Arctic Edible Brown Alga Fucus distichus L.: Biochemical Composition, Antiradical Potential and Human Health Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Chemicals
2.3. Carbohydrates Composition
2.4. Analysis of Total Phenolic, Total Flavonoids, and Antiradical Activity
2.5. Element Analysis
2.6. Metal Pollution Index
2.7. Assessments of Human Health Risk
2.8. Statistical Analysis
3. Results and Discussion
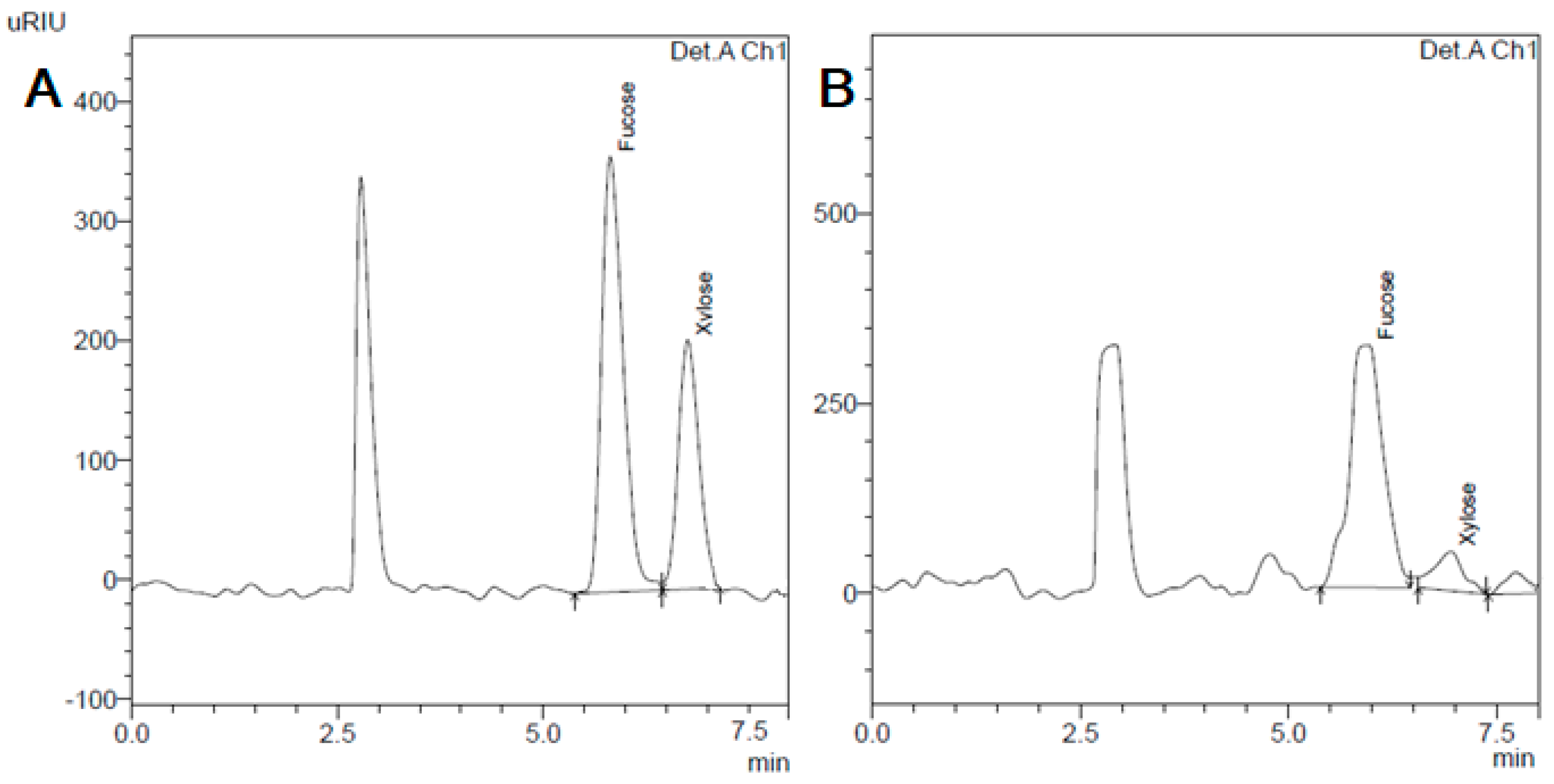
3.1. Carbohydrates Composition
3.2. Polyphenols and Flavonoids Content
3.3. DPPH Radical Scavenging Activity
3.4. Element Contents
3.5. Metal Pollution Index
3.6. Human Health Risk
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2023; Available online: https://www.algaebase.org (accessed on 2 May 2023).
