Phenolic Content and Antioxidant Capacity of Synthetic Hexaploid Wheats
Abstract
1. Introduction
2. Results and Discussions
2.1. The Free, Bound, and Total Phenolics
2.2. Individual Phenolic Contents
2.3. Antioxidant Activities with DPPH, ABTS and CUPRAC Assays
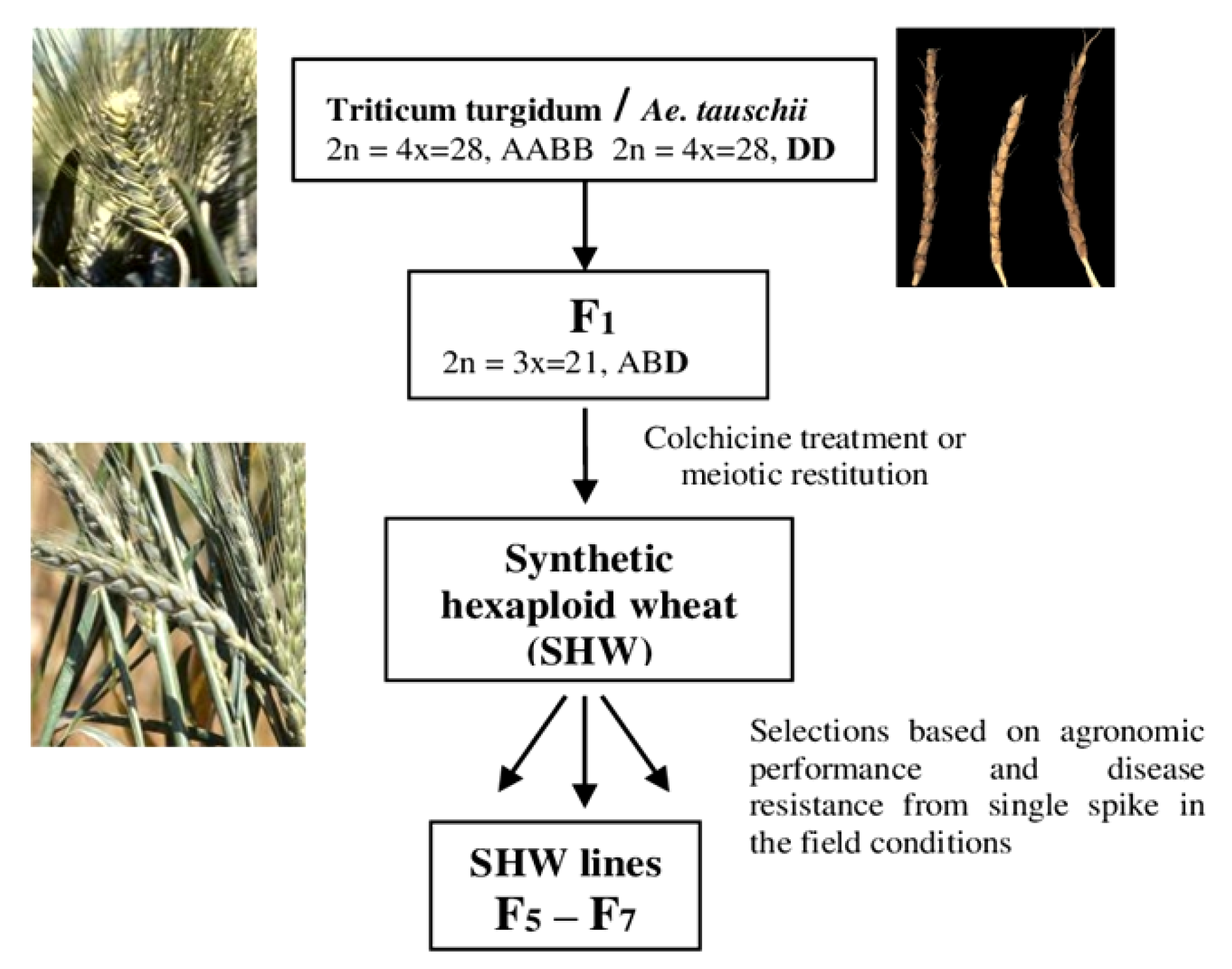
3. Materials and Methods
3.1. Raw Material
3.2. Chemicals
3.3. Extraction
3.3.1. Removal of Oil from Wheat Samples
3.3.2. Extraction of Free Phenolic Compounds
3.3.3. Extraction of Bound Phenolic Compounds
3.4. Free, Bound, and Total Phenolic Contents
3.5. HPLC Determination of Individual Phenolics
3.6. DPPH Radical Scavenging Activity
3.7. ABTS Scavenging Activity
3.8. CUPRAC (CUPric Reducing Antioxidant Capacity) Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of Antioxidant Activities of Different Colored Wheat Grains and Analysis of Phenolic Compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Van Slageren, M. Wild wheats: A monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae); Agricultural University Wageningen: Wageningen, The Netherlands, 1994. [Google Scholar]
- Li, J.; Wan, H.S.; Yang, W.Y. Synthetic hexaploid wheat enhances variation and adaptive evolution of bread wheat in breeding processes. J. Syst. Evol. 2014, 52, 735–742. [Google Scholar] [CrossRef]
- Ogbonnaya, F.C.; Abdalla, O.; Mujeeb-Kazi, A.; Kazi, A.G.; Xu, S.S.; Gosman, N.; Lagudah, E.S.; Bonnett, D.; Sorrells, M.E.; Tsujimoto, H. Synthetic hexaploids: Harnessing species of the primary gene pool for wheat improvement. Plant Breed. Rev. 2013, 37, 35–122. [Google Scholar]
- Khan, Z.; Qazi, J.; Rasheed, A.; Mujeeb-Kazi, A. Diversity in D-genome synthetic hexaploid wheat association panel for seedling emergence traits under salinity stress. Plant Genet. Resour. 2017, 15, 488–495. [Google Scholar] [CrossRef]
- Mitrofanova, O.; AG, K. Novye geneticheskie resursy v selektsii pshenitsy na uvelichenie soderzhaniya belka v zerne [New genetic resources in wheat breeding to increase the protein content in grain]. Vavilovskii Zhurnal Genet. I Sel. 2016, 20, 545–554. [Google Scholar]
- Lillemo, M.; Chen, F.; Xia, X.; William, M.; Peña, R.J.; Trethowan, R.; He, Z. Puroindoline grain hardness alleles in CIMMYT bread wheat germplasm. J. Cereal Sci. 2006, 44, 86–92. [Google Scholar] [CrossRef]
- Xu, S.; Khan, K.; Klindworth, D.; Nygard, G. Evaluation and characterization of high-molecular weight 1D glutenin subunits from Aegilops tauschii in synthetic hexaploid wheats. J. Cereal Sci. 2010, 52, 333–336. [Google Scholar] [CrossRef]
