Response of Soil Microenvironment and Crop Growth to Cyclic Irrigation Using Reclaimed Water and Brackish Water
Abstract
:1. Introduction
2. Results
2.1. Variations in Physical and Chemical Properties of Soil under Cyclic Irrigation
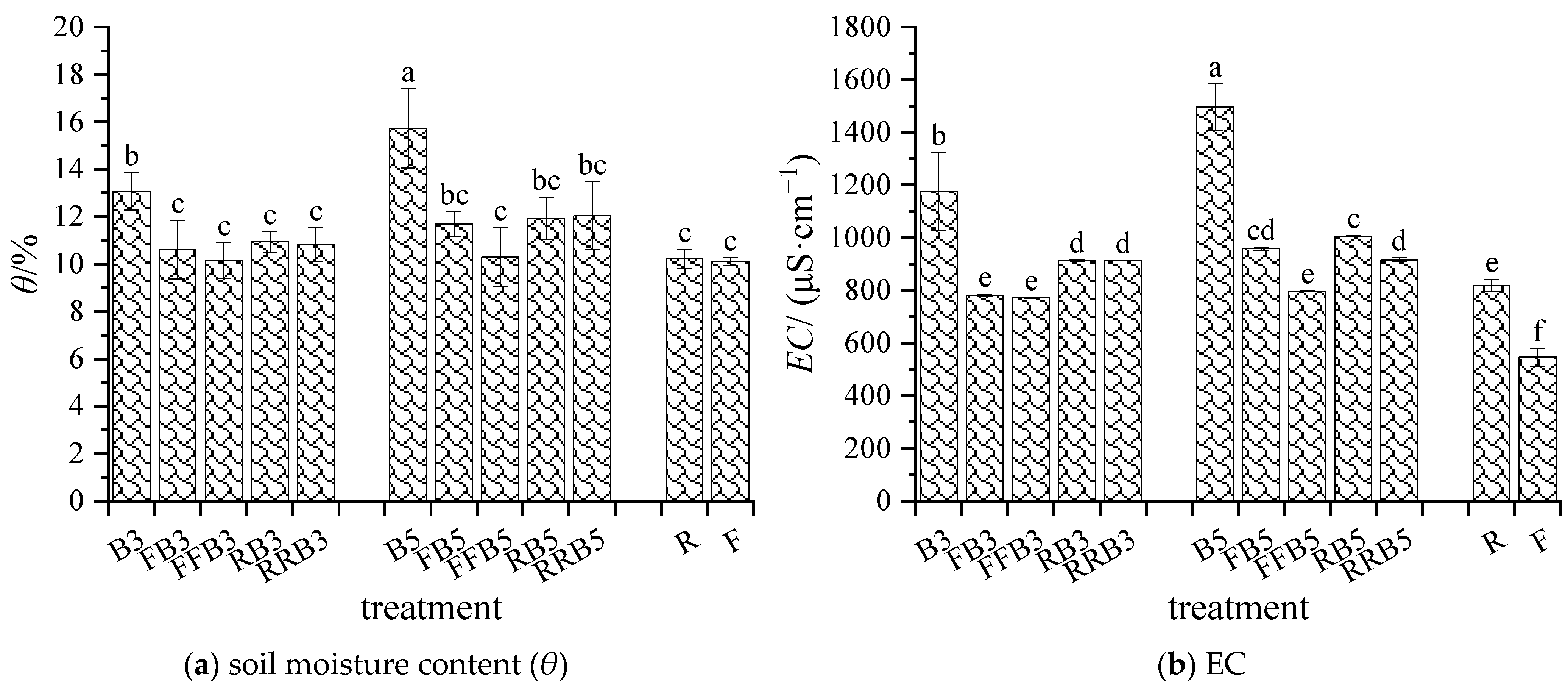
2.1.1. Soil Moisture Content and EC
2.1.2. Contents of Water-Soluble Na+ and Cl− in Soil
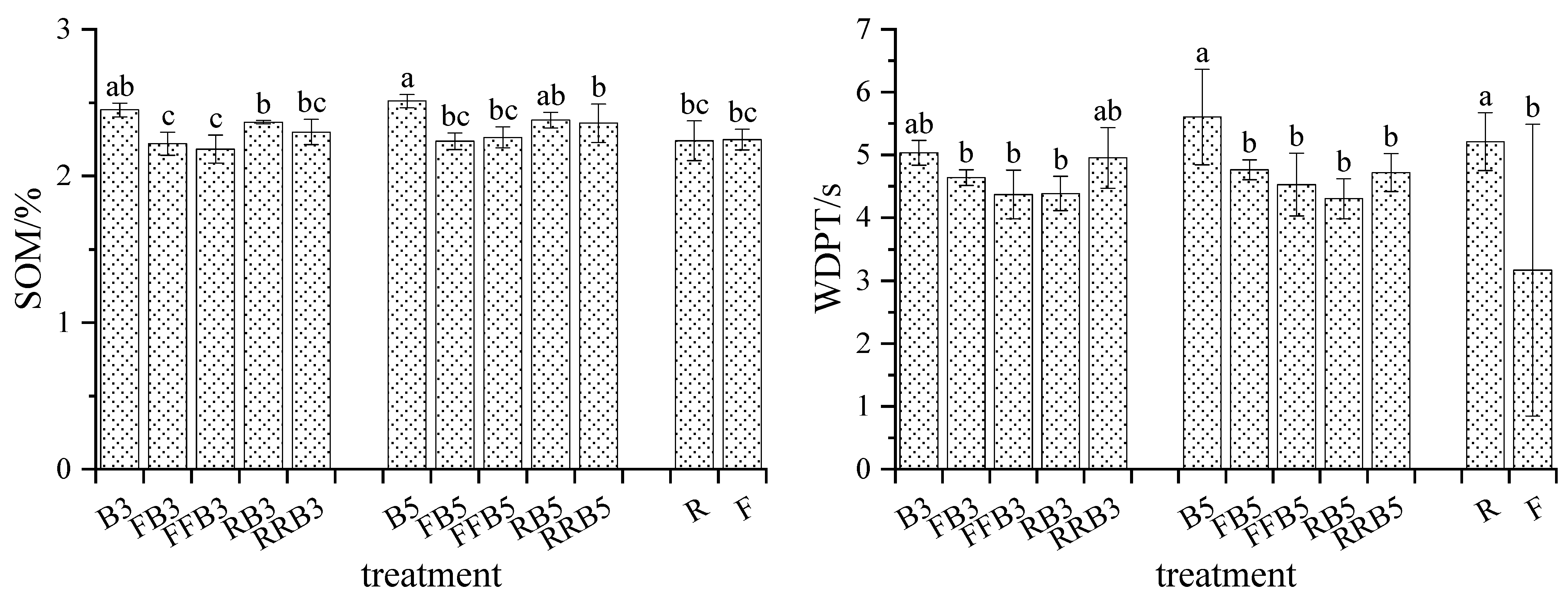
2.1.3. SOM and WDPT of Soil
2.2. Soil Enzyme Activity
2.3. Risk Analysis of Secondary Soil Salinization
2.4. Physiological and Growth Characteristics of Crops
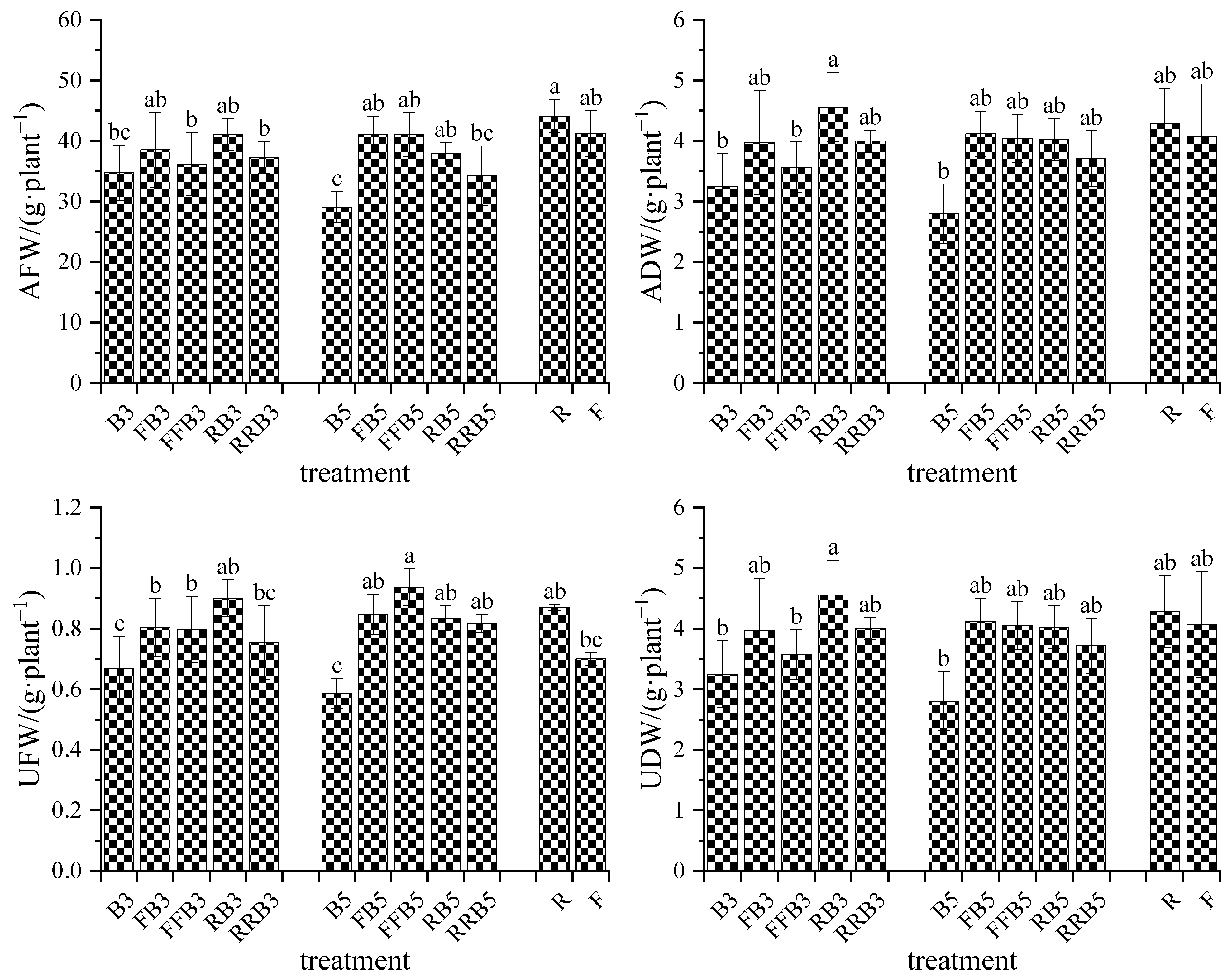
2.4.1. Biomass of Crops
2.4.2. Chlorophyll Content
2.5. Antioxidant Characteristics of Crops
2.5.1. Antioxidant Enzymes of Leaves
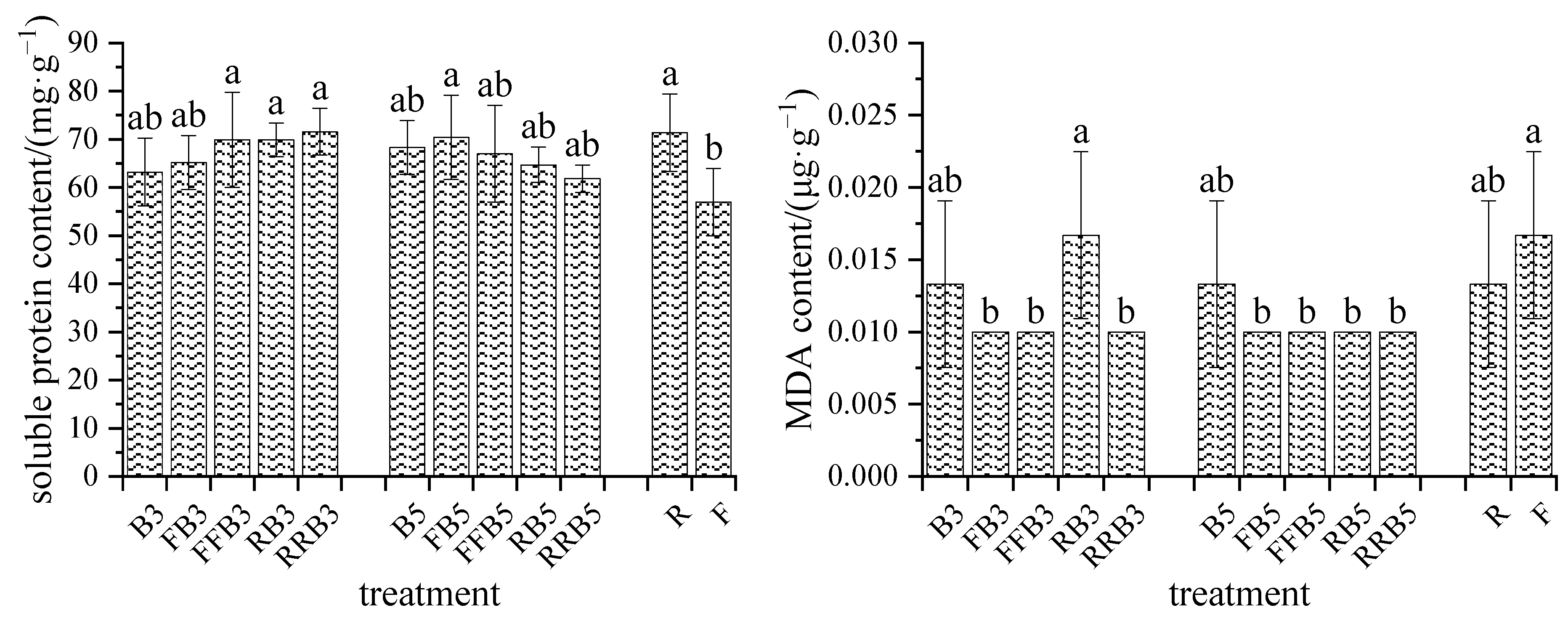
2.5.2. MDA
2.5.3. Soluble Protein Content
2.6. Distribution of Na+ in Soil–Crop
2.6.1. Na+ Content in Soil and Leaves
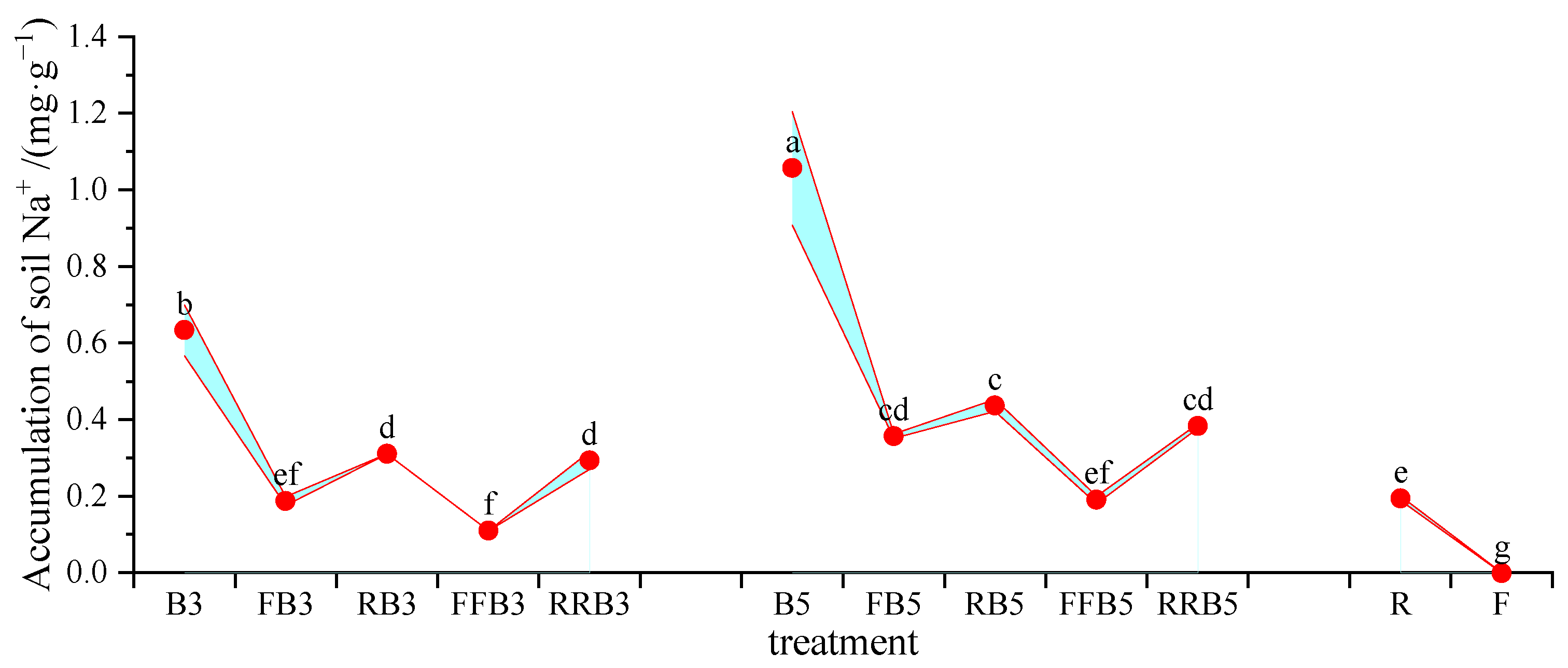
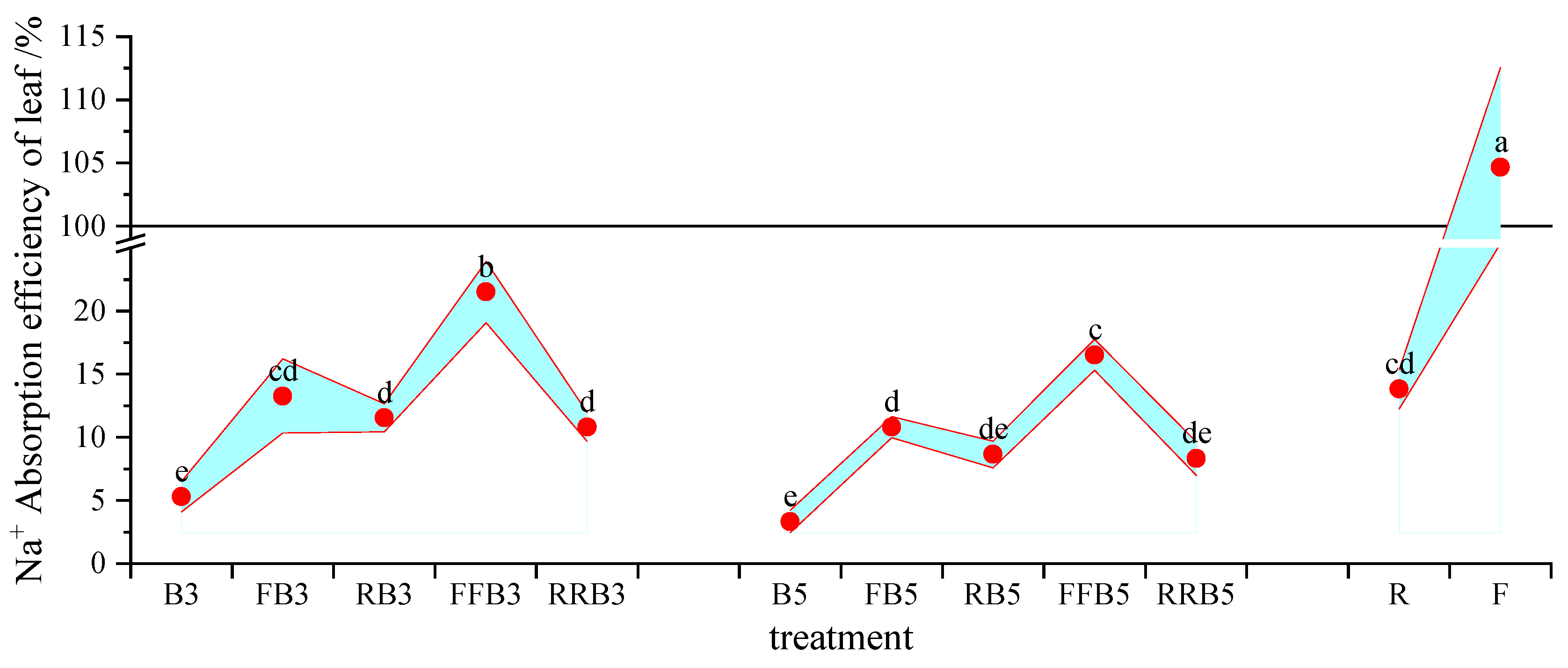
2.6.2. Accumulation of Na+ in Soil and Na+ Uptake Efficiency of Leaves
3. Discussion
3.1. Response of Soil Physicochemical Properties to RBCI
3.2. Response of Secondary Soil Salinization to RBCI