- WoRMS Editorial Board; World Register of Marine Species. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=145544 (accessed on 2 May 2023).
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Jueterbock, A.; Smolina, I.; Coyer, J.A.; Hoarau, G. The fate of the Arctic seaweed Fucus distichus under climate change: An ecological niche modeling approach. Ecol. Evol. 2016, 6, 1712–1724. [Google Scholar] [CrossRef] [Green Version]
- Sukhoveeva, M.V.; Podkorytova, A.V. Commercial Algae and Grasses of the Seas of the Far East: Biology, Distribution, Stocks, Processing Technology; Tinro-Center: Vladivostok, Russia, 2006. [Google Scholar]
- Aimakov, O.A. Biological value of extractives of brown algae. J. Agric. Environ. 2022, 6, 26. [Google Scholar] [CrossRef]
- Aminina, N.M.; Vishnevskaya, T.I.; Guruleva, O.N.; Kovekovdova, L.T. Biological value and parameters of safety of brown seaweeds in the Far-Eastern seas. Bulletin FEB RAS 2007, 6, 123–130. [Google Scholar]
- Catarino, M.D.; Silva, A.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podkorytova, A.V.; Roshchina, A.N.; Evseeva, N.V.; Usov, A.I.; Golovin, G.Y.; Popov, A.M. Brown algae of the orders Laminariales and Fucales from the Sakhalin-Kuril region: Stocks, extraction, use. Trudy VNIRO 2020, 181, 235–256. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.V.; Trang, T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-assisted fucoidan extraction from brown macroalgae Fucus distichus subsp. evanescens and Saccharina latissimi. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Romoth, K.; Nowak, P.; Kempke, D.; Dietrich, A.; Porsche, C.; Schubert, H. Acclimation limits of Fucus evanescens along the salinity gradient of the southwestern Baltic Sea. Bot. Mar. 2019, 62, 31–42. [Google Scholar] [CrossRef]
- Imbs, T.I.; Krasovskaya, N.P.; Ermakova, S.P.; Makarieva, T.N.; Shevchenko, N.M.; Zvyagintseva, T.N. Comparative study of chemical composition and antitumor activity of aqueous-ethanol extracts of brown algae Laminaria cichorioides, Costaria costata, and Fucus evanescens. Russ. J. Mar. Biol. 2009, 35, 164–170. [Google Scholar] [CrossRef]
- Kreissig, K.J.; Hansen, L.T.; Jensen, P.E.; Wegeberg, S.; Geertz-Hansen, O.; Sloth, J.J. Characterisation and chemometric evaluation of 17 elements in ten seaweed species from Greenland. PLoS ONE 2021, 16, e0243672. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Bilan, M.I.; Ustyuzhanina, N.E.; Nifantiev, N.E. Fucoidans of brown algae: Comparison of sulfated polysaccharides from Fucus vesiculosus and Ascophyllum nodosum. Mar. Drugs 2022, 20, 638. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural characterization and in vivo anti-inflammatory activity of fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef]
- Rohwer, K.; Neupane, S.; Bittkau, K.S.; Pérez, M.G.; Dörschmann, P.; Roider, J.; Alban, S.; Klettner, A. Effects of crude Fucus distichus subspecies evanescens fucoidan extract on retinal pigment epithelium cells—Implications for use in age-related macular degeneration. Mar. Drugs 2019, 17, 538. [Google Scholar] [CrossRef] [Green Version]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive properties of marine phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Raven, J.A.; Craigie, J.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Kashutin, A.N.; Egorova, E.V.; Kashutina, I.A.; Rogalyova, N.L. Influence of anthropogenic pollution on macrophyte algae of Avacha Bay (Southeastern Kamchatka). Ekosistemy 2020, 24, 130–141. [Google Scholar] [CrossRef]
- Kumar, M.S.; Sharma, S.A. Toxicological effects of marine seaweeds: A cautious insight for human consumption. Crit. Rev. Food Sci. Nutr. 2020, 61, 500–521. [Google Scholar] [CrossRef]