- Bibi, A.; Rasheed, A.; Kazi, A.G.; Mahmood, T.; Ajmal, S.; Ahmed, I.; Mujeeb-Kazi, A. High-molecular-weight (HMW) glutenin subunit composition of the Elite-II synthetic hexaploid wheat subset (Triticum turgidum × Aegilops tauschii; 2n = 6x = 42; AABBDD). Plant Genet. Resour. 2012, 10, 1–4. [Google Scholar] [CrossRef]
- Yan, L.; Liang, F.; Xu, H.; Zhang, X.; Zhai, H.; Sun, Q.; Ni, Z. Identification of QTL for grain size and shape on the D genome of natural and synthetic allohexaploid wheats with near-identical AABB genomes. Front. Plant Sci. 2017, 8, 1705. [Google Scholar] [CrossRef]
- Igrejas, G.; Branlard, G. The importance of wheat. In Wheat Quality for Improving Processing and Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar]
- Zeibig, F.; Kilian, B.; Frei, M. The grain quality of wheat wild relatives in the evolutionary context. Theor. Appl. Genet. 2022, 135, 4029–4048. [Google Scholar] [CrossRef]
- Shelenga, T.; Malyshev, L.; Kerv, Y.A.; Diubenko, T.; Konarev, A.; Horeva, V.; Belousova, M.; Kolesova, M.; Chikida, N. Metabolomic approach to search for fungal resistant forms of Aegilops tauschii Coss. from the VIR collection. Vavilov J. Genet. Breed. 2020, 24, 252. [Google Scholar] [CrossRef]
- Cox, T.S.; Wu, J.; Wang, S.; Cai, J.; Zhong, Q.; Fu, B. Comparing two approaches for introgression of germplasm from Aegilops tauschii into common wheat. Crop J. 2017, 5, 355–362. [Google Scholar] [CrossRef]
- Saini, P.; Kumar, N.; Kumar, S.; Mwaurah, P.W.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K.; Singh, V. Bioactive compounds, nutritional benefits and food applications of colored wheat: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.-S.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Lacko-Bartošová, M.; Lacko-Bartošová, L.; Kobida, Ľ.; Kaur, A.; Moudrý, J. Phenolic Acids Profiles and Phenolic Concentrations of Emmer Cultivars in Response to Growing Year under Organic Management. Foods 2023, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Dinelli, G.; Segura Carretero, A.; Di Silvestro, R.; Marotti, I.; Fu, S.; Benedettelli, S.; Ghiselli, L.; Fernández Gutiérrez, A. Determination of phenolic compounds in modern and old varieties of durum wheat using liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 7229–7240. [Google Scholar] [CrossRef]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparative analysis of phenolic compound characterization and their biosynthesis genes between two diverse bread wheat (Triticum aestivum) varieties differing for chapatti (unleavened flat bread) quality. Front. Plant Sci. 2016, 7, 1870. [Google Scholar] [CrossRef]