3.3. Response of Growth and Physiological Index of Crops to RBCI
3.4. Response of Antioxidant Enzymes of Leaves to RBCI
4. Materials and Methods
4.1. Tested Soil
4.2. Experimental Device and Scheme
4.3. Measured Indices and Methods
- (1)
- Physicochemical properties of soil. After crop harvest, soil samples were collected and then air-dried, ground and sieved (2 mm). The soil moisture content was measured with the oven-drying method. The soil sample was extracted with a soil-to-water ratio of 1:5. Then, the extracts were used to measure the electrical conductivity (EC) with a conductivity meter, Na+ by flame photometry, Cl− by AgNO3 titration, and the soil organic matter (SOM) was determined using a low-temperature external-heat potassium dichromate oxidation-colorimetric method according to the methods of [82]; the water drop penetration time (WDPT) was measured via the water drop penetration time method according to the methods of [22].
- (2)
- Soil salinization index. The soil pH, exchangeable ions, exchangeable soil sodium percentage (ESP) and effective action exchange capacity (ECEC) were determined and calculated according to the methods of [83].
- (3)
- Soil enzyme activity. The activities of soil alkaline phosphatase (S-AKP/ALP) were determined with detection kits (Solarbio, Beijing), and the daily release of 1 nmol phenol per gram of soil at 37 °C was used as an enzyme activity unit. The soil sucrase (S-SC) activity was determined using 3,5-dinitrosalicylic acid colorimetry, and its activity was expressed as milligrams of 1 g of soil glucose after 24 h. The activity of soil urease (S-UE) was determined with indophenol-blue colorimetry, and its activity was expressed by the number of milligrams of NH3-N in 1 g of soil after 24 h.
- (4)
- Growth and physiological index of crop. After harvesting (14 December), the aboveground fresh weight (AFW), aboveground dry weight (ADW), underground fresh weight (UFW) and underground dry weight (UDW) were determined, referring to the methods described in [84]. A detection kit (Suolebao, Beijing) was used to measure the leaf chlorophyll contents.
- (5)
- Antioxidant index of crop. Using methods outlined in [84], the soluble protein content, catalase (CAT), superoxide dismutase (SOD), peroxidase (POD) activities and malondialdehyde (MDA) content were determined.
- (6)
- Na+ content in leaves. The Na+ content in leaves was determined via a flame photometer method.
4.4. Data Analysis
5. Conclusions
- (1)
- Compared with FBCI, the soil water content increased without a significant level, while the soil EC, sodium and chloride ions contents improved obviously under RBCI. The contents of soil EC, sodium and chloride ions gradually declined, while the soil moisture content decreased gradually as irrigation times were increased using reclaimed water under RBCI.
- (2)
- The responses of different soil enzyme activities to RBCI were different. As irrigation times increased using reclaimed water, the difference in S-UE activity reached a significant level; for the same irrigation sequence, the activities of soil alkaline phosphatase, sucrase and urease increased with the increase in salinity in brackish water.
- (3)
- RBCI can alleviate the risk of secondary soil salinization to some extent. The soil pH values were all below 8.5 without any soil alkalization risk. The ESP did not exceed 15%, and there was no possible risk of soil alkalization except that the ESP of B3 and B5 went beyond the limit of 15%.