- Hahn, J.L.; Van Alstyne, K.L.; Gaydos, J.K.; Wallis, L.K.; West, J.E.; Hollenhorst, S.J.; Ylitalo, G.M.; Poppenga, R.H.; Bolton, J.L.; McBride, D.E.; et al. Chemical contaminant levels in edible seaweeds of the Salish Sea and implications for their consumption. PLoS ONE. 2022, 17, e0269269. [Google Scholar] [CrossRef]
- García-Seoane, R.; Fernández, J.A.; Villares, R.; Aboal, J.R. Use of macroalgae to biomonitor pollutants in coastal waters: Optimization of the methodology. Ecol. Indic. 2018, 84, 710–726. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Zakharova, L. Metal concentrations in three species of Fucus L. on the Murmansk coast of the Barents Sea. Polar Sci. 2021, 28, 100646. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Klochkova, N.G. Polysaccharides of algae: 55. Polysaccharide composition of several brown algae from Kamchatka. Russ. J. Bioorg. Chem. 2001, 27, 395–399. [Google Scholar] [CrossRef]
- Dische, Z.; Shettles, L.B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 1948, 175, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.; Daurtseva, A. Effects of air drying and freezing and long-term storage on phytochemical composition of brown seaweeds. J. Appl. Phycol. 2020, 32, 4235–4249. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Obluchinskaya, E.D. Comparative chemical composition of the Barents Sea brown algae. Appl. Biochem. Microbiol. 2008, 44, 305–309. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea antarctica. Dry Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Vargas, N.; Pasten, A.; Rodríguez, K.; Ah-Hen, K.S. Phytochemical components and amino acid profile of brown seaweed Durvillaea antarctica as affected by air drying temperature. J. Food Sci. Technol. 2018, 55, 4792–4801. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Botić, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Čagalj, M.; Skroza, D. Bioactive phenolic metabolites from Adriatic brown algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant capacity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Flores, É.M.D.M.; Barin, J.S.; Paniz, J.N.G.; Medeiros, J.A.; Knapp, G. Microwave-assisted sample combustion: A technique for sample preparation in trace element determination. Anal. Chem. 2004, 76, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Obluchinskaya, E.D.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Pozharitskaya, O.N. The Impact of natural deep eutectic solvents and extraction method on the co-extraction of trace metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef]
- Usero, J.; Morillo, J.; Gracia, I. Heavy metal concentrations in molluscs from the Atlantic coast of southern Spain. Chemosphere 2005, 59, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Norms of Physiological Needs Energy and Nutrients for Various Groups of the Population of the Russian Federation. In Methodical Recommendations; MR 2.3.1.2432−08; Federal Center for Hygiene and Epidemiology of Rospotrebnadzor: Moscow, Russia, 2009.
- EFSA European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals. February 2006. Available online: www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 2 May 2023).
- WHO. World Health Organization Evaluation of Certain Food Additives and Contaminants: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series No. 959; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. World Health Organization Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, 2010; WHO Technical Report Series No. 960; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- JECFA. Safety Evaluation of Certain Food Additives and Contaminants. 67th Joint FAO/WHO Expert Committee on Food Additives, WHO, Rome, Italy. 2006. Available online: http://www.who.int/ipcs/food/jecfa/summaries/summary67.pdf (accessed on 2 May 2023).
- USEPA, Human Health Risk Assessment. Environmental Protection Agency, USA. 2020. Available online: https://www.epa.gov/risk/human-health-risk-assessment (accessed on 25 April 2023).