- Stuper, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Quantitative profile of phenolic acids and antioxidant activity of wheat grain exposed to stress. Eur. Food Res. Technol. 2019, 245, 1595–1603. [Google Scholar] [CrossRef]
- Mattila, P.; Pihlava, J.-m.; Hellström, J. Contents of phenolic acids, alkyl-and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef] [PubMed]
- Di Loreto, A.; Bosi, S.; Montero, L.; Bregola, V.; Marotti, I.; Sferrazza, R.E.; Dinelli, G.; Herrero, M.; Cifuentes, A. Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis 2018, 39, 2001–2010. [Google Scholar] [CrossRef]
- Di Silvestro, R.; Marotti, I.; Bosi, S.; Bregola, V.; Carretero, A.S.; Sedej, I.; Mandic, A.; Sakac, M.; Benedettelli, S.; Dinelli, G. Health-promoting phytochemicals of Italian common wheat varieties grown under low-input agricultural management. J. Sci. Food Agric. 2012, 92, 2800–2810. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Comparison of wheat flours grown at different locations for their antioxidant properties. Food Chem. 2004, 86, 11–16. [Google Scholar] [CrossRef]
- Zengin, G.; Nithiyanantham, S.; Sarikurkcu, C.; Uysal, S.; Ceylan, R.; Ramya, K.S.; Maskovic, P.; Aktumsek, A. Identification of phenolic profiles, fatty acid compositions, antioxidant activities, and enzyme inhibition effects of seven wheat cultivars grown in Turkey: A phytochemical approach for their nutritional value. Int. J. Food Prop. 2017, 20, 2373–2382. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant Capacity and Profiles of Phenolic Acids in Various Genotypes of Purple Wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Cebi, N.; Sagdic, O. Characterization of Feijoa sellowiana leaves based on volatile and phenolic compound compositions and antimicrobial properties. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Singh, R.; Chidambara Murthy, K.; Jayaprakasha, G. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
| Sample | Free Phenolics (mg GAE/100 g) | Bound Phenolics (mg GAE/100 g) | Total Phenolics (mg GAE/100 g) |
|---|---|---|---|
| W1 | 258.55 ± 0.1 a | 304.49 ± 0.6 f,g | 563.04 ± 0.7 a,b |
| W2 | 180.78 ± 1.8 h,i | 241.00 ± 0.5 l | 421.78 ± 2.3 h,i |
| W3 | 184.17 ± 0.5 g,h,i | 228.03 ± 0.8 n | 412.20 ± 1.3 h,i,j |
| W4 | 198.58 ± 0.3 d,e,f | 257.28 ± 0.2 k | 455.86 ± 0.5 f |
| W5 | 198.18 ± 0.1 d,e,f | 238.84 ± 0.2 l | 437.01 ± 0.3 g |
| W6 | 183.74 ± 0.7 g,h,i | 227.61 ± 0.4 n | 411.35 ± 1.1 i,j |
