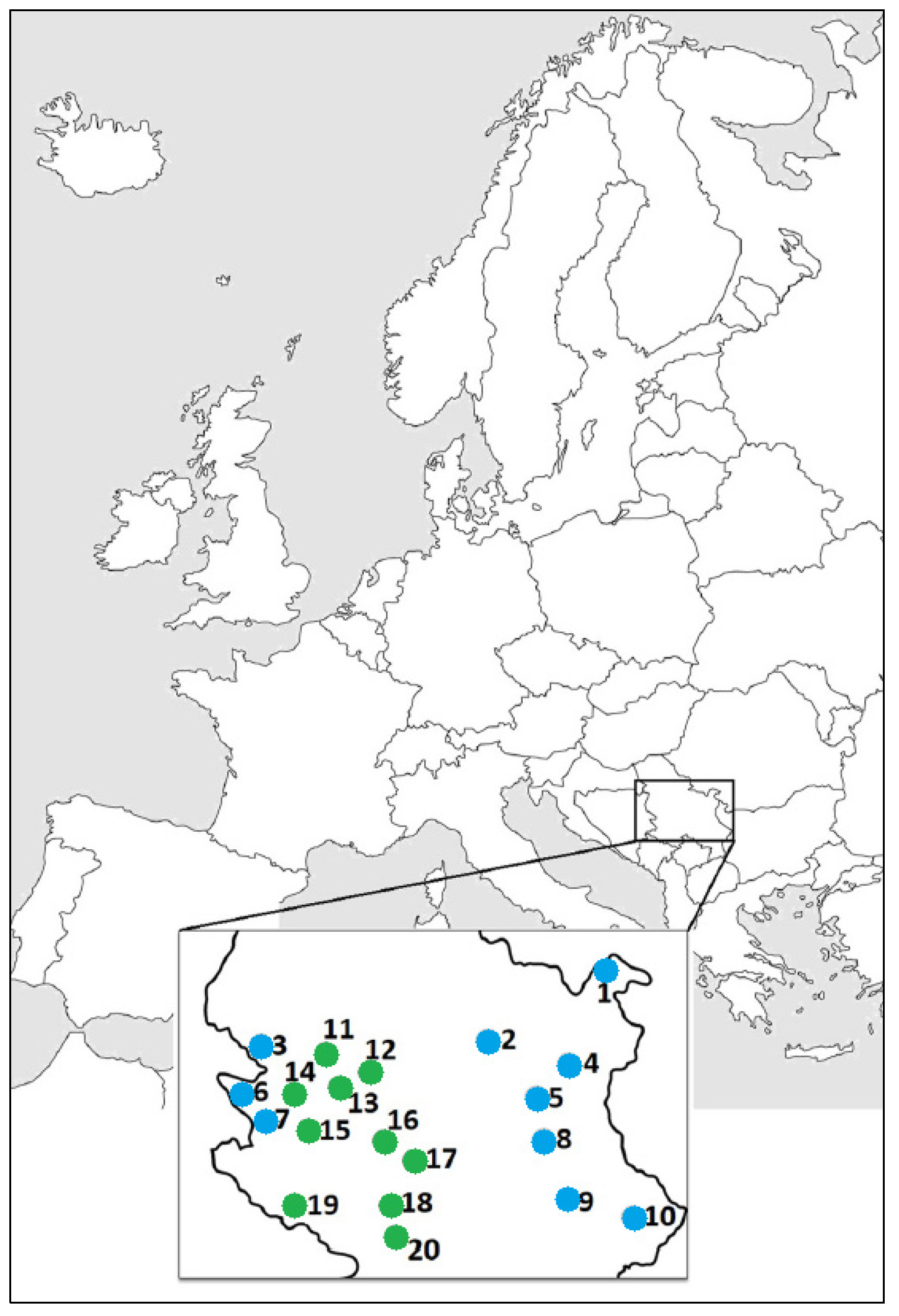



| No. | Locality/Abbreviation | Altitude | Latitude (N) | Longitude (E) |
|---|---|---|---|---|
| 1 * | Miroč Mt., Mali Štrbac/MS | 576 m | 44°38′3.065″ | 22°18′48.022″ |
| 2 | Homolje Mts., Ježevac/JE | 675 m | 44°16′0.396″ | 21°31′1.672″ |
| 3 | Trešnjica Canyon/KT | 476 m | 44°8′40.877″ | 19°31′52.859″ |
| 4 | Veliki Krš Mt./VK | 872 m | 44°9′34.597″ | 22°5′48.285″ |
| 5 | Lazar’s Canyon/KL | 368 m | 44°1′70.940″ | 21°57′27.009″ |
| 6 | Tara Mt., Rastište/RA | 477 m | 43°56′36.132″ | 19°21′30.533″ |
| 7 | Rzav Gorge, Kotroman/KO | 517 m | 43°46′23.747″ | 19°27′51.856″ |
| 8 | Rtanj Mt.—near Šiljak/RT | 1323 m | 43°46′21.516″ | 21°53′39.029″ |
| 9 | Jelašnička Gorge/JK | 373 m | 43°16′43.619″ | 22°4′6.975″ |
| 10 | Vidlič Mt., Basarski kamen/BK | 1014 m | 43°10′32.524″ | 22°40′13.964″ |
| 11 | Maljen Mt., Divčibare/DI | 873 m | 44°5′12.199″ | 20°0′39.43″ |
| 12 | Brđanska gorge/BR | 325 m | 43°59′24.545″ | 20°25′14.454″ |
| 13 | Orovica Mt.—near Kablar Mt./OR | 538 m | 43°54′51.852″ | 20°7′40.082″ |
| 14 | Zlatibor Mt., Kremna/KR | 815 m | 43°50′7.305″ | 19°37′7.45″ |
| 15 | Zlatibor Mt., Smiljanski zakosi/SZ | 1044 m | 43°40′15.066″ | 19°42′19.579″ |
| 16 | Ibar Gorge, Maglič/MA | 305 m | 43°36′46.218″ | 20°33′5.414″ |
| 17 | Goč Mt., Kamenica/KA | 447 m | 43°33′22.878″ | 20°42′20.631″ |
| 18 | Raška, Trnava/TR | 516 m | 43°17′19.665″ | 20°35′53.466″ |
| 19 | Giljeva Mt., Krajinoviće/KJ | 1264 m | 43°11′4.859″ | 19°51′43.409″ |
| 20 | Novi Pazar, Negotinac/NE | 1095 m | 43°6′23.842″ | 20°39′2.104″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatić, N.; Budečević, S.; Stanković, M. Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach. Plants 2023, 12, 2381. https://doi.org/10.3390/plants12122381
Zlatić N, Budečević S, Stanković M. Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach. Plants. 2023; 12(12):2381. https://doi.org/10.3390/plants12122381
Chicago/Turabian StyleZlatić, Nenad, Sanja Budečević, and Milan Stanković. 2023. "Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach" Plants 12, no. 12: 2381. https://doi.org/10.3390/plants12122381
APA StyleZlatić, N., Budečević, S., & Stanković, M. (2023). Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach. Plants, 12(12), 2381. https://doi.org/10.3390/plants12122381








