The Protective Role of Cranberries and Blueberries in Oral Cancer
Abstract
1. Background
2. Materials and Methods
3. Introduction
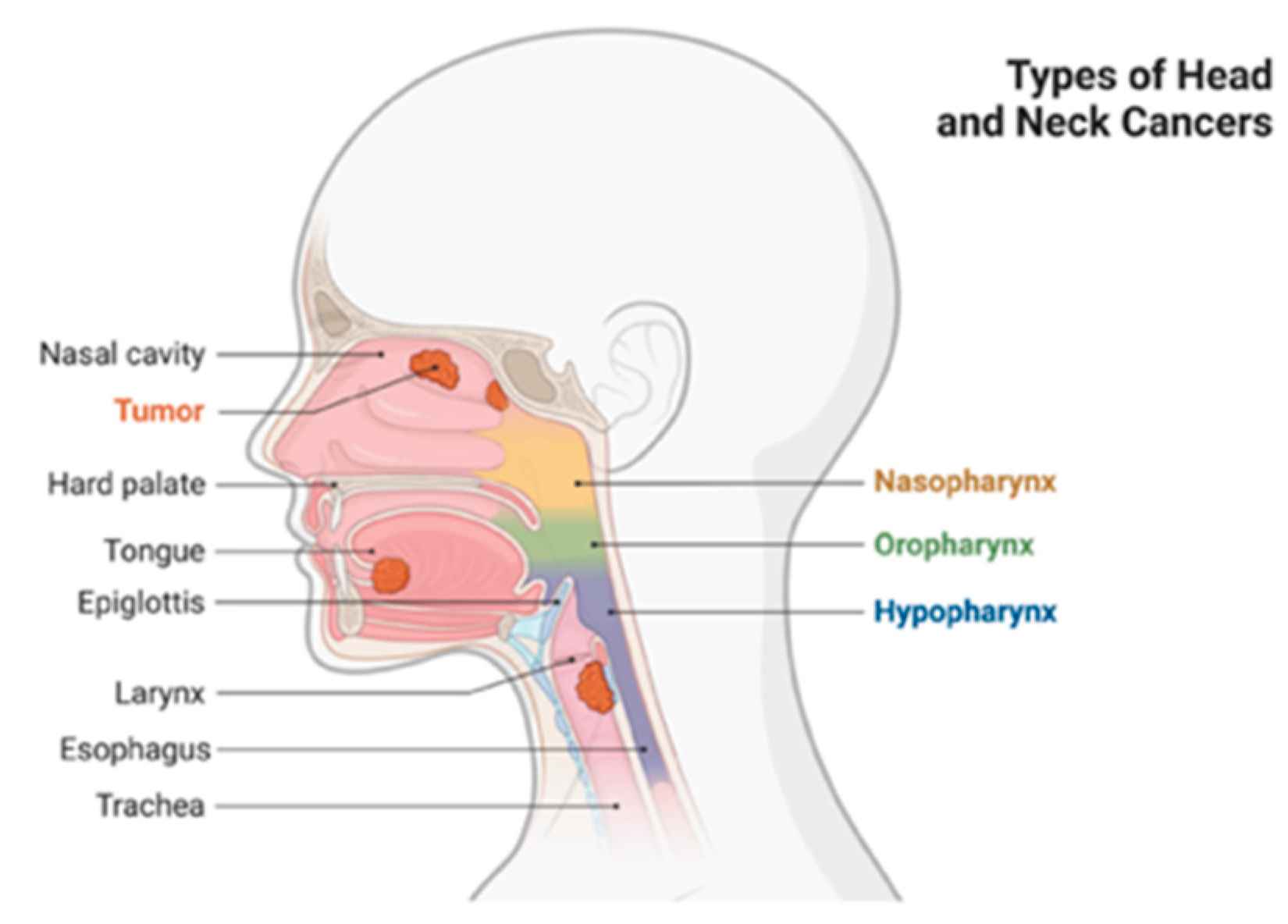
3.1. Head and Neck Cancer
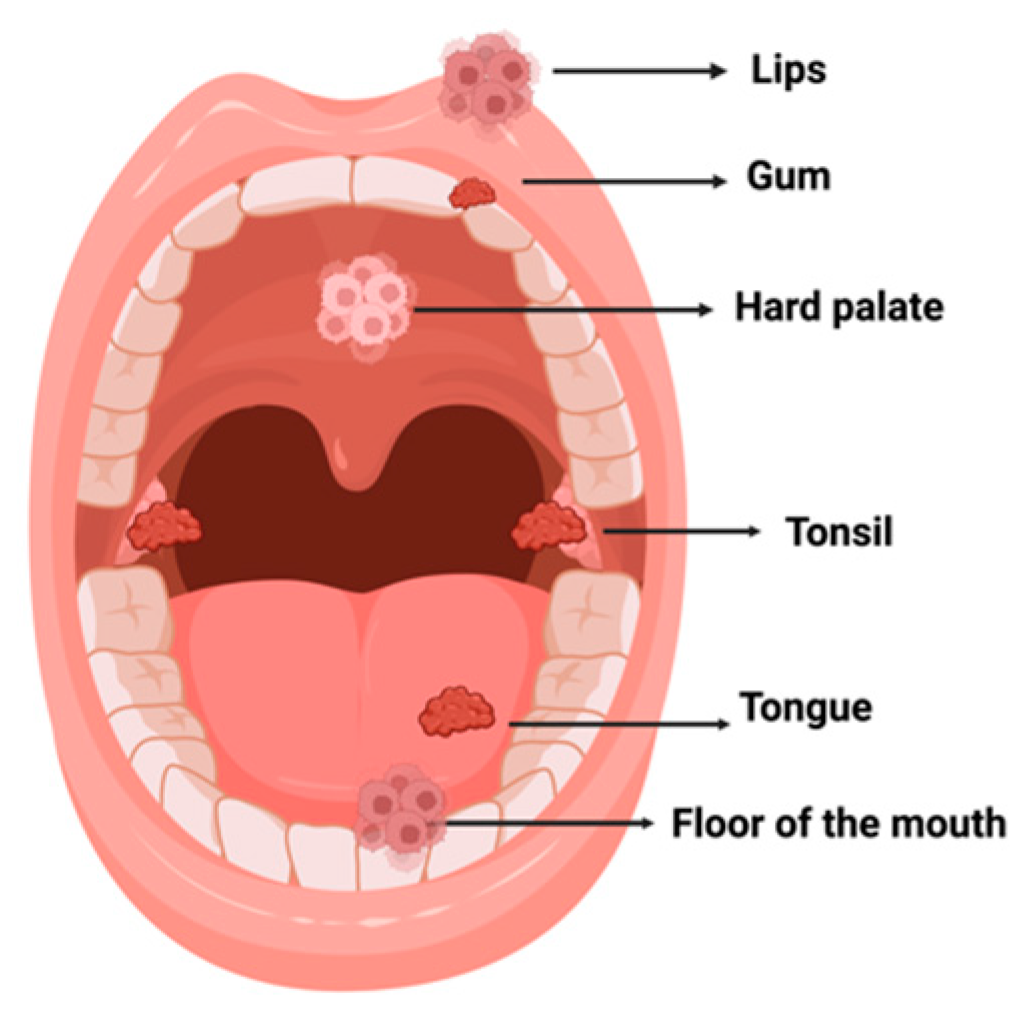
3.2. Oral Cancer
3.3. Epidemiology
3.4. Etiology and Risk Factors
3.4.1. Tobacco
3.4.2. Alcohol
3.4.3. Viral Infections
Human Papillomavirus
Epstein–Barr Virus
3.4.4. Oral Health
3.4.5. Diet and Nutrition
3.5. Premalignant Lesions
3.5.1. Leucoplakia
3.5.2. Erythroplasia
3.5.3. Lichen Planus
3.6. Histological Aspects of Oral Cancer
3.7. Carcinogenesis of Oral Cancer
3.7.1. Oncogenes and Tumor Suppressor Genes
3.7.2. Epigenetic Alterations
4. Treatment
Prevention and Antioxidants
5. Phytochemical Compounds
5.1. Family Ericaceae
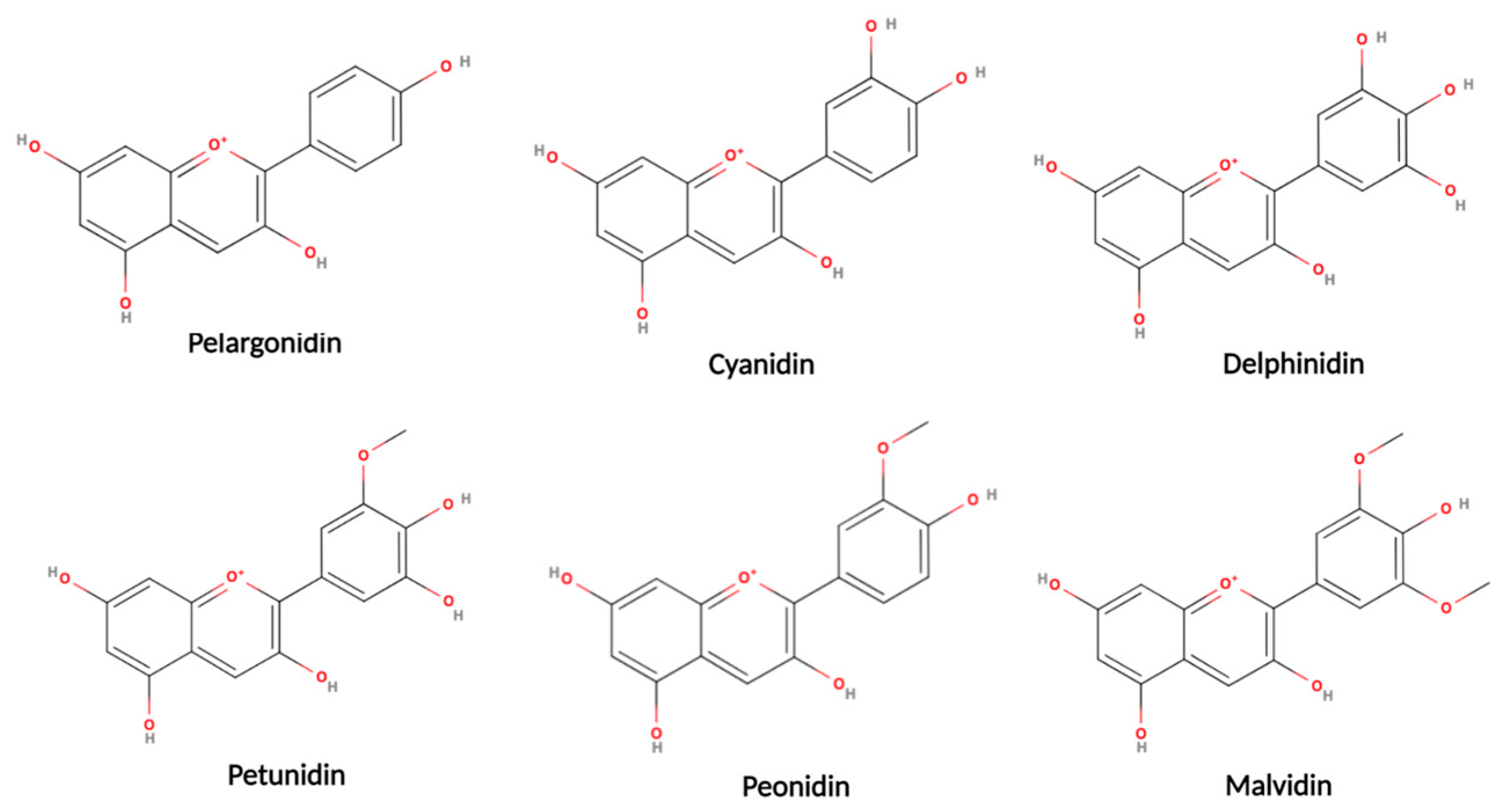
5.2. Anthocyanidins
5.3. Anthocyanins
5.4. Flavan-3-ols and Proanthocyanidins
5.5. Phenolic Acids
5.6. Triterpenoids
6. Berries and Cancer
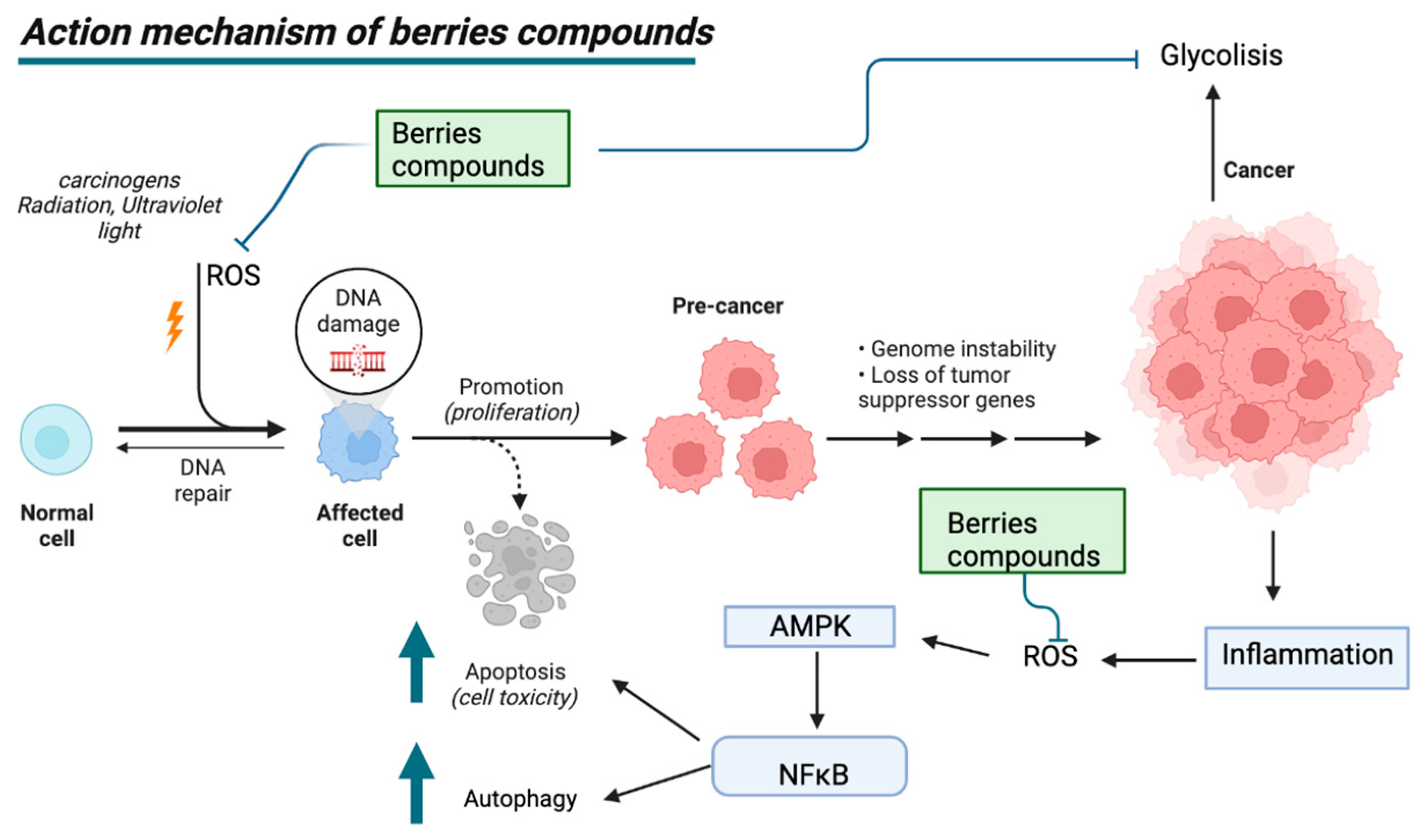
6.1. Mechanism of Action of Berries in Cancer
6.2. Mechanism of Action of Berry-Derived Phytochemicals in Oral Cancer
6.3. Consumption of Cranberries and Blueberries in Protection against Risk Factors for Oral Cancer
6.3.1. Cranberries and Blueberries and Their Protection against Tobacco-Induced Oral Cancer
6.3.2. Cranberries and Blueberries and Their Protection against Alcohol-Induced Oral Cancer
6.4. Consumption of Cranberries and Blueberries Protects against the Effects of Bacteria and Poor Oral Hygiene
6.5. Cranberries and Blueberries and Their Protection against Oral Cancer Induced by Viral Infections
| Cranberry Type | Type of Study Conducted (In Vitro/In Vivo/Clinical Study) | Evidence against Oral Cancer | Reference |
|---|---|---|---|
| Vaccinium corymbosum L. (blueberries) | In vitro | The methanolic extract of blueberries inhibits cell proliferation in the oral cancer line KB. | [128,188] |