- Siddique, M.A.M.; Hossain, M.S.; Islam, M.M.; Rahman, M.; Kibria, G. Heavy metals and metalloids in edible seaweeds of Saint Martin’s Island, Bay of Bengal, and their potential health risks. Mar. Pollut. Bull. 2022, 181, 113866. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives. Rome, 28–29 October 2021; Food Safety and Quality Series No. 13; FAO; WHO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 2003, 294, 1–13. [Google Scholar] [CrossRef]
- Kiseleva, M.I.; Imbs, T.I.; Avilov, S.A.; Bakunina, I.Y. The effects of polyphenolic impurities in fucoidan samples from the brown alga Fucus distichus subsp. evanescens (C. Agardh) HT Powell, 1957 on the embryogenesis in the sea urchin Strongylocentrotus intermedius (A. Agassiz, 1864) and on the embryotoxic action of cucumarioside. Russ. J. Mar. Biol. 2021, 47, 290–299. [Google Scholar] [CrossRef]
- Dörschmann, P.; Klettner, A. Fucoidans as potential therapeutics for age-related macular degeneration—Current evidence from in vitro research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Neupane, S.; Ptak, S.H.; Römer, R.; Xiong, J.; Ohmes, J.; Seekamp, A.; Frette, X.; Alban, S.; et al. Influence of fucoidan extracts from different fucus species on adult stem cells and molecular mediators in in vitro models for bone formation and vascularization. Mar. Drugs 2021, 19, 194. [Google Scholar] [CrossRef]
- Ohmes, J.; Mikkelsen, M.D.; Nguyen, T.T.; Tran, V.H.N.; Meier, S.; Nielsen, M.S.; Ding, M.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Depolymerization of fucoidan with endo-fucoidanase changes bioactivity in processes relevant for bone regeneration. Carbohydr. Polym. 2022, 286, 119286. [Google Scholar] [CrossRef]
- Dörschmann, P.; Apitz, S.; Hellige, I.; Neupane, S.; Alban, S.; Kopplin, G.; Ptak, S.; Fretté, X.; Roider, J.; Zille, M.; et al. Evaluation of the effects of fucoidans from Fucus species and Laminaria hyperborea against oxidative stress and iron-dependent cell death. Mar. Drugs 2021, 19, 557. [Google Scholar] [CrossRef] [PubMed]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Shahrestani, R.G. Innovative strategies for engineering mannitol production. Trends Food Sci. Technol. 2009, 20, 263–270. [Google Scholar] [CrossRef]
- Usov, A.I.; Klochkova, N.G. Brown–algae from Kamchatka as a source of mannitol. Bioorganicheskaya Khimiya 1994, 20, 1234–1241. [Google Scholar]
- Klindukh, M.P.; Obluchinskaya, E.D.; Matishov, G.G. Seasonal changes in the mannitol and proline contents of the brown alga Fucus vesiculosus L. on the Murman coast of the Barents Sea. Dokl. Biol. Sci. 2021, 441, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Mandel, K.G.; Daggy, D.A.; Brodie, H.I. Jacoby, Review article: Alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment. Pharmacol. Ther. 2000, 14, 669–690. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Valentão, P.; Trindade, P.; Gomes, D.; Guedes de Pinho, P.; Mouga, T.; Andrade, P.B. Codium tomentosum and Plocamium cartilagineum: Chemistry and antioxidant potential. Food Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Francisco, J.; Horta, A.; Pedrosa, R.; Afonso, C.; Cardoso, C.; Bandarra, N.M.; Gil, M.M. Bioaccessibility of antioxidants and fatty acids from Fucus spiralis. Foods 2020, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Krause-Jensen, D.; Duarte, C.M. Expansion of vegetated coastal ecosystems in the future Arctic. Front. Mar. Sci. 2014, 1, 77. [Google Scholar] [CrossRef] [Green Version]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Shikov, A.N. Biochemical composition, antiradical potential and human health risk of the Arctic edible brown seaweed Fucus spiralis L. J. Appl. Phycol. 2023, 35, 365–380. [Google Scholar] [CrossRef]
- Podkorytova, A.V.; Roshchina, A.N. Marine brown algae—Perspective source of BAS for medical, pharmaceutical and food use. Trudy VNIRO 2021, 186, 156–172. [Google Scholar]
- Chernova, E.N. The biogeochemical background and trace metal accumulation by brown algae of the genus Fucus in coastal waters of the Sea of Japan, the Sea of Okhotsk, and the White Sea. Russ. J. Mar. Biol. 2016, 42, 87–96. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, L.; Wu, M.; Yu, H.; Shang, T.; Zhang, T.; Zhao, M. Total and inorganic arsenic contents in seaweeds: Absorption, accumulation, transformation and toxicity. Aquaculture 2018, 497, 49–55. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Güven, K.C.; Akyüz, K.; Yurdun, T. Selectivity of heavy metal binding by algal polysaccharides. Toxicol. Environ. Chem. 1995, 47, 65–70. [Google Scholar] [CrossRef]
- SFT. Classification of Environmental Quality in Fjords and Coastal Waters; Guidance Document 97:03; TA-1467/1997; Norwegian State Pollution Control Authority: Oslo, Norway, 1997; p. 36. (In Norwegian) [Google Scholar]
- European Union Commission Regulation (EC) No 629/2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. OJEU 2008, L 173, 6–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2008.173.01.0006.01.ENG (accessed on 2 May 2023).