- (4)
- Neither the aboveground or underground biomass reached an obvious difference between FBCI and RBCI. RBCI was conducive to improving the aboveground biomass compared to irrigating crops with brackish water.
- (5)
- Cyclic irrigation using reclaimed-reclaimed-brackish water at 3 g·L−1 was recommended under the experimental conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seymen, M.; Yavuz, D.; Dursun, A.; Kurtar, E.S.; Türkmen, Ö. Identification of drought-tolerant pumpkin (Cucurbita pepo L.) genotypes associated with certain fruit characteristics, seed yield, and quality. Agric. Water Manag. 2019, 221, 150–159. [Google Scholar] [CrossRef]
- Yavuz, D.; Kılıç, E.; Seymen, M.; Dal, Y.; Kayak, N.; Kal, Ü.; Yavuz, N. The effect of irrigation water salinity on the morph-physiological and biochemical properties of spinach under deficit irrigation conditions. Sci. Hortic. 2022, 304, 111272. [Google Scholar] [CrossRef]
- Yavuz, D.; Rashid, B.A.R.; Seymen, M. The influence of NaCl salinity on evapotranspiration, yield traits, antioxidant status, and mineral composition of lettuce grown under deficit irrigation. Sci. Hortic. 2023, 310, e111776. [Google Scholar] [CrossRef]
- Guo, X.; Du, S.; Guo, H.; Min, W. Long-term saline water drip irrigation alters soil physicochemical properties, bacterial community structure, and nitrogen transformations in cotton. Appl. Soil Ecol. 2023, 182, e104719. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, J. Effect of brackish water irrigation on the movement of water and salt in salinized soil. Open Geosci. 2022, 14, 404–413. [Google Scholar] [CrossRef]
- Huang, C.H.; Xue, X.; Wang, T.; De Mascellis, R.; Mele, G.; You, Q.G.; Peng, F.; Tedeschi, A. Effects of saline water irrigation on soil properties in northwest China. Environ. Earth Sci. 2011, 63, 701–708. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene Expression Profiling of Plants under Salt Stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving Salinity Tolerance in Cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef]
- Wei, K.; Zhang, J.H.; Wang, Q.J.; Chen, Y.; Guo, Y.; Sun, Y. Effects of potassium humate on cotton (Gossypium hirsutum L.) growth and yield and soil salinity under film-mulched drip irrigation with brackish water in Northwest China. Appl. Ecol. Environ. Res. 2021, 19, 3879–3895. [Google Scholar] [CrossRef]
- Yasuor, H.; Yermiyahu, U.; Ben-Gal, A. Consequences of irrigation and fertigation of vegetable crops with variable quality water: Israel as a case study. Agric. Water Manag. 2020, 242, e106362. [Google Scholar] [CrossRef]
- Sietz, D.; Van Dijk, H. Land-based adaptation to global change: What drives soil and water conservation in western Africa? Glob. Environ. Chang. 2015, 33, 131–141. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.D.; Borin, M.; Adams, M.A. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Moreno, F.; Cabrera, F.; Fernández-Boy, E.; Girón, I.F.; Fernández, J.E.; Bellido, B. Irrigation with saline water in the reclaimed marsh soils of south-west Spain: Impact on soil properties and cotton and sugar beet crops. Agric. Water Manag. 2001, 48, 133–150. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Y.; Xu, Q.; Sun, H.; Li, C.; Zhu, H.; Bai, C. Effects of brackish water and reclaimed water on soil physical properties and root system of potted cotton. Water Sav. Irrig. 2016, 122–126. [Google Scholar]
- Yuan, C.; Feng, S.; Huo, Z.; Ji, Q. Effects of deficit irrigation with saline water on soil water-salt distribution and water use efficiency of maize for seed production in arid Northwest China. Agric. Water Manag. 2019, 212, 424–432. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Jin, M.; Ferre, T.P.A.; Wang, J.; Huang, J.; Wang, X. Effect of Sodium Chloride and Manganese in Irrigation Water on Cotton Growth. Agron. J. 2018, 110, 900–909. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Z.; Feng, G.; Lu, P.; Huang, M.; Zhao, X. Biochar Amendment Combined with Straw Mulching Increases Winter Wheat Yield by Optimizing Soil Water-Salt Condition under Saline Irrigation. Agriculture 2022, 12, 1681. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Tahtouh, J.; Mohtar, R.; Assi, A.; Schwab, P.; Jantrania, A.; Deng, Y.; Munster, C. Impact of brackish groundwater and treated wastewater on soil chemical and mineralogical properties. Sci. Total Environ. 2019, 647, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Guo, L.; Guan, B.; Liao, Y.; Wang, J. Effect of brackish water irrigation on water and salt movement in repellent soils. Trans. Chin. Soc. Agric. Eng. 2011, 27, 39–45. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, J.; Sun, C.; Liu, X.; Dang, H.; Cao, C.; Zheng, C.; Sun, J.; Li, K.; Ma, J. Effects of Long-term Irrigation with Saline Water on Soil Physical-chemical Properties and Activities of Soil Enzyme. J. Soil Water Conserv. 2014, 28, 171–176. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, C.; Cao, C.; Dang, H.; Li, K.; Ma, J. Effect of Long-term Saline Water Irrigation on Photosynthetic Characteristics of Winter Wheat and Soil Salt Content. Trans. Chin. Soc. Agric. Mach. 2017, 48, 183–190. [Google Scholar] [CrossRef]