| In vivo/In vitro | Dietary administration of blueberry produces significant effects on the SCC131 cancer cell line through the inhibition of TGF-β and NF-κB, as well as act against invasion and angiogenesis at doses higher than 200 mg/kg. | [20,167] | |
| In vitro | The phytochemical pterostilbene present in blueberries induces apoptotic cell death and, through autophagy in cisplatin-resistant human oral cancer cells (CAR cells), which is related to the AKT pathway, are mediated by the suppression of MDR1. | [100] | |
| Vaccinium macrocarpon A. (lingonberries) | In vitro | The methanol extract of the cranberries inhibits cellular proliferation in the line of oral cancer KB. | [128,188] |
| In vivo/In vitro | The extract composed of proanthocyanidins (C-PAC) derived from cranberries inhibits the growth of resistant and acid-sensitive esophageal adenocarcinoma (EAC) cells, both in cell lines and xenotransplant mice, inducing caspase-independent cell death, mainly by the autophagic pathway. | [131] | |
| In vitro | The hydroethanolic extract of cranberries produces an antiproliferative effect on the caspase-independent KB cell line, mainly by the autophagic route. | [190] | |
| In vitro | Cranberry extract produces an inhibitory effect on the proliferation of OSCC lines cAL27 and SCC25 at an optimal concentration of 40 μg/mL, producing the upstream regulation of caspases 2 and 8, and effects cell adhesion, cell morphology, and the cell cycle. | [191] |
7. Limitations and Perspectives of the Consumption of Cranberries and Blueberries for Protection against Oral Cancer
Limitations
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous carcinoma cell |
| HPV | Human papillomavirus |
| EBV | Epstein–Barr virus |
| NNN | N′-nitrosonornicotine |
| NNK | 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone |
| ROS | Reactive oxygen species |
| BMI | Body mass index |
| EGFR/c-erb | Epidermal growth factor receptor |
| TNF | tumor necrosis factor |
| MMP | matrix metalloproteinase |
| CAL-27 | oral cancer cell line |
| C3G | cyanidin-3-glucoside |
| PACs | Proanthocyanidins |