| W7 | 197.31 ± 0.2 d,e,f,g | 201.33 ± 0.3 p | 398.64 ± 0.5 j |
| W8 | 187.13 ± 0.3 f,g,h,i | 213.20 ± 0.8 o | 400.28 ± 1.3 j |
| W9 | 145.38 ± 0.1 j | 188.19 ± 0.1 q | 333.57 ± 0.2 k |
| W10 | 240.32 ± 0.1 b,c | 299.24 ± 0.1 h | 539.56 ± 0.2 c |
| W11 | 193.49 ± 0.3 e,f,g,h | 356.03 ± 0.9 c | 549.51 ± 1.2 b,c |
| W12 | 205.99 ± 0.9 d,e | 361.40 ± 0.7 b | 567.39 ± 1.6 a |
| W13 | 199.21 ± 0.9 d,e,f | 369.38 ± 0.6 a | 568.59 ± 1.5 a |
| W14 | 192.22 ± 0.5 f,g,h | 234.53 ± 0.9 m | 426.75 ± 1.4 g,h |
| W15 | 188.62 ± 0.0 i | 233.54 ± 1.0 m | 411.16 ± 1.0 i,j |
| W16 | 229.09 ± 0.1 c | 274.66 ± 0.3 j | 503.75 ± 0.3 d |
| W17 | 210.66 ± 0.3 d | 306.02 ± 0.0 f | 516.68 ± 0.3 d |
| W18 | 248.38 ± 0.2 a,b | 302.49 ± 0.5 g | 550.88 ± 0.7 b,c |
| W19 | 193.49 ± 0.0 e,f,g,h | 320.85 ± 0.2 e | 514.34 ± 0.2 d |
| W20 | 231.64 ± 0.5 c | 345.30 ± 0.1 d | 576.94 ± 0.6 a |
| W21 | 200.48 ± 0.2 d,e,f | 285.68 ± 0.4 i | 486.16 ± 0.6 e |
| Gallic Acid | Protocatechuic Acid | Catechin | Syringic Acid | Ellagic Acid | Chrysin | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Quercetin | Kaempferol | Chlorogenic Acid | Rutin | Sinapic Acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 28.69 | 18.31 | 4.85 | 1.46 | 12.24 | 1.20 | 4.81 | 2.03 | 7.09 | 17.14 | 6.51 | 2.19 | 9.95 | 6.07 |
| W2 | 21.10 | 15.84 | 4.67 | 0.29 | 8.87 | 0.72 | 4.32 | 2.13 | 6.07 | 16.97 | 6.71 | 1.18 | 10.56 | 4.99 |
| W3 | 25.77 | 14.41 | 4.06 | 0.44 | 16.51 | 0.60 | 4.23 | 1.67 | 5.07 | 16.81 | 6.43 | 1.22 | 7.85 | 3.92 |
| W4 | 29.46 | 17.35 | 6.19 | 0.26 | 19.16 | 0.66 | 4.45 | 2.46 | 6.40 | 17.23 | 7.95 | 1.34 | 12.47 | 5.34 |
| W5 | 87.35 | 13.56 | 7.22 | 0.28 | 13.16 | 1.00 | 4.29 | 1.97 | 5.89 | 16.87 | 6.68 | 1.42 | 9.64 | 4.80 |
| W6 | 92.73 | 20.32 | 0.00 | 1.37 | 9.60 | 1.09 | 4.27 | 1.07 | 6.20 | 18.16 | 6.69 | 1.15 | 4.43 | 5.13 |
| W7 | 45.11 | 26.48 | 5.68 | 0.32 | 10.54 | 3.19 | 6.21 | 3.67 | 13.10 | 17.60 | 7.58 | 2.78 | 19.47 | 12.49 |
| W8 | 39.39 | 13.23 | 2.77 | 0.82 | 8.06 | 0.00 | 4.90 | 0.92 | 6.22 | 16.83 | 6.48 | 1.04 | 3.56 | 5.15 |
| W9 | 41.23 | 12.50 | 3.38 | 0.73 | 18.65 | 15.92 | 5.14 | 1.94 | 6.03 | 16.81 | 6.53 | 0.91 | 9.43 | 4.95 |
| W10 | 14.54 | 15.02 | 3.25 | 0.45 | 11.18 | 2.17 | 4.43 | 1.73 | 7.81 | 17.08 | 6.50 | 1.51 | 8.22 | 6.84 |
| W11 | 49.51 | 11.79 | 9.64 | 1.23 | 12.33 | 4.63 | 4.38 | 1.50 | 4.14 | 17.07 | 6.52 | 1.19 | 6.87 | 2.94 |
| W12 | 46.90 | 26.99 | 3.70 | 0.72 | 26.07 | 4.38 | 4.30 | 4.33 | 8.67 | 16.73 | 6.50 | 2.45 | 23.25 | 7.76 |