- Liu, X.; Gao, P.; Yang, D.; Zhang, Q.; Zhang, Y. Effects of Intermittent Combined Irrigation on the Characteristics of Soil Water and Salt Movement in Farm Land of Salt-affected Soil. Acta Pedol. Sin. 2017, 54, 1404–1413. [Google Scholar] [CrossRef]
- Han, H. Research of Brackishe Water Supplementary Irrigation for Crop in North Shandong Province. Master’s Thesis, Shandong University, Ji′nan, China, 2012. [Google Scholar]
- Chandel, S.; Datta, A.; Yadav, R.K.; Dheri, G.S. Does Saline Water Irrigation Influence Soil Carbon Pools and Nutrient Distribution in Soil under Seed Spices? J. Soil Sci. Plant Nutr. 2021, 21, 949–966. [Google Scholar] [CrossRef]
- Petousi, I.; Daskalakis, G.; Fountoulakis, M.S.; Lydakis, D.; Fletcher, L.; Stentiford, E.I.; Manios, T. Effects of treated wastewater irrigation on the establishment of young grapevines. Sci. Total Environ. 2019, 658, 485–492. [Google Scholar] [CrossRef]
- Pedrero, F.; Camposeo, S.; Pace, B.; Cefola, M.; Vivaldi, G.A. Use of reclaimed wastewater on fruit quality of nectarine in Southern Italy. Agric. Water Manag. 2018, 203, 186–192. [Google Scholar] [CrossRef]
- Han, Y.; Li, P.; Qi, X.-B.; Cui, B.-J.; Chang, D.; Ma, H.-H. Effects of Different Levels of Irrigation with Reclaimed Water on Soil Enzyme Activity and Distribution of Thermotolerant Coliforms. Environ. Sci. 2018, 39, 4366–4374. [Google Scholar] [CrossRef]
- Guo, W.; Qi, X.; Li, P.; Li, Z.; Zhou, Y.; Xiao, Y. Impact of reclaimed water irrigation and nitrogen fertilization on soil bacterial community structure. Acta Sci. Circumstantiae 2017, 37, 280–287. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Pina, H. Survey of 218 organic contaminants in groundwater derived from the world‘s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, X.; Liang, P. Influence of drip irrigation by reclaimed water on the dynamic change of the nitrogen element in soil and tomato yield and quality. J. Clean. Prod. 2016, 139, 561–566. [Google Scholar] [CrossRef]
- Zalacain, D.; Sastre-Merlin, A.; Martinez-Perez, S.; Bienes, R.; Garcia-Diaz, A. Assessment on micronutrient concentration after reclaimed water irrigation: A CASE study in green areas of Madrid. Irrig. Drain. 2021, 70, 668–678. [Google Scholar] [CrossRef]
- Perulli, G.D.; Gaggia, F.; Sorrenti, G.; Donati, I.; Boini, A.; Bresilla, K.; Manfrini, L.; Baffoni, L.; Di Gioia, D.; Grappadelli, L.C.; et al. Treated wastewater as irrigation source: A microbiological and chemical evaluation in apple and nectarine trees. Agric. Water Manag. 2021, 244, 106403. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Cabanero, J.J.A.; Tortosa, P.A.N.; Gambin, J.M.B.; Maestre-Valero, J.F.; Nicolas, E.N. medium-long term effects of saline reclaimed water and regulated deficit irrigation on fruit quality of citrus. J. Sci. Food Agric. 2020, 100, 1350–1357. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Pan, N.; Jiao, W. Impacts of long-term reclaimed water irrigation on soil salinity accumulation in urban green land in Beijing. Water Resour. Res. 2013, 49, 7401–7410. [Google Scholar] [CrossRef]
- Yuan, C.; Feng, S.; Wang, J.; Huo, Z.; Ji, Q. Effects of irrigation water salinity on soil salt content distribution, soil physical properties and water use efficiency of maize for seed production in arid Northwest China. Int. J. Agric. Biol. Eng. 2018, 11, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Mavi, M.S.; Singh, G.; Choudhary, O.P.; Dey, D. Moderation of nitrogen availability through the application of pyrolyzed and unpyrolyzed organic materials in saline water irrigated soil. Environ. Monit. Assess. 2023, 195, 467. [Google Scholar] [CrossRef]
- Amanifar, S.; Toghranegar, Z. The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind. Crops Prod. 2020, 147, 112234. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Shabala, L.; Zhou, M.; Meinke, H.; Venkataraman, G.; Chen, Z.; Zeng, F.; Zhao, Q. Tissue-Specific Regulation of Na+ and K+ Transporters Explains Genotypic Differences in Salinity Stress Tolerance in Rice. Front. Plant Sci. 2019, 10, 1361. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ors, S.; Dursun, A. Physiological, morphological and biochemical responses of exogenous hydrogen sulfide in salt-stressed tomato seedlings. Sustainability 2023, 15, 1098. [Google Scholar] [CrossRef]
- Kida, M.; Tomotsune, M.; Iimura, Y.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. High salinity leads to accumulation of soil organic carbon in mangrove soil. Chemosphere 2017, 177, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, J.; Fengmin, L. Influence of Salinity, Sodicity and Organic Matter on Some Physical Properties of Salt -affected Soils. Chin. J. Soil Sci. 2004, 35, 64–72. [Google Scholar]
- Zhang, J.; Wang, Q.; Wang, W.; Zhang, X. The dispersion mechanism of dispersive seasonally frozen soil in western Jilin Province. Bull. Eng. Geol. Environ. 2021, 80, 5493–5503. [Google Scholar] [CrossRef]