| COX-2 | Cyclooxygenase 2 |
| MMP-9 | Matrix metalloproteinase-9 |
| VEGF-A | Vascular endothelial growth factor A |
| NF-κB | Nuclear transcription factor |
| NOSs | Nitric oxide synthases |
| P3G | pelargonidin-3-glucoside |
| SCC131 | oral cancer cell line 131 |
| PI3K | phosphoinositide-3-kinase |
| AKT | Protein kinase B |
| mTOR | the mammalian target of rapamycin |
| MAP | Mitogen-activated protein kinase |
| PKA | cAMP-dependent protein kinaseB |
| AMPK | the AMP-activated protein kinase |
| TGF-β | Transforming growth factor-β |
| STAT-3 | Signal transducer and activator of transcription 3 |
| JAK/STAT | The Janus-Kinase signal transducer and the transcription activation pathway |
References
- Hammer, W. What Is Cancer? Available online: https://www.aacr.org/patients-caregivers/about-cancer/what-is-cancer/ (accessed on 8 July 2022).
- Head and Neck Cancers—NCI. Available online: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet (accessed on 12 July 2022).
- Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020—IARC. Available online: https://www.iarc.who.int/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/ (accessed on 12 July 2022).
- Module 5: What Is Cancer? Available online: https://www.tn.gov/health/health-program-areas/tcr/cancer-reporting-facility-training/module5.html (accessed on 8 July 2022).
- Research Areas—Global Health—NCI. Available online: https://www.cancer.gov/research/areas/global-health (accessed on 8 July 2022).
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 8 July 2022).
- Conway, D.I.; Purkayastha, M.; Chestnutt, I.G. The changing epidemiology of oral cancer: Definitions, trends, and risk factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef]
- Westra, W.H.; Lewis, J.S. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Oropharynx. Head Neck Pathol. 2017, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Mouth and Oral Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/mouth-and-oral-cancer-statistics/ (accessed on 7 August 2022).