| W13 | 20.93 | 11.68 | 3.58 | 0.19 | 17.08 | 3.16 | 4.54 | 1.12 | 5.92 | 17.29 | 6.47 | 1.27 | 4.69 | 4.83 |
| W14 | 41.25 | 17.19 | 0.00 | 1.10 | 11.53 | 4.13 | 4.45 | 0.75 | 7.28 | 19.63 | 6.40 | 1.97 | 2.54 | 6.28 |
| W15 | 24.07 | 22.81 | 5.38 | 2.40 | 11.74 | 3.36 | 4.74 | 1.30 | 8.13 | 18.72 | 6.48 | 1.19 | 5.74 | 7.18 |
| W16 | 30.44 | 19.12 | 4.38 | 2.38 | 12.79 | 3.93 | 5.04 | 2.43 | 9.93 | 21.84 | 6.70 | 1.50 | 12.28 | 9.10 |
| W17 | 32.02 | 15.19 | 3.41 | 0.70 | 10.11 | 3.70 | 4.75 | 1.48 | 8.76 | 16.87 | 6.68 | 2.36 | 6.76 | 7.86 |
| W18 | 28.57 | 17.22 | 0.00 | 0.09 | 8.03 | 0.95 | 5.15 | 1.47 | 5.53 | 17.12 | 6.66 | 0.86 | 6.73 | 4.42 |
| W19 | 33.08 | 15.91 | 0.00 | 0.91 | 35.04 | 0.00 | 4.84 | 1.63 | 11.74 | 16.83 | 6.66 | 1.08 | 7.63 | 11.04 |
| W20 | 30.38 | 8.37 | 7.03 | 1.41 | 8.67 | 1.03 | 4.83 | 3.19 | 10.74 | 16.90 | 6.50 | 0.81 | 16.68 | 9.97 |
| W21 | 11.20 | 16.66 | 0.00 | 1.98 | 9.58 | 0.16 | 4.42 | 1.22 | 5.76 | 16.72 | 6.50 | 0.96 | 5.28 | 4.66 |
| Gallic Acid | Protocatechuic Acid | Catechin | Syringic Acid | Ellagic Acid | Chrysin | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Quercetin | Kaempferol | Chlorogenic Acid | Rutin | Sinapic Acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 14.98 | 3.28 | 17.59 | 0.00 | 0.00 | 0.36 | 4.30 | 16.74 | 6.34 | 0.28 | 3.10 | 14.98 | 3.28 | 17.59 |
| W2 | 8.94 | 0.00 | 27.06 | 0.69 | 0.00 | 0.77 | 6.72 | 0.00 | 0.00 | 2.66 | 5.69 | 8.94 | 0.00 | 27.06 |
| W3 | 8.32 | 0.00 | 9.50 | 1.11 | 4.12 | 12.69 | 39.77 | 16.68 | 7.57 | 10.59 | 32.51 | 8.32 | 0.00 | 9.50 |
| W4 | 15.10 | 58.97 | 21.87 | 3.02 | 0.00 | 0.62 | 5.46 | 16.71 | 6.47 | 1.80 | 4.34 | 15.10 | 58.97 | 21.87 |
| W5 | 9.25 | 0.00 | 41.93 | 1.96 | 0.00 | 1.19 | 13.13 | 17.06 | 6.33 | 5.13 | 12.52 | 9.25 | 0.00 | 41.93 |
| W6 | 7.97 | 0.00 | 22.90 | 2.65 | 0.00 | 0.50 | 5.55 | 16.70 | 6.40 | 1.11 | 4.43 | 7.97 | 0.00 | 22.90 |
| W7 | 17.16 | 51.30 | 0.00 | 0.62 | 0.00 | 0.38 | 1.91 | 0.00 | 6.70 | 0.42 | 0.56 | 17.16 | 51.30 | 0.00 |
| W8 | 11.36 | 85.55 | 0.00 | 0.65 | 0.00 | 0.38 | 1.86 | 0.00 | 6.61 | 0.41 | 0.50 | 11.36 | 85.55 | 0.00 |
| W9 | 8.37 | 0.00 | 33.59 | 0.00 | 4.27 | 3.55 | 12.85 | 16.89 | 6.58 | 18.76 | 18.82 | 8.37 | 0.00 | 33.59 |
| W10 | 9.07 | 0.00 | 8.67 | 2.58 | 0.00 | 0.50 | 4.21 | 16.68 | 6.47 | 1.13 | 3.01 | 9.07 | 0.00 | 8.67 |
| W11 | 8.15 | 31.54 | 17.66 | 3.10 | 0.00 | 0.31 | 2.88 | 0.00 | 6.55 | 0.01 | 1.58 | 8.15 | 31.54 | 17.66 |
| W12 | 9.84 | 0.00 | 41.70 | 1.46 | 4.18 | 1.65 | 32.29 | 17.02 | 6.41 | 7.75 | 32.94 | 9.84 | 0.00 | 41.70 |