- SanPiN 2.3.2.1078-01; Food Raw Materials and Food Products Hygiene Requirements for Safety and Nutritional Food. Sanitary-Epidemiological Rules and Standards: Moscow, Russia, 2002.
- SCF: Scientific Committee for Food of the European Communities. 2011. Available online: http://ec.europa.eu/food/fs/sc/scf/index_en.html (accessed on 2 May 2023).
- Wang, W.; Xie, Z.; Lin, Y.; Zhang, D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J. Epidemiol. Community Health 2014, 68, 176–184. [Google Scholar] [CrossRef]
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef] [Green Version]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C. 2012. Arsenic, Metals, Fibres, and Dusts. Available online: https://publications.iarc.fr/120 (accessed on 2 May 2023).
- Sartal, C.G.; Alonso, M.C.B.; Barrera, P.B. Arsenic in seaweed: Presence, bioavailability and speciation. In Seafood Science: Advances in Chemistry Technology and Applications; Kim, S.-K., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 276–351. [Google Scholar]
- Chien, L.C.; Hung, T.C.; Choang, K.Y.; Yeh, C.Y.; Meng, P.J.; Shieh, M.J.; Ha, B.C. Daily intake of TBT, Cu, Zn, Cd and as for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef]
- U.S. EPA. Concepts, Methods, and Data Sources For Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report, 2008); EPA/600/R-06/013F; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=190187 (accessed on 2 May 2023).
- Rahman, M.S.; Hossain, M.B.; Babu, S.; Rahman, M.; Ahmed, A.S.S.; Jolly, Y.N.; Choudhury, T.R.; Begum, B.A.; Kabir, J.; Akter, S. Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Mar. Pollut. Bull. 2019, 141, 137–146. [Google Scholar] [CrossRef] [PubMed]
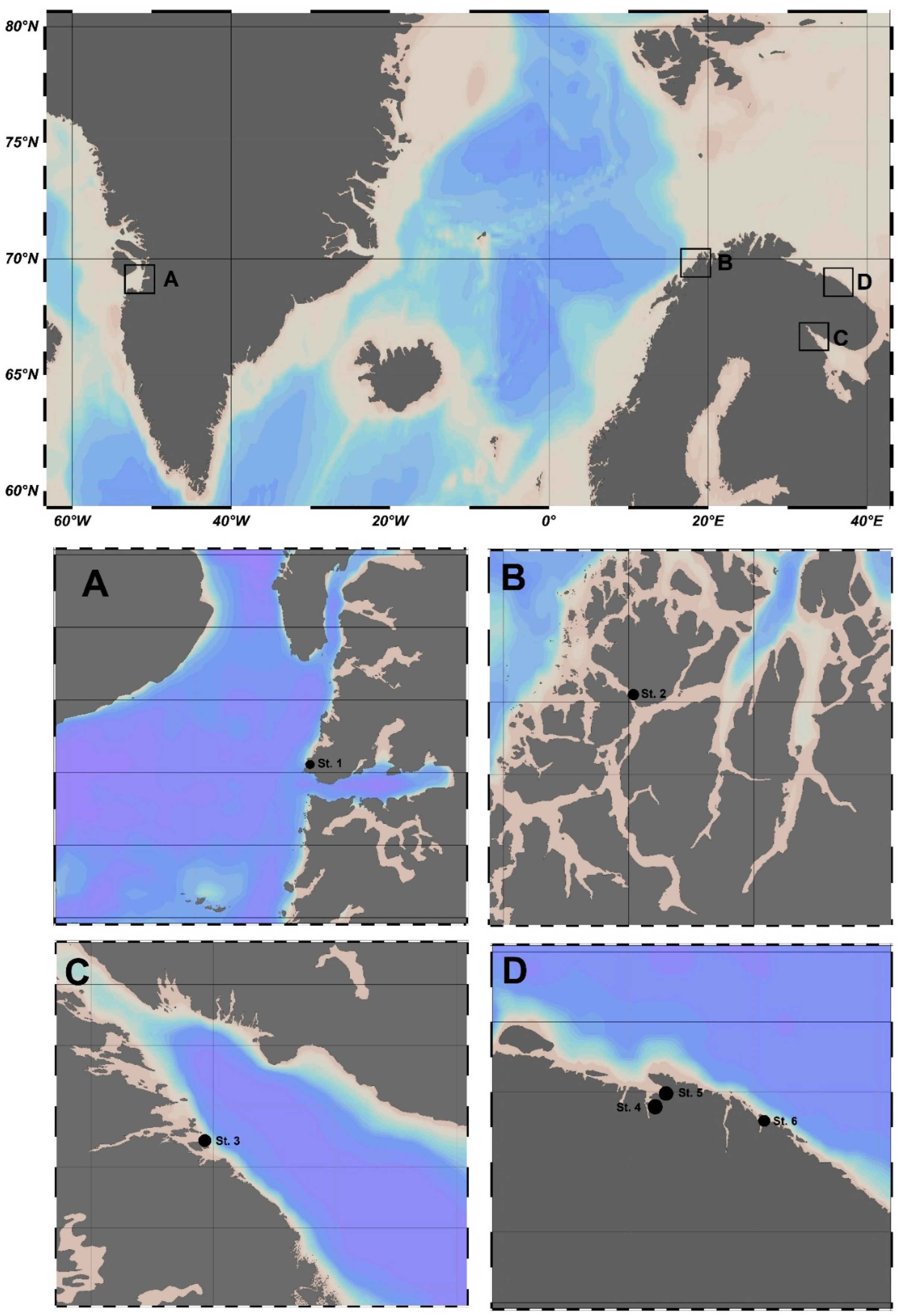




| Sea Area | Sampling Site | Coordinates | Station on Figure 1 | Mean Water Temperature, °C | Range of Salinity, ‰ |