- Li, C.C.; Shen, Z.; Bavarian, R.; Yang, F.; Bhattacharya, A. Oral Cancer: Genetics and the Role of Precision Medicine. Dent. Clin. N. Am. 2018, 62, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Oral Cavity & Oropharyngeal Cancer Key Statistics 2021. Available online: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html (accessed on 7 August 2022).
- Watters, C.; Brar, S.; Pepper, T. Oral Mucosa Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pavia, M.; Pileggi, C.; Nobile, C.G.A.; Angelillo, I.F. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2006, 83, 1126–1134. [Google Scholar] [CrossRef]
- Cancer Treatment|SEER Training. Available online: https://training.seer.cancer.gov/treatment/ (accessed on 7 August 2022).
- Porro, C.; La Torre, M.E.; Tartaglia, N.; Benameur, T.; Santini, M.; Ambrosi, A.; Messina, G.; Cibelli, G.; Fiorelli, A.; Polito, R.; et al. The Potential Role of Nutrition in Lung Cancer Establishment and Progression. Life 2022, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, R.; Singh, K.; Sharma, V.; Thakur, M. Physicochemical Properties and Antioxidant Potential of Curry Leaf Chutney Powder: A Traditional Functional Food Adjunct. In Bioactive Components: A Sustainable System for Good Health and Well-Being; Springer: Berlin/Heidelberg, Germany, 2022; pp. 595–609. [Google Scholar]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Brewczyński, A.; Jabłońska, B.; Kentnowski, M.; Mrowiec, S.; Składowski, K.; Rutkowski, T. The Association between Carotenoids and Head and Neck Cancer Risk. Nutrients 2021, 14, 88. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.S.; Casto, B.C. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef]
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Ellington, T.D. Trends in Incidence of Cancers of the Oral Cavity and Pharynx—United States 2007–2016. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Rajagopal, V.; Thankam, F.G. Chapter 9—Oral tissue regeneration: Current status and future perspectives. In Regenerated Organs; Sharma, C.P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 169–187. [Google Scholar]
- Cancer Today. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 7 August 2022).
- Ali, K. Oral Cancer—The Fight Must Go on against All Odds…. Evid.-Based Dent. 2022, 23, 4–5. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Xie, L.; Shang, Z.-J. Burden of Oral Cancer on the 10 Most Populous Countries from 1990 to 2019: Estimates from the Global Burden of Disease Study 2019. Int. J. Environ. Res. Public Health 2022, 19, 875. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef]
- McKeon, M.G.; Gallant, J.-N.; Kim, Y.J.; Das, S.R. It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer. Cancers 2022, 14, 3120. [Google Scholar] [CrossRef] [PubMed]
- Irani, S. New Insights into Oral Cancer-Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- Cancer Net. Oral and Oropharyngeal Cancer—Risk Factors and Prevention. 2012. Available online: https://www.cancer.net/cancer-types/oral-and-oropharyngeal-cancer/risk-factors-and-prevention (accessed on 7 August 2022).
- Kavarthapu, A.; Gurumoorthy, K. Linking chronic periodontitis and oral cancer: A review. Oral Oncol. 2021, 121, 105375. [Google Scholar] [CrossRef]
- Tabaco. Available online: https://www.who.int/es/news-room/fact-sheets/detail/tobacco (accessed on 27 October 2022).
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, M.; Nargis, N.; Tursan d’Espaignet, E. Global economic cost of smoking-attributable diseases. Tob. Control 2018, 27, 58–64. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol. Res. Health 2006, 29, 193–198. [Google Scholar] [PubMed]
- Pérez-Ortuño, R.; Martínez-Sánchez, J.M.; Fu, M.; Fernández, E.; Pascual, J.A. Evaluation of tobacco specific nitrosamines exposure by quantification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human hair of non-smokers. Sci. Rep. 2016, 6, 25043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, M.; Villalta, P.W.; Lindgren, B.R.; Upadhyaya, P.; Lao, Y.; Hecht, S.S. Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2009, 22, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ronai, Z.A.; Gradia, S.; Peterson, L.A.; Hecht, S.S. G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis 1993, 14, 2419–2422. [Google Scholar] [CrossRef]
- Balbo, S.; Johnson, C.S.; Kovi, R.C.; James-Yi, S.A.; O’Sullivan, M.G.; Wang, M.; Le, C.T.; Khariwala, S.S.; Upadhyaya, P.; Hecht, S.S. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis 2014, 35, 2798–2806. [Google Scholar] [CrossRef]
- Watters, C.; Brar, S.; Pepper, T. Oral Mucosa Cancer. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Niaz, K.; Maqbool, F.; Khan, F.; Bahadar, H.; Ismail Hassan, F.; Abdollahi, M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol. Health 2017, 39, e2017009. [Google Scholar] [CrossRef]
- Rossini, A.R.; Hashimoto, C.L.; Iriya, K.; Zerbini, C.; Baba, E.R.; Moraes-Filho, J.P. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis. Esophagus 2008, 21, 316–321. [Google Scholar] [CrossRef]
- Feng, L.; Wang, L. Effects of alcohol on the morphological and structural changes in oral mucosa. Pak. J. Med. Sci. 2013, 29, 1046–1049. [Google Scholar] [CrossRef]