| W13 | 15.92 | 19.13 | 9.78 | 0.00 | 4.22 | 1.37 | 24.74 | 19.51 | 7.50 | 6.17 | 24.89 | 15.92 | 19.13 | 9.78 |
| W14 | 13.11 | 0.00 | 0.00 | 0.48 | 0.00 | 0.32 | 1.89 | 17.21 | 0.00 | 0.09 | 0.53 | 13.11 | 0.00 | 0.00 |
| W15 | 9.62 | 0.00 | 9.74 | 1.58 | 0.00 | 0.37 | 2.01 | 16.71 | 0.00 | 0.35 | 0.66 | 9.62 | 0.00 | 9.74 |
| W16 | 8.23 | 10.12 | 11.13 | 0.67 | 0.00 | 0.35 | 2.00 | 0.00 | 0.00 | 0.27 | 0.65 | 8.23 | 10.12 | 11.13 |
| W17 | 11.62 | 5.04 | 12.07 | 0.66 | 4.17 | 1.17 | 26.41 | 17.98 | 7.15 | 5.01 | 26.67 | 11.62 | 5.04 | 12.07 |
| W18 | 14.99 | 75.28 | 10.78 | 0.85 | 0.00 | 0.38 | 2.63 | 16.72 | 6.36 | 0.39 | 1.32 | 14.99 | 75.28 | 10.78 |
| W19 | 12.62 | 0.00 | 9.91 | 2.27 | 4.16 | 0.68 | 10.03 | 17.01 | 6.61 | 2.15 | 9.21 | 12.62 | 0.00 | 9.91 |
| W20 | 8.33 | 0.00 | 17.35 | 2.72 | 0.00 | 0.40 | 1.96 | 0.00 | 0.00 | 0.52 | 0.61 | 8.33 | 0.00 | 17.35 |
| W21 | 9.52 | 7.37 | 10.53 | 0.30 | 0.00 | 0.35 | 1.88 | 16.72 | 0.00 | 0.24 | 0.53 | 9.52 | 7.37 | 10.53 |
| # of Sample | Free (%AA) | Bound (%AA) |
|---|---|---|
| W1 | 36.5 ± 0.1 b | 50.6 ± 0.2 a |
| W2 | 33.8 ± 0.0 h | 35.7 ± 0.1 h,i |
| W3 | 34.0 ± 0.2 f,g,h | 41.8 ± 0.0 c |
| W4 | 34.4 ± 0.0 e,f,g,h | 35.8 ± 0.0 h,i |
| W5 | 33.8 ± 0.1 h,i | 36.1 ± 0.2 f,g,h |
| W6 | 35.1 ± 0.0 d,e | 42.7 ± 0.0 b,c |
| W7 | 35.1 ± 0.1 d,e,f | 36.7 ± 0.2 f,g |
| W8 | 33.0 ± 0.0 i | 35.9 ± 0.0 g,h |
| W9 | 34.0 ± 0.1 g,h | 35.0 ± 0.2 i,j |
| W10 | 33.8 ± 0.1 h | 39.4 ± 0.3 e |
| W11 | 33.3 ± 0.0 h,i | 40.6 ± 0.0 d |
| W12 | 35.4 ± 0.0 c,d | 36.4 ± 0.0 f,g,h |
| W13 | 33.8 ± 0.1 h,i | 39.9 ± 0.1 d,e |
| W14 | 34.7 ± 0.0 d,e,f,g | 34.4 ± 0.0 j |
| W15 | 35.4 ± 0.1 c,d | 39.2 ± 0.1 e |
| W16 | 35.9 ± 0.0 b,c | 35.5 ± 0.2 h,i |
| W17 | 40.5 ± 0.1 a | 40.7 ± 0.0 d |
| W18 | 40.3 ± 0.0 a | 43.5 ± 0.1 b |
| W19 | 35.9 ± 0.4 b,c | 36.9 ± 0.0 f |
| W20 | 34.2 ± 0.0 f,g,h | 42.6 ± 0.4 c |
| W21 | 34.3 ± 0.1 e,f,g,h | 36.2 ± 0.0 f,g,h |
| # of Sample | Free (mg TE/100 g) | Bound (mg TE/100 g) | Total (mg TE/100 g) |
|---|---|---|---|
| W1 | 37.93 ± 0.03 k | 93.84 ± 0.04 n | 131.77 ± 0.07 n |
| W2 | 31.13 ± 0.01 q | 62.82 ± 0.04 s | 93.94 ± 0.05 u |
| W3 | 73.96 ± 0.02 c | 112.91 ± 0.01 l | 186.87 ± 0.03 k |
| W4 | 123.18 ± 0.02 a | 166.19 ± 0.01 i | 289.37 ± 0.03 d |
| W5 | 34.39 ± 0.00 n | 71.12 ± 0.05 r | 105.50 ± 0.05 s |
| W6 | 45.52 ± 0.02 h | 76.76 ± 0.02 q | 122.27 ± 0.04 o |
| W7 | 61.25 ± 0.01 e | 101.49 ± 0.07 m | 162.74 ± 0.08 m |
| W8 | 40.63 ± 0.02 j | 77.07 ± 0.02 p | 117.70 ± 0.04 p |
| W9 | 66.42 ± 0.04 d | 241.64 ± 0.04 b | 308.07 ± 0.08 a |