|---|---|---|---|---|---|
| Baffin Sea | Disko Bay | 69.219858 N 51.111819 W | St. 1 | 11.0 | 25.8–26.2 |
| Norwegian Sea | Ringvassøya Island | 69.815097 N 19.027894 E | St. 2 | 10.6 | 33.7–34.3 |
| White Sea | Pezhostrov Island | 66.273315 N 33.934406 E | St. 3 | 17.2 | 22.1–22.3 |
| Barents Sea | Teriberskaya Bay (Korabelnaya Bay) | 69.173088 N 35.168468 E | St. 4 | 11.2 | 14.7–15.5 |
| Barents Sea | Teriberskaya Bay (Zavalishina Bay) | 69.184068 N 35.259487 E | St. 5 | 9.1 | 19.9–20.7 |
| Barents Sea | Zelenetskaya Bay | 69.117150 N 36.070790 E | St. 6 | 10.3 | 31.0–32.0 |
| Sea, Station | Fucose, mg/g DW | Xylose, mg/g DW | Fucose/Xylose Ratio |
|---|---|---|---|
| BfS, St. 1 | 43.5 ± 1.5 | 5.4 ± 0.3 | 8.05 ± 0.57 |
| NS, St. 2 | 58.1 ± 1.8 | 8.9 ± 0.8 | 6.53 ± 0.41 |
| WS, St. 3 | 59.9 ± 1.8 | 9.8 ± 0.6 | 6.12 ± 0.31 |
| BS, St. 4 | 75.2 ± 1.3 | 13.6 ± 1.0 | 5.57 ± 0.35 |
| BS, St. 5 | 62.1 ± 0.5 | 14.2 ± 0.4 | 4.38 ± 0.13 |
| BS, St. 6 | 90.3 ± 0.4 | 17.5 ± 0.4 | 5.16 ± 0.10 |
| Element | LOQ | Mean ± sd | Range (min–max) | St. 1 | St. 2 | St. 3 | St. 4 | St. 5 | St. 6 |
|---|---|---|---|---|---|---|---|---|---|
| Al | 1.6 | 68.9 ± 37.5 | 33.3–126.2 | 58.7 ± 4.8 | 38.0 ± 2.8 | 103.4 ± 4.0 | 33.3 ± 1.2 | 53.7 ± 4.3 | 126.2 ± 18.9 |
| As | 6.3 | 32.4 ± 15.4 | 19.2–58.5 | 19.2 ± 3.3 | 27.2 ± 1.7 | 21.6 ± 0.7 | 58.5 ± 0.7 | 43.4 ± 2.9 | 24.7 ± 0.9 |
| Ba | 0.016 | 13.0 ±7.7 | 7.3–28.0 | 10.4 ± 0.2 | 7.3 ± 1.6 | 28.0 ± 0.2 | 14.0 ± 0.6 | 10.4 ± 0.2 | 7.9 ± 0.2 |
| Ca | 1.9 | 14,774 ± 5565 | 9490–25,476 | 15,029 ± 177 | 13,816 ± 509 | 12,037 ± 268 | 12,795 ± 255 | 25,476 ± 580 | 9490 ± 17 |
| Cd | 0.23 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Co | 0.12 | 1.8 ± 2.3 | 0.6–6.5 | 0.96 ± 0.02 | 0.71 ± 0.06 | 6.46 ± 0.02 | 1.03 ± 0.05 | 1.31 ± 0.01 | 0.62 ± 0.01 |