- González-López, L.L.; Morales-González, Á.; Sosa-Gómez, A.; Madrigal-Santillán, E.O.; Anguiano-Robledo, L.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Delgado-Olivares, L.; Valadez-Vega, C.; Esquivel-Chirino, C.; et al. Damage to Oral Mucosae Induced by Weekend Alcohol Consumption: The Role of Gender and Alcohol Concentration. Appl. Sci. 2022, 12, 3464. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Maeng, J.-S. Food, nutrition, physical activity and cancer: A global perspective and analysis of the research. Bull. Food Technol. 2012, 25, 2–26. [Google Scholar]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Piemonte, E.D.; Lazos, J.P.; Gilligan, G.M.; Panico, R.L.; Werner, L.C.; Yang, Y.H.; Warnakulasuriya, S. Chronic mechanical irritation enhances the effect of tobacco and alcohol on the risk of oral squamous cell carcinoma: A case-control study in Argentina. Clin. Oral Investig. 2022, 26, 6317–6326. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, A.; Agliardi, E.; Peri, A.; Marchioni, R.; Abati, S. Oral microbiome and mucosal trauma as risk factors for oral cancer: Beyond alcohol and tobacco. A literature review. J. Biol. Regul. Homeost. Agents 2020, 34, 11–18. [Google Scholar]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Jain, M. Assesment of Correlation of Herpes Simplex Virus-1 with Oral Cancer and Precancer—A Comparative Study. J. Clin. Diagn. Res. 2016, 10, Zc14–Zc17. [Google Scholar] [CrossRef]
- Dylawerska, A.; Barczak, W.; Wegner, A.; Golusinski, W.; Suchorska, W.M. Association of DNA repair genes polymorphisms and mutations with increased risk of head and neck cancer: A review. Med. Oncol. 2017, 34, 197. [Google Scholar] [CrossRef]
- Giraldi, L.; Collatuzzo, G.; Hashim, D.; Franceschi, S.; Herrero, R.; Chen, C.; Schwartz, S.M.; Smith, E.; Kelsey, K.; McClean, M.; et al. Infection with Human Papilloma Virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol. 2021, 75, 102020. [Google Scholar] [CrossRef]
- Kim, Y.; Joo, Y.H.; Kim, M.S.; Lee, Y.S. Prevalence of high-risk human papillomavirus and its genotype distribution in head and neck squamous cell carcinomas. J. Pathol. Transl. Med. 2020, 54, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.A.C.; Vilar, L.G.; Oliveira, N.R.; Lima, P.O.; Pinheiro, M.B.; Domingueti, C.P.; Pereira, M.C. Human papillomavirus infection and oral squamous cell carcinoma—A systematic review. Braz. J. Otorhinolaryngol. 2021, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Montes-Mojarro, I.A.; Fend, F.; Quintanilla-Martinez, L. Epstein-Barr Virus-Associated T and NK-Cell Lymphoproliferative Diseases. Front. Pediatr. 2019, 7, 71. [Google Scholar] [CrossRef]
- Núñez-Acurio, D.; Bravo, D.; Aguayo, F. Epstein-Barr Virus-Oral Bacterial Link in the Development of Oral Squamous Cell Carcinoma. Pathogens 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef]
- Singhvi, H.R.; Malik, A.; Chaturvedi, P. The Role of Chronic Mucosal Trauma in Oral Cancer: A Review of Literature. Indian J. Med. Paediatr. Oncol. 2017, 38, 44–50. [Google Scholar] [CrossRef]
- Aceves Argemí, R.; González Navarro, B.; Ochoa García-Seisdedos, P.; Estrugo Devesa, A.; López-López, J. Mouthwash With Alcohol and Oral Carcinogenesis: Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101407. [Google Scholar] [CrossRef]
- Carr, E.; Aslam-Pervez, B. Does the use of alcohol mouthwash increase the risk of developing oral cancer? Evid. Based Dent. 2022, 23, 28–29. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Jafari, A.; Najafi, S.; Moradi, F.; Kharazifard, M.; Khami, M. Delay in the diagnosis and treatment of oral cancer. J. Dent. (Shiraz) 2013, 14, 146–150. [Google Scholar] [PubMed]
- Maymone, M.B.C.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.D.; Maymone, A.C.; Vashi, N.A. Premalignant and malignant oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wils, L.J.; Poell, J.B.; Brink, A.; Evren, I.; Brouns, E.R.; De Visscher, J.G.A.M.; Bloemena, E.; Brakenhoff, R.H. Elucidating the Genetic Landscape of Oral Leukoplakia to Predict Malignant Transformation. Clin. Cancer Res. 2023, 29, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Udaltsova, N.; Engels, E.A.; Katzel, J.A.; Yanik, E.L.; Katki, H.A.; Lingen, M.W.; Silverberg, M.J. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2020, 112, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Parakh, M.K.; Ulaganambi, S.; Ashifa, N.; Premkumar, R.; Jain, A.L. Oral potentially malignant disorders: Clinical diagnosis and current screening aids: A narrative review. Eur. J. Cancer Prev. 2020, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Sreenivasan, P.; Öhman, J.; Wallström, M.; Braz-Silva, P.H.; Giglio, D.; Kjeller, G.; Hasséus, B. Potentially Malignant Oral Disorders and Cancer Transformation. Anticancer Res. 2018, 38, 3223–3229. [Google Scholar] [CrossRef]
- Woo, S.B. Oral Epithelial Dysplasia and Premalignancy. Head Neck Pathol. 2019, 13, 423–439. [Google Scholar] [CrossRef]
- Gupta, S.; Jawanda, M.K. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J. Dermatol. 2015, 60, 222–229. [Google Scholar] [CrossRef]
- Dionne, K.R.; Warnakulasuriya, S.; Zain, R.B.; Cheong, S.C. Potentially malignant disorders of the oral cavity: Current practice and future directions in the clinic and laboratory. Int. J. Cancer 2015, 136, 503–515. [Google Scholar] [CrossRef]
- Nosratzehi, T. Oral Lichen Planus: An Overview of Potential Risk Factors, Biomarkers and Treatments. Asian Pac. J. Cancer Prev. 2018, 19, 1161–1167. [Google Scholar] [CrossRef]
- Nagaraju, K.; Prasad, S.; Ashok, L. Diagnostic efficiency of toluidine blue with Lugol’s iodine in oral premalignant and malignant lesions. Indian J. Dent. Res. 2010, 21, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bittar, R.F.; Ferraro, H.P.; Ribas, M.H.; Lehn, C.N. Predictive factors of occult neck metastasis in patients with oral squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2016, 82, 543–547. [Google Scholar] [CrossRef]