| W10 | 27.31 ± 0.01 t | 184.09 ± 0.06 g | 211.39 ± 0.07 j |
| W11 | 32.64 ± 0.01 o | 144.07 ± 0.09 k | 176.71 ± 0.10 l |
| W12 | 43.66 ± 0.00 i | 224.56 ± 0.02 d | 268.22 ± 0.02 f |
| W13 | 66.48 ± 0.02 d | 157.11 ± 0.07 j | 223.59 ± 0.09 i |
| W14 | 51.92 ± 0.03 g | 198.03 ± 0.02 f | 249.95 ± 0.05 g |
| W15 | 37.09 ± 0.01 l | 238.50 ± 0.05 c | 275.58 ± 0.06 e |
| W16 | 29.67 ± 0.02 r | 263.23 ± 0.02 a | 292.89 ± 0.04 c |
| W17 | 114.19 ± 0.03 b | 179.59 ± 0.04 h | 293.78 ± 0.07 b |
| W18 | 36.07 ± 0.01 m | 202.52 ± 0.08 e | 238.60 ± 0.09 h |
| W19 | 52.15 ± 0.02 f | 61.65 ± 0.01 t | 113.80 ± 0.03 q |
| W20 | 32.08 ± 0.01 p | 62.88 ± 0.02 s | 94.97 ± 0.03 t |
| W21 | 27.87 ± 0.00 s | 82.99 ± 0.03 o | 110.85 ± 0.03 r |
| # of Sample | Free (mg TE/100 g) | Bound (mg TE/100 g) | Total (mg TE/100 g) |
|---|---|---|---|
| W1 | 90.21 ± 0.06 h | 128.13 ± 0.11 i | 218.34 ± 0.17 g |
| W2 | 25.78 ± 0.05 r | 81.73 ± 0.01 s | 107.51 ± 0.06 q |
| W3 | 58.53 ± 0.00 l | 155.62 ± 0.06 f | 214.15 ± 0.11 i |
| W4 | 114.27 ± 0.01 e | 122.53 ± 0.20 k | 236.80 ± 0.21 f |
| W5 | 43.04 ± 0.02 o | 75.35 ± 0.10 t | 118.39 ± 0.12 p |
| W6 | 43.87 ± 0.01 o | 88.99 ± 0.08 q | 132.86 ± 0.09 o |
| W7 | 127.03 ± 0.05 d | 146.55 ± 0.10 h | 273.56 ± 0.15 e |
| W8 | 51.33 ± 0.15 n | 97.45 ± 0.11 o | 148.78 ± 0.26 m |
| W9 | 89.73 ± 0.09 h | 112.93 ± 0.05 m | 202.66 ± 0.14 j |
| W10 | 35.60 ± 0.14 q | 82.53 ± 0.05 r | 118.13 ± 0.19 p |
| W11 | 61.73 ± 0.21 k | 113.41 ± 0.08 m | 175.14 ± 0.29 l |
| W12 | 91.86 ± 0.19 g | 125.45 ± 0.07 j | 217.31 ± 0.26 h |
| W13 | 77.63 ± 0.06 j | 158.55 ± 0.05 e | 236.18 ± 0.11 f |
| W14 | 56.54 ± 0.15 m | 120.82 ± 0.22 l | 177.36 ± 0.37 k |
| W15 | 131.30 ± 0.11 c | 147.62 ± 0.04 g | 278.95 ± 0.15 d |
| W16 | 110.00 ± 0.33 f | 163.84 ± 0.15 d | 273.84 ± 0.48 e |
| W17 | 160.94 ± 0.04 a | 203.86 ± 0.20 b | 364.79 ± 0.24 a |
| W18 | 52.13 ± 0.10 n | 308.13 ± 0.03 a | 360.26 ± 0.13 b |
| W19 | 39.52 ± 0.40 p | 107.80 ± 0.03 n | 147.13 ± 0.43 n |
| W20 | 134.53 ± 0.10 b | 187.88 ± 0.15 c | 322.41 ± 0.26 c |
| W21 | 80.67 ± 0.12 i | 96.41 ± 0.17 p | 177.08 ± 0.29 k |
| Pedigree | Number of Lines | Cross ID | Ae. tauschii Origin | Subspecies |
|---|---|---|---|---|
| CIMMYT spring synthetics * | ||||
| Aisberg/Ae.sq.(369) | 2 | C04GH3 | Mazandaran, Iran | tauschii |
| Aisberg/Ae.sq.(511) | 3 | C04GH5 | Unknown | Unknown |
| Ukr.-od.952.92/Ae.sq.(1031) | 3 | C04GH61 | Zanjan, Iran | tauschii |
| Ukr.-od.1530.94/Ae.sq.(310) | 1 | C04GH68 | Gilan, Iran | strangulata |
| Ukr.-od.1530.94/Ae.sq.(392) | 3 | C04GH71 | Shemakha, Azerbaijan | tauschii |