| Cr | 0.13 | <LOQ | < LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Cu | 0.37 | 1.7 ± 1.1 | 0.6–3.2 | 3.23 ± 0.12 | 1.46 ± 0.09 | 2.86 ± 0.18 | 1.11 ± 0.24 | 0.97 ± 0.04 | 0.60 ± 0.01 |
| Fe | 0.098 | 214 ± 190 | 74–562 | 73.8 ± 10.7 | 112 ± 21 | 562 ± 15 | 97.1 ± 6.6 | 132 ± 19 | 310 ± 10 |
| Mg | 1.7 | 9304 ± 700 | 8400–10,222 | 10222 ± 124 | 9510 ± 31 | 9904 ± 171 | 9056 ± 20 | 8731 ± 67 | 8400 ± 28 |
| Mn | 0.058 | 45.8 ± 27.8 | 15.3–90.1 | 59.7 ± 1.7 | 15.3 ± 1.4 | 90.1 ± 4.4 | 32.7 ± 0.6 | 54.3 ± 0.3 | 22.8 ± 0.2 |
| Ni | 0.3 | 10.2 ± 1.1 | <LOQ–10.9 | <LOQ | <LOQ | <LOQ | 9.5 ± 0.09 | 10.9 ± 0.04 | <LOQ |
| Pb | 4.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Rb | 0.55 | 36.4 ± 12.8 | 22.5–54.1 | 54.1 ± 1.5 | 35.8 ± 0.7 | 49.8 ± 1.2 | 28.9 ± 0.9 | 22.5 ± 0.6 | 27.3 ± 3.0 |
| Sr | 0.026 | 875 ± 130 | 704–1051 | 828 ± 35 | 833 ± 21 | 1009 ± 30 | 1051 ± 32 | 828 ± 8 | 704 ± 9 |
| Zn | 0.17 | 33.8 ± 8.1 | 26.9–44.6 | 27.3 ± 1.0 | 27.7 ± 1.7 | 33.1 ± 0.5 | 42.9 ± 0.9 | 44.6 ±0.3 | 26.9 ±1.0 |
| Element | Sampling Site with a Maximum Concentration | Mean–Max Concentration (mg/kg) | Single Dose for 3.3 g Consumption (mg/Day) | Daily Dose for 12.5 g Consumption (mg/Day) | Daily Dose from Risk Estimators | Daily Nutritional Requirements |
|---|---|---|---|---|---|---|
| Al | BS, St. 6 | 68.9–126.2 | 0.23–0.42 | 0.86–1.58 | 70 1 | 10 5 |
| As (total) | BS, St. 4 | 32.4–58.5 | 0.11–0.19 | 0.41–0.73 | 0.15 1 (inorganic) | 5.0 6 |
| Ba | WS, St. 3 | 13.0–28.0 | 0.04–0.09 | 0.16–0.35 | 200 | 0.75 5 |
| Ca | BS, St. 5 | 14,774–25,476 | 49–84 | 185–318 | 2500 2 | 1000 3 |
| Co | WS, St. 3 | 1.8–6.5 | 0.006–0.021 | 0.023–0.081 | 30 5 | 10 5 |
| Cu | BfS, St. 1 | 1.7–3.2 | 0.006–0.011 | 0.021–0.040 | 5 2,5 | 0.9 4/1.0 5 |
| Fe | WS, St. 3 | 214–562 | 0.71–1.86 | 2.68–7.03 | 45 5 | 10 3,5 |