- Lin, N.C.; Hsien, S.I.; Hsu, J.T.; Chen, M.Y.C. Impact on patients with oral squamous cell carcinoma in different anatomical subsites: A single-center study in Taiwan. Sci. Rep. 2021, 11, 15446. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Georgaki, M.; Theofilou, V.I.; Pettas, E.; Stoufi, E.; Younis, R.H.; Kolokotronis, A.; Sauk, J.J.; Nikitakis, N.G. Understanding the complex pathogenesis of oral cancer: A comprehensive review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 566–579. [Google Scholar] [CrossRef]
- Gasche, J.A.; Goel, A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol. 2012, 8, 1407–1425. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Kirita, T. Hallmarks of Cancer-Related Newly Prognostic Factors of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef]
- Usman, S.; Jamal, A.; Teh, M.T.; Waseem, A. Major Molecular Signaling Pathways in Oral Cancer Associated With Therapeutic Resistance. Front. Oral Health 2020, 1, 603160. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Sonea, L.; Zimta, A.A.; Cojocneanu-Petric, R.; Tonchev, K.; Mehterov, N.; Diudea, D.; Buduru, S.; Berindan-Neagoe, I. A Looking-Glass of Non-coding RNAs in oral cancer. Int. J. Mol. Sci. 2017, 18, 2620. [Google Scholar] [CrossRef]
- Gabusi, A.; Gissi, D.B.; Grillini, S.; Stefanini, M.; Tarsitano, A.; Marchetti, C.; Foschini, M.P.; Montebugnoli, L.; Morandi, L. Shared epigenetic alterations between oral cancer and periodontitis: A preliminary study. Oral Dis. 2022, 29, 2052–2060. [Google Scholar] [CrossRef]
- Flausino, C.S.; Daniel, F.I.; Modolo, F. DNA methylation in oral squamous cell carcinoma: From its role in carcinogenesis to potential inhibitor drugs. Crit. Rev. Oncol. Hematol. 2021, 164, 103399. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Nguyen, Q.T. Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 2014, 66, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer--surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; de Wit, J.G.; Voskuil, F.J.; Witjes, M.J.H. Improving oral cavity cancer diagnosis and treatment with fluorescence molecular imaging. Oral Dis. 2021, 27, 21–26. [Google Scholar] [CrossRef]
- González-Moles, M.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef]
- Ali Khani, J.; Fatemeh, J. Oral Cancer: Epidemiology, Prevention, Early Detection, and Treatment. In Oral Cancer; Gokul, S., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 1. [Google Scholar] [CrossRef]
- May, S.; Parry, C.; Parry, L. Berry chemoprevention: Do berries decrease the window of opportunity for tumorigenesis. Food Front. 2020, 1, 260–275. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tseng, Y.-H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Juan, Y.N.; Tsao, J.W.; Chiu, H.Y.; Yang, J.S. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. Int. J. Oncol. 2018, 52, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Qiu, Y.; Wang, J.; Chen, Q.; Wang, S.; Wang, Y.; Li, Y.; Weng, Y.; Qian, J.; Chen, F.; et al. Association Between Dietary Fatty Acid Pattern and Risk of Oral Cancer. Front. Nutr. 2022, 9, 864098. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.J. Anthocyanins and heart health. Ann. Dell’istituto Super. Sanita 2007, 43, 369–374. [Google Scholar]
- Greenwald, P.; Milner, J.A.; Clifford, C.K. Creating a New Paradigm in Nutrition Research within the National Cancer Institute. J. Nutr. 2000, 130, 3103–3105. [Google Scholar] [CrossRef]
- Wei, H.; Li, H.; Wan, S.P.; Zeng, Q.T.; Cheng, L.X.; Jiang, L.L.; Peng, Y.D. Cardioprotective Effects of Malvidin against Isoproterenol-Induced Myocardial Infarction in Rats: A Mechanistic Study. Med. Sci. Monit. 2017, 23, 2007–2016. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.K.; Tang, Y.; Hwang, J.W.; Natarajan, S.B.; Kim, W.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant and anticancer effects of extracts from fermented Haliotis discus hannai with Cordyceps militaris mycelia. Food Sci. Biotechnol. 2016, 25, 1775–1782. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Quantitation and Distribution of Simple and Acylated Anthocyanins and Other Phenolics in Blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in Chronic Diseases: The Power of Purple. Nutrients 2022, 14, 2161. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Oliveira, H.; Correia, P.; Pereira, A.R.; Araújo, P.; Mateus, N.; de Freitas, V.; Oliveira, J.; Fernandes, I. Exploring the Applications of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020, 21, 7464. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Milner, J.A. Foods and health promotion: The case for cranberry. Crit. Rev. Food Sci. Nutr. 2002, 42, 265–266. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Fan, M.J.; Wang, I.C.; Hsiao, Y.T.; Lin, H.Y.; Tang, N.Y.; Hung, T.C.; Quan, C.; Lien, J.C.; Chung, J.G. Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing MMPs and NF-κB expressions in human oral cancer CAL 27 cells. Nutr. Cancer 2015, 67, 327–338. [Google Scholar] [CrossRef]
- Huang, Y.W.; Chuang, C.Y.; Hsieh, Y.S.; Chen, P.N.; Yang, S.F.; Shih Hsuan, L.; Chen, Y.Y.; Lin, C.W.; Chang, Y.C. Rubus idaeus extract suppresses migration and invasion of human oral cancer by inhibiting MMP-2 through modulation of the Erk1/2 signaling pathway. Environ. Toxicol. 2017, 32, 1037–1046. [Google Scholar] [CrossRef]
- Qi, C.; Li, S.; Jia, Y.; Wang, L. Blueberry anthocyanins induce G2/M cell cycle arrest and apoptosis of oral cancer KB cells through down-regulation methylation of p53. Yi Chuan 2014, 36, 566–573. [Google Scholar] [PubMed]
- Chen, J.L.; Lai, C.Y.; Ying, T.H.; Lin, C.W.; Wang, P.H.; Yu, F.J.; Liu, C.J.; Hsieh, Y.H. Modulating the ERK1/2-MMP1 Axis through Corosolic Acid Inhibits Metastasis of Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 8641. [Google Scholar] [CrossRef] [PubMed]