| Ukr.-od.1530.94/Ae.sq.(458) | 1 | C04GH74 | Unknown | Unknown |
| Ukr.-od.1530.94/Ae.sq.(629) | 1 | C04GH76 | Mazandaran, Iran | strangulata |
| Ukr.-od.1530.94/Ae.sq.(1027) | 5 | C04GH78 | Mazandaran, Iran | tauschii |
| Pandur/Ae.sq.(223) | 1 | C04GH79 | Gilan, Iran | tauschii |
| Japanese synthetics ** | ||||
| Langdon/Ae.sq. IG 126387 | 1 | – | Ashkhabad, Turkmenistan | Unknown |
| # of Samples | Pedigree |
|---|---|
| W1 | Ukr.-Od. 1530.94/Ae.squarrosa (629) |
| W2 | Ukr.-Od. 952.92/Ae.squarrosa (1031) |
| W3 | Ukr.-Od. 1530.94/Ae.squarrosa (458) |
| W4 | Ukr.-Od. 952.92/Ae.squarrosa (1031) |
| W5 | Ukr.-Od. 1530.94/Ae.squarrosa (392) |
| W6 | Ukr.-Od. 1530.94/Ae.squarrosa (392) |
| W7 | Ukr.-Od. 1530.94/Ae.squarrosa (1027) |
| W8 | Aisberg/Ae.squarrosa (511) |
| W9 | Aisberg/Ae.squarrosa (511) |
| W10 | Pandur/Ae.squarrosa (223) |
| W11 | Aisberg/Ae.squarrosa (369) |
| W12 | Langdon/IG 126387 |
| W13 | Ukr.-Od. 1530.94/Ae.squarrosa (310) |
| W14 | Aisberg/Ae.squarrosa (369) |
| W15 | Ukr.-Od. 952.92/Ae.squarrosa (1031) |
| W16 | Ukr.-Od. 1530.94/Ae.squarrosa (1027) |
| W17 | Ukr.-Od. 1530.94/Ae.squarrosa (1027) |
| W18 | Ukr.-Od. 1530.94/Ae.squarrosa (1027) |
| W19 | Aisberg/Ae.squarrosa (511) |
| W20 | Ukr.-Od. 1530.94/Ae.squarrosa (392) |
| W21 | Ukr.-Od. 1530.94/Ae.squarrosa (1027) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanin, V.P.; Tekin-Cakmak, Z.H.; Karasu, S.; Pototskaya, I.V.; Shepelev, S.S.; Chursin, A.S.; Morgounov, A.I.; Sagdic, O.; Koksel, H. Phenolic Content and Antioxidant Capacity of Synthetic Hexaploid Wheats. Plants 2023, 12, 2301. https://doi.org/10.3390/plants12122301
Shamanin VP, Tekin-Cakmak ZH, Karasu S, Pototskaya IV, Shepelev SS, Chursin AS, Morgounov AI, Sagdic O, Koksel H. Phenolic Content and Antioxidant Capacity of Synthetic Hexaploid Wheats. Plants. 2023; 12(12):2301. https://doi.org/10.3390/plants12122301
Chicago/Turabian StyleShamanin, Vladimir P., Zeynep H. Tekin-Cakmak, Salih Karasu, Inna V. Pototskaya, Sergey S. Shepelev, Alexandr S. Chursin, Alexey I. Morgounov, Osman Sagdic, and Hamit Koksel. 2023. "Phenolic Content and Antioxidant Capacity of Synthetic Hexaploid Wheats" Plants 12, no. 12: 2301. https://doi.org/10.3390/plants12122301
APA StyleShamanin, V. P., Tekin-Cakmak, Z. H., Karasu, S., Pototskaya, I. V., Shepelev, S. S., Chursin, A. S., Morgounov, A. I., Sagdic, O., & Koksel, H. (2023). Phenolic Content and Antioxidant Capacity of Synthetic Hexaploid Wheats. Plants, 12(12), 2301. https://doi.org/10.3390/plants12122301