| Mg | BfS, St. 1 | 9304–10,222 | 31–34 | 116–128 | 800 5 | 400 5 |
| Mn | WS, St. 3 | 45.8–90.1 | 0.15–0.30 | 0.57–1.13 | 11 5 | 2.7 3/2.0 5 |
| Ni | BS, St. 5 | 3.6–11.0 | 0.012–0.036 | 0.045–0.14 | 20 5 | 0.2 5 |
| Rb | BfS, St. 1 | 36.4–56.1 | 0.12–0.18 | 0.45–0.68 | 200 | 2.2 5 |
| Sr | BS, St. 4 | 875–1051 | 2.89–3.47 | 10.9–13.1 | 11 5 | 1.9 5 |
| Zn | BS, St. 5 | 33.8–44.6 | 0.11–0.15 | 0.42–0.56 | 25 2/40 5 | 12 3,5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obluchinskaya, E.D.; Pozharitskaya, O.N.; Gorshenina, E.V.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Shikov, A.N. Arctic Edible Brown Alga Fucus distichus L.: Biochemical Composition, Antiradical Potential and Human Health Risk. Plants 2023, 12, 2380. https://doi.org/10.3390/plants12122380
Obluchinskaya ED, Pozharitskaya ON, Gorshenina EV, Zakharov DV, Flisyuk EV, Terninko II, Generalova YE, Shikov AN. Arctic Edible Brown Alga Fucus distichus L.: Biochemical Composition, Antiradical Potential and Human Health Risk. Plants. 2023; 12(12):2380. https://doi.org/10.3390/plants12122380
Chicago/Turabian StyleObluchinskaya, Ekaterina D., Olga N. Pozharitskaya, Elena V. Gorshenina, Denis V. Zakharov, Elena V. Flisyuk, Inna I. Terninko, Yuliya E. Generalova, and Alexander N. Shikov. 2023. "Arctic Edible Brown Alga Fucus distichus L.: Biochemical Composition, Antiradical Potential and Human Health Risk" Plants 12, no. 12: 2380. https://doi.org/10.3390/plants12122380
APA StyleObluchinskaya, E. D., Pozharitskaya, O. N., Gorshenina, E. V., Zakharov, D. V., Flisyuk, E. V., Terninko, I. I., Generalova, Y. E., & Shikov, A. N. (2023). Arctic Edible Brown Alga Fucus distichus L.: Biochemical Composition, Antiradical Potential and Human Health Risk. Plants, 12(12), 2380. https://doi.org/10.3390/plants12122380