- Maarten, J.M.C.; James, W.B. The Number of Known Plants Species in the World and Its Annual Increase; Magnolia Press: Waco, TX, USA, 2016; Volume 261, p. 201. [Google Scholar] [CrossRef]
- Kresty, L.A.; Weh, K.M.; Zeyzus-Johns, B.; Perez, L.N.; Howell, A.B. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget 2015, 6, 33438–33455. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef]
- Howell, A.B.; Vorsa, N.; Der Marderosian, A.; Foo, L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Engl. J. Med. 1998, 339, 1085–1086. [Google Scholar] [CrossRef]
- Ferguson, P.J.; Kurowska, E.M.; Freeman, D.J.; Chambers, A.F.; Koropatnick, J. In vivo inhibition of growth of human tumor lines by flavonoid fractions from cranberry extract. Nutr. Cancer 2006, 56, 86–94. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef]
- de Moura, C.F.; Noguti, J.; de Jesus, G.P.; Ribeiro, F.A.; Garcia, F.A.; Gollucke, A.P.; Aguiar, O., Jr.; Ribeiro, D.A. Polyphenols as a chemopreventive agent in oral carcinogenesis: Putative mechanisms of action using in-vitro and in-vivo test systems. Eur. J. Cancer Prev. 2013, 22, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shu, Y.; Chen, Y.; Ge, Z.; Zhang, C.; Cao, J.; Li, X.; Wang, Y.; Sun, C. Evaluation of Antioxidant Capacity and Gut Microbiota Modulatory Effects of Different Kinds of Berries. Antioxidants 2022, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Chi, L.; Bian, X.; Gao, B.; Ru, H.; Lu, K. A Black Raspberry-Rich Diet Protects From Dextran Sulfate Sodium-Induced Intestinal Inflammation and Host Metabolic Perturbation in Association With Increased Aryl Hydrocarbon Receptor Ligands in the Gut Microbiota of Mice. Front. Nutr. 2022, 9, 842298. [Google Scholar] [CrossRef]
- Singh, V.; Singh, R.; Kujur, P.K.; Singh, R.P. Combination of Resveratrol and Quercetin Causes Cell Growth Inhibition, DNA Damage, Cell Cycle Arrest, and Apoptosis in Oral Cancer Cells. Assay Drug Dev. Technol. 2020, 18, 226–238. [Google Scholar] [CrossRef]
- Wang, P.; Shang, R.; Ma, Y.; Wang, D.; Zhao, W.; Chen, F.; Hu, X.; Zhao, X. Targeting microbiota-host interactions with resveratrol on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, C.Y.; Lu, C.C.; Tsai, F.J.; Hsu, Y.M.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Yang, J.S.; Wang, C.C. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: A key role of AMPK and Akt/mTOR signaling. Int. J. Oncol. 2017, 50, 873–882. [Google Scholar] [CrossRef]
- Shi, P.; Li, B.; Chen, H.; Song, C.; Meng, J.; Xi, Z.; Zhang, Z. Iron Supply Affects Anthocyanin Content and Related Gene Expression in Berries of Vitis vinifera cv. Cabernet Sauvignon. Molecules 2017, 22, 283. [Google Scholar] [CrossRef]
- Weh, K.M.; Zhang, Y.; Howard, C.L.; Howell, A.B.; Clarke, J.L.; Kresty, L.A. Cranberry Polyphenols in Esophageal Cancer Inhibition: New Insights. Nutrients 2022, 14, 969. [Google Scholar] [CrossRef]
- Oghumu, S.; Casto, B.C.; Ahn-Jarvis, J.; Weghorst, L.C.; Maloney, J.; Geuy, P.; Horvath, K.Z.; Bollinger, C.E.; Warner, B.M.; Summersgill, K.F.; et al. Inhibition of Pro-inflammatory and Anti-apoptotic Biomarkers during Experimental Oral Cancer Chemoprevention by Dietary Black Raspberries. Front. Immunol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Knobloch, T.J.; Ryan, N.M.; Bruschweiler-Li, L.; Wang, C.; Bernier, M.C.; Somogyi, A.; Yan, P.S.; Cooperstone, J.L.; Mo, X.; Brüschweiler, R.P.; et al. Metabolic Regulation of Glycolysis and AMP Activated Protein Kinase Pathways during Black Raspberry-Mediated Oral Cancer Chemoprevention. Metabolites 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4β-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; Wiley-Vch: Hoboken, NJ, USA, 2006. [Google Scholar]
- D’Souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mangalath, U.; Aslam, S.A.; Abdul Khadar, A.H.; Francis, P.G.; Mikacha, M.S.; Kalathingal, J.H. Recent trends in prevention of oral cancer. J. Int. Soc. Prev. Community Dent. 2014, 4, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, Y.-J.; Shin, Y. Assessment of Physicochemical Quality, Antioxidant Content and Activity, and Inhibition of Cholinesterase between Unripe and Ripe Blueberry Fruit. Foods 2020, 9, 690. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen Radical Absorbing Capacity, Anthocyanin and Phenolic Content of Highbush Blueberries (Vaccinium corymbosum L.) during Ripening and Storage. J. Am. Soc. Hortic. Sci. Jashs 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Liu, Y.; Fillmore, S.A.E. Human anthocyanin bioavailability: Effect of intake duration and dosing. Food Funct. 2017, 8, 4563–4569. [Google Scholar] [CrossRef]
- Poei-Langston, M.S.; Wrolstad, R.E. Color Degradation in an Ascorbic Acid-Anthocyanin-Flavanol Model System. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Piacente, S.; Pizza, C.; Montoro, P. Metabolomics of Healthy Berry Fruits. Curr. Med. Chem. 2018, 25, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, A.; Warnakulasuriya, S. Oral health consequences of smokeless tobacco use. Indian J. Med. Res. 2018, 148, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef]
- G, M.S.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2021, 12, 809308. [Google Scholar] [CrossRef]