- Guedes, P.; Martins, C.; Couto, N.; Silva, J.; Mateus, E.P.; Ribeiro, A.B.; Pereira, C.S. Irrigation of soil with reclaimed wastewater acts as a buffer of microbial taxonomic and functional biodiversity. Sci. Total Environ. 2022, 802, 149671. [Google Scholar] [CrossRef]
- Yilmaz, A.M.; Alpozen, C.M.; Kaplan, G. Irrigation water quality assessments for irrigated lands of Konya—Sarayonugozlu Agricultural Enterprise. Selcuk J. Agric. Food Sci. 2020, 34, 31–41. [Google Scholar]
- Jamshidi Goharrizi, K.; Riahi-Madvar, A.; Rezaee, F.; Pakzad, R.; Jadid Bonyad, F.; Ghazizadeh Ahsaei, M. Effect of Salinity Stress on Enzymes’ Activity, Ions Concentration, Oxidative Stress Parameters, Biochemical Traits, Content of Sulforaphane, and CYP79F1 Gene Expression Level in Lepidium draba Plant. J. Plant Growth Regul. 2020, 39, 1075–1094. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Baghizadeh, A.; Kalantar, M.; Fatehi, F. Assessment of Changes in Some Biochemical Traits and Proteomic Profile of UCB-1 Pistachio Rootstock Leaf under Salinity Stress. J. Plant Growth Regul. 2020, 39, 608–630. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Amirmahani, F.; Salehi, F. Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought + salinity stresses. Physiol. Plant. 2020, 168, 973–989. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Baghizadeh, A.; Kalantar, M.; Fatehi, F. Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci. Hortic. 2019, 261, 108970. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.E. Nitrogen fertilizer alleviated negative impacts of nacl on some physiological parameters of wheat. Pak. J. Bot. 2018, 50, 2097–2104. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.P.; Silva-Santos, L.; Souza, L.; Morais, M.; Corte-Real, N.; Monte Junior, I.P.; da Camara, C.A.G.; de Moraes, M.M.; Ulisses, C. Mycorrhization changes the antioxidant response and chemical profile of Lippia alba (Verbenaceae) essential oil under salinity conditions. S. Afr. J. Bot. 2023, 152, 264–277. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [Green Version]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress Memory and the Inevitable Effects of Drought: A Physiological Perspective. Front. Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Galviz, Y.C.F.; Ribeiro, R.V.; Souza, G.M. Yes, plants do have memory. Theor. Exp. Plant Physiol. 2020, 32, 195–202. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S. Salinity effect on plant growth, PSII photochemistry and chlorophyll content in sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea Capitata L.). Pak. J. Bot. 2007, 39, 753–760. [Google Scholar]
- Misra, A.N.; Sahu, S.M.; Misra, M.; Singh, P.; Meera, I.; Das, N.; Kar, M.; Sahu, P. Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 1997, 39, 257–262. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Ali, Q.; Alam, M.W.; Haider, M.S.; Mehboob, W. Triacontanol modulates salt stress tolerance in cucumber by altering the physiological and biochemical status of plant cells. Sci. Rep. 2021, 11, e24504. [Google Scholar] [CrossRef] [PubMed]
- Khademian, R.; Asghari, B.; Sedaghati, B.; Yaghoubian, Y. Plant beneficial rhizospheric microorganisms (PBRMs) mitigate deleterious effects of salinity in sesame (Sesamum indicum L.): Physio-biochemical properties, fatty acids composition and secondary metabolites content. Ind. Crops Prod. 2019, 136, 129–139. [Google Scholar] [CrossRef]
- Li, Y. Physiological Responses of Tomato Seedlings (Lycopersicon esculentum) to Salt Stress. Mod. Appl. Sci. 2009, 3, p171. [Google Scholar] [CrossRef] [Green Version]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.M.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 2019, 141, 466–476. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, S.; Roy, S.K.; Woo, S.H.; Sonawane, K.D.; Shohael, A.M. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar] [CrossRef]
- Yavuz, N.; Seymen, M.; Kal, Ü. Impacts of water stress and harvest time on physio-biochemical characteristics of lettuce. Int. J. Agric. Nat. Sci. 2021, 14, 61–77. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Zhang, W.; Du, T. Fresh/brackish watering at growth period provided a trade-off between lettuce growth and resistance to NaCl-induced damage. Sci. Hortic. 2022, 304, 111283. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Freitas, W.E.d.S.; Oliveira, A.B.d.; Mesquita, R.O.; Carvalho, H.H.d.; Prisco, J.T.; Gomes-Filho, E. Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake, lower Na/K ratio and an efficient antioxidative defense system. Sci. Hortic. 2019, 257, 108764. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Zheng, C.; Cao, C.; Sun, C.; Dang, H.; Feng, D.; Sun, J. Cotton Responses to Saline Water Irrigation in the Low Plain around the Bohai Sea in China. J. Irrig. Drain. Eng. 2018, 144, 04018027. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Liu, C.; Cui, B.; Zeleke, K.T.; Hu, C.; Wu, H.; Cui, E.; Huang, P.; Gao, F. Risk of Secondary Soil Salinization under Mixed Irrigation Using Brackish Water and Reclaimed Water. Agronomy 2021, 11, 2039. [Google Scholar] [CrossRef]