- Baba, A.B.; Kowshik, J.; Krishnaraj, J.; Sophia, J.; Dixit, M.; Nagini, S. Blueberry inhibits invasion and angiogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-κB signaling pathways. J. Nutr. Biochem. 2016, 35, 37–47. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway. Antioxidants 2020, 9, 871. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Chen, K.M.; Zhang, S.M.; Sun, Y.W.; Amin, S.; Stoner, G.; Guttenplan, J.B. Carcinogenesis of the Oral Cavity: Environmental Causes and Potential Prevention by Black Raspberry. Chem. Res. Toxicol. 2017, 30, 126–144. [Google Scholar] [CrossRef]
- Ogden, G.R. Alcohol and oral cancer. Alcohol 2005, 35, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Red Wine May Reduce Oral Cancer Risks. J. Am. Dent. Assoc. 2000, 131, 729–731. [CrossRef] [PubMed]
- Bhandari, A.; Bhatta, N. Tobacco and its Relationship with Oral Health. JNMA J. Nepal. Med. Assoc. 2021, 59, 1204–1206. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lodi, G.; Iriti, M. Ethanol versus Phytochemicals in Wine: Oral Cancer Risk in a Light Drinking Perspective. Int. J. Mol. Sci. 2015, 16, 17029–17047. [Google Scholar] [CrossRef]
- Stornetta, A.; Guidolin, V.; Balbo, S. Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers 2018, 10, 20. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2021, 13, e0314421. [Google Scholar] [CrossRef]
- Dutreix, L.; Bernard, C.; Juin, C.; Imbert, C.; Girardot, M. Do raspberry extracts and fractions have antifungal or anti-adherent potential against Candida spp.? Int. J. Antimicrob. Agents 2018, 52, 947–953. [Google Scholar] [CrossRef]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Girardot, M.; Guerineau, A.; Boudesocque, L.; Costa, D.; Bazinet, L.; Enguehard-Gueiffier, C.; Imbert, C. Promising results of cranberry in the prevention of oral Candida biofilms. Pathog. Dis. 2014, 70, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics (Sao Paulo) 2012, 67, 661–668. [Google Scholar] [CrossRef]
- La, V.D.; Howell, A.B.; Grenier, D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob. Agents Chemother. 2010, 54, 1778–1784. [Google Scholar] [CrossRef]
- Vitkov, L.; Singh, J.; Schauer, C.; Minnich, B.; Krunić, J.; Oberthaler, H.; Gamsjaeger, S.; Herrmann, M.; Knopf, J.; Hannig, M. Breaking the Gingival Barrier in Periodontitis. Int. J. Mol. Sci. 2023, 24, 4544. [Google Scholar] [CrossRef]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef]
- Chaitanya, N.C.; Allam, N.S.; Gandhi Babu, D.B.; Waghray, S.; Badam, R.K.; Lavanya, R. Systematic meta-analysis on association of human papilloma virus and oral cancer. J. Cancer Res. Ther. 2016, 12, 969–974. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Khuayjarernpanishk, T.; Sookying, S.; Duangjai, A.; Saokaew, S.; Sanbua, A.; Bunteong, O.; Rungruangsri, N.; Suepsai, W.; Sodsai, P.; Soylaiad, J.; et al. Anticancer Activities of Polygonum odoratum Lour.: A Systematic Review. Front. Pharmacol. 2022, 13, 875016. [Google Scholar] [CrossRef]
- Ankola, A.V.; Kumar, V.; Thakur, S.; Singhal, R.; Smitha, T.; Sankeshwari, R. Anticancer and antiproliferative efficacy of a standardized extract of Vaccinium macrocarpon on the highly differentiating oral cancer KB cell line athwart the cytotoxicity evaluation of the same on the normal fibroblast L929 cell line. J. Oral Maxillofac. Pathol. 2020, 24, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S.; Kingsley, K. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid. Based Complement. Altern. Med. 2011, 2011, 467691. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. (Phila) 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
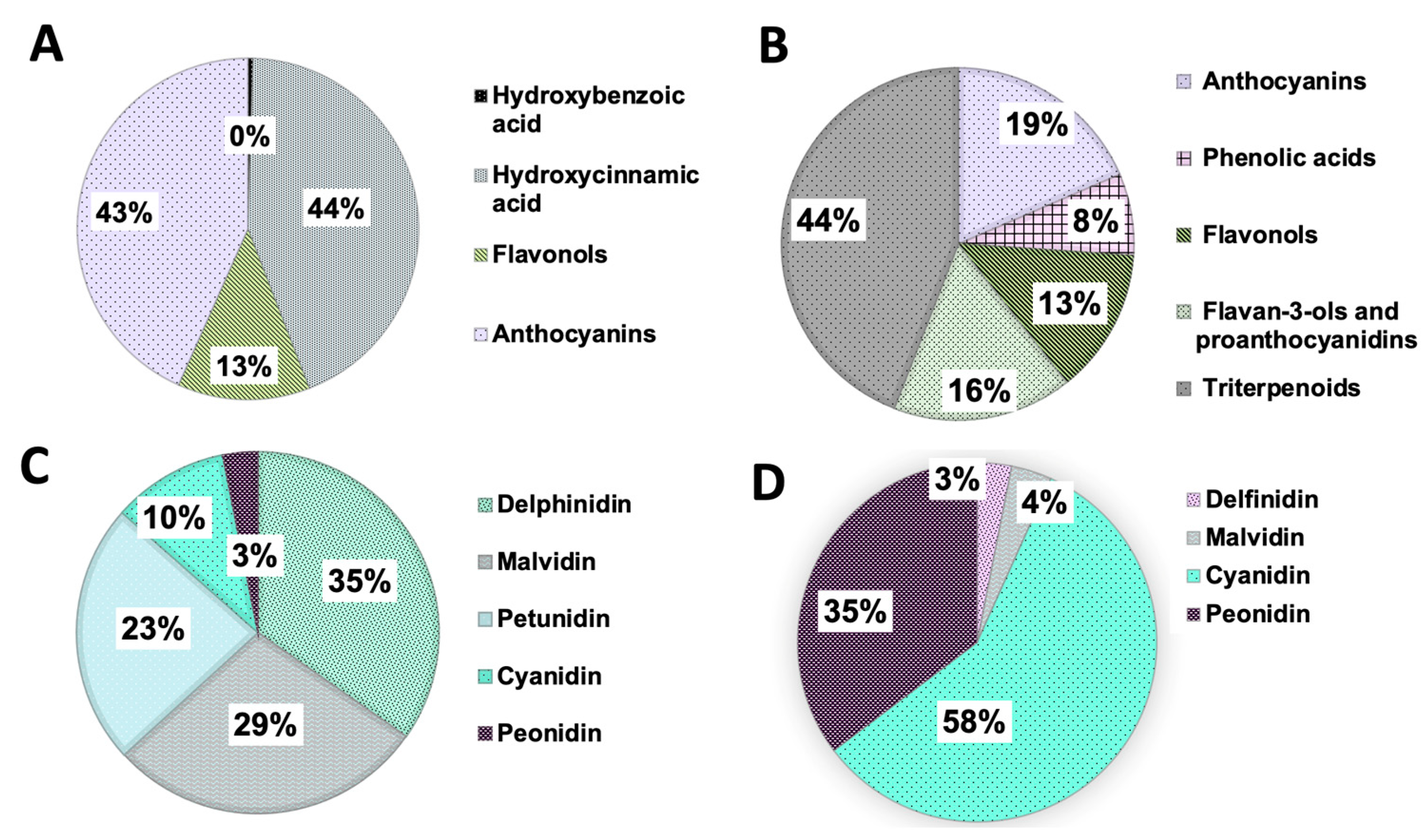
| Secondary Metabolite | Average Concentration in the Extract * | Reference: |
|---|---|---|
| Vaccinium corymbosum L. (blueberries) | ||
| Phenolic acids | ||
| Hydroxybenzoic acid | 1.5 mg/kg fw | [132] |
| Hydroxycinnamic acid | 135 mg/kg fw | [132] |
| Flavonoids | [132] | |
| Flavonols | 38.7 mg/kg fw | [132] |
| Anthocyanins | 134 mg/kg fw | [132] |
| Vaccinium macrocarpon A. (lingonberries) | ||
| Anthocyanins | 695–1716 mg/100 g dm | [99] |
| Phenolic acids | 327–649 mg/100 g dm | [99] |
| Flavonols | 643–1088 mg/100 g dm | [99] |
| Flavan-3-ols and proanthocyanidins | 860–1283 mg/100 g dm | [99] |
| Triterpenoids | 2528–3201.5 mg/kg dm | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquivel-Chirino, C.; Bolaños-Carrillo, M.A.; Carmona-Ruiz, D.; Lopéz-Macay, A.; Hernández-Sánchez, F.; Montés-Sánchez, D.; Escuadra-Landeros, M.; Gaitán-Cepeda, L.A.; Maldonado-Frías, S.; Yáñez-Ocampo, B.R.; et al. The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants 2023, 12, 2330. https://doi.org/10.3390/plants12122330
Esquivel-Chirino C, Bolaños-Carrillo MA, Carmona-Ruiz D, Lopéz-Macay A, Hernández-Sánchez F, Montés-Sánchez D, Escuadra-Landeros M, Gaitán-Cepeda LA, Maldonado-Frías S, Yáñez-Ocampo BR, et al. The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants. 2023; 12(12):2330. https://doi.org/10.3390/plants12122330
Chicago/Turabian StyleEsquivel-Chirino, César, Mario Augusto Bolaños-Carrillo, Daniela Carmona-Ruiz, Ambar Lopéz-Macay, Fernando Hernández-Sánchez, Delina Montés-Sánchez, Montserrat Escuadra-Landeros, Luis Alberto Gaitán-Cepeda, Silvia Maldonado-Frías, Beatriz Raquel Yáñez-Ocampo, and et al. 2023. "The Protective Role of Cranberries and Blueberries in Oral Cancer" Plants 12, no. 12: 2330. https://doi.org/10.3390/plants12122330
APA StyleEsquivel-Chirino, C., Bolaños-Carrillo, M. A., Carmona-Ruiz, D., Lopéz-Macay, A., Hernández-Sánchez, F., Montés-Sánchez, D., Escuadra-Landeros, M., Gaitán-Cepeda, L. A., Maldonado-Frías, S., Yáñez-Ocampo, B. R., Ventura-Gallegos, J. L., Laparra-Escareño, H., Mejía-Velázquez, C. P., & Zentella-Dehesa, A. (2023). The Protective Role of Cranberries and Blueberries in Oral Cancer. Plants, 12(12), 2330. https://doi.org/10.3390/plants12122330