- Liu, C.; Cui, B.; Hu, C.; Wu, H.; Gao, F. Effects of mixed irrigation using brackish water with different salinities and reclaimed water on a soil-crop system. J. Water Reuse Desalination 2021, 11, 632–648. [Google Scholar] [CrossRef]


| Treatment | Na+ Content/(mg·kg−1) | Cl− Content/(mg·kg−1) |
|---|---|---|
| B3 | 676.67 ± 68.25 b | 175.92 ± 17.68 b |
| FB3 | 230.00 ± 8.66 ef | 96.90 ± 0.68 ef |
| RB3 | 351.67 ± 2.89 d | 112.55 ± 1.33 de |
| FFB3 | 152.17 ± 0.76 f | 95.71 ± 0.77 ef |
| RRB3 | 338.33 ± 23.09 d | 107.83 ± 0.93 de |
| B5 | 1098.33 ± 146.32 a | 257.75 ± 9.57 a |
| FB5 | 398.33 ± 7.64 d | 131.31 ± 1.79 c |
| RB5 | 480.00 ± 13.23 c | 132.79 ± 1.11 c |
| FFB5 | 231.67 ± 7.64 ef | 105.61 ± 2.71 e |
| RRB5 | 425.00 ± 5.00 cd | 118.61 ± 3.58 d |
| R | 235.00 ± 5.00 e | 93.21 ± 7.29 f |
| F | 43.67 ± 1.04 g | 61.74 ± 3.69 g |
| Treatment | S-AKP/ALP Activity /(U·g−1) | S-SC Activity /(mg·g−1·24 h−1) | S-UE Activity /(mg·g−1·24 h−1) |
|---|---|---|---|
| B3 | 2105.95 ± 344.81 bc | 9.59 ± 0.40 c | 0.39 ± 0.01 e |
| FB3 | 1881.55 ± 431.21 c | 11.37 ± 0.44 ab | 0.46 ± 0.00 c |
| RB3 | 2744.64 ± 719.43 b | 10.73 ± 0.26 bc | 0.42 ± 0.01 d |
| FFB3 | 2641.07 ± 773.33 bc | 10.91 ± 0.64 bc | 0.45 ± 0.01 c |
| RRB3 | 2623.81 ± 837.16 bc | 12.16 ± 1.16 a | 0.44 ± 0.01 c |
| B5 | 2313.09 ± 367.4 bc | 10.43 ± 0.55 bc | 0.39 ± 0.01 e |
| FB5 | 3935.71 ± 499.41 a | 10.04 ± 0.84 bc | 0.48 ± 0.01 b |
| RB5 | 3521.43 ± 404.46 ab | 11.69 ± 0.41 ab | 0.45 ± 0.01 c |
| FFB5 | 2364.88 ± 239.18 bc | 11.71 ± 0.68 ab | 0.48 ± 0.01 b |
| RRB5 | 2606.55 ± 374.63 bc | 10.92 ± 0.89 bc | 0.51 ± 0.02 a |
| R | 3901.19 ± 528.96 a | 11.01 ± 0.39 b | 0.45 ± 0.02 c |
| F | 3348.81 ± 29.9 ab | 10.86 ± 1.13 bc | 0.41 ± 0.01 de |
| Treatment | Content of Chlorophyll a/(mg·g−1) | Content of Chlorophyll b/(mg·g−1) | Content of Total Chlorophyll/(mg·g−1) |
|---|---|---|---|
| B3 | 1.67 ± 0.18 ab | 0.43 ± 0.01 cd | 2.1 ± 0.18 ab |
| FB3 | 1.5 ± 0.08 b | 0.47 ± 0.02 c | 1.96 ± 0.1 b |
| RB3 | 1.92 ± 0.14 a | 0.52 ± 0.02 b | 2.44 ± 0.13 a |
| FFB3 | 1.59 ± 0.08 ab | 0.5 ± 0.02 bc | 2.08 ± 0.1 ab |
| RRB3 | 1.73 ± 0.03 ab | 0.56 ± 0.07 a | 2.29 ± 0.1 ab |
| B5 | 1.78 ± 0.11 ab | 0.44 ± 0.01 cd | 2.22 ± 0.1 ab |
| FB5 | 1.65 ± 0.07 ab | 0.49 ± 0.02 bc | 2.14 ± 0.09 ab |
| RB5 | 1.78 ± 0.29 ab | 0.47 ± 0.02 c | 2.25 ± 0.29 ab |
| FFB5 | 1.46 ± 0.07 b | 0.46 ± 0.02 c | 1.92 ± 0.09 b |
| RRB5 | 1.77 ± 0.3 ab | 0.45 ± 0.02 cd | 2.21 ± 0.29 ab |
| R | 1.62 ± 0.38 ab | 0.44 ± 0.02 cd | 2.07 ± 0.39 b |
| F | 1.85 ± 0.26 a | 0.41 ± 0.03 d | 2.26 ± 0.25 ab |
| Treatment | Na+ Content in Soil/(mg·g−1) | Na+ Content in Leaf/(mg·g−1) |
|---|---|---|
| B3 | 0.68 ± 0.0683 b | 15.07 ± 0.26 b |
| FB3 | 0.23 ± 0.0087 ef | 10.01 ± 0.04 e |
| RB3 | 0.35 ± 0.0029 d | 12.36 ± 0.33 cd |
| FFB3 | 0.15 ± 0.0008 f | 11.60 ± 0.43 d |
| RRB3 | 0.34 ± 0.0231 d | 12.43 ± 0.25 cd |
| B5 | 1.1 ± 0.1463 a | 17.85 ± 1.26 a |
| FB5 | 0.4 ± 0.0076 cd | 14.63 ± 0.54 b |
| RB5 | 0.48 ± 0.0132 c | 14.34 ± 1.06 b |
| FFB5 | 0.23 ± 0.0076 ef | 12.86 ± 0.83 c |
| RRB5 | 0.43 ± 0.005 cd | 13.01 ± 0.67 c |
| R | 0.24 ± 0.005 e | 10.02 ± 0.18 e |
| F | 0.04 ± 0.001 g | 6.85 ± 0.73 f |
| Treatment | B3 | FB3 | FFB3 | B5 | FB5 | FFB5 | F | RB3 | RRB3 | RB5 | RRB5 | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation water | BW3 | FW- BW3 | FW-FW- BW3 | BW5 | FW- BW5 | FW-FW- BW5 | FW | RW- BW3 | RW-RW- BW3 | RW- BW5 | RW-RW- BW5 | RW |
| Water Source | EC | pH | Na+ | K+ | HCO3− | Cl− | Ca2+ | Mg2+ | SO42− | SAR | TN | TP | Pb | Cu | Zn | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW | 321 | 8.31 | 0.4 | 0.04 | 1.96 | 0.85 | 0.98 | 0.61 | 1.08 | 0.34 | 1.17 | 0.02 | - | - | - | - |
| RW | 2120 | 8.17 | 13.5 | 0.36 | 4.56 | 8.85 | 2.28 | 3.10 | 5.28 | 5.81 | 0.52 | 0.05 | - | - | - | - |
| BW3 | 6100 | 8.41 | 57.8 | 0.05 | 2.32 | 54.20 | 1.08 | 0.71 | 0.96 | 43.21 | 1.31 | 0.02 | - | - | - | - |
| BW5 | 9432 | 8.44 | 87.0 | 0.07 | 2.28 | 90.90 | 0.92 | 0.77 | 1.14 | 66.86 | 1.18 | 0.02 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, J.; Huang, P.; Hu, C.; Gao, F.; Liu, Y.; Li, Z.; Cui, B. Response of Soil Microenvironment and Crop Growth to Cyclic Irrigation Using Reclaimed Water and Brackish Water. Plants 2023, 12, 2285. https://doi.org/10.3390/plants12122285
Liu C, Wang J, Huang P, Hu C, Gao F, Liu Y, Li Z, Cui B. Response of Soil Microenvironment and Crop Growth to Cyclic Irrigation Using Reclaimed Water and Brackish Water. Plants. 2023; 12(12):2285. https://doi.org/10.3390/plants12122285
Chicago/Turabian StyleLiu, Chuncheng, Juan Wang, Pengfei Huang, Chao Hu, Feng Gao, Yuan Liu, Zhongyang Li, and Bingjian Cui. 2023. "Response of Soil Microenvironment and Crop Growth to Cyclic Irrigation Using Reclaimed Water and Brackish Water" Plants 12, no. 12: 2285. https://doi.org/10.3390/plants12122285
APA StyleLiu, C., Wang, J., Huang, P., Hu, C., Gao, F., Liu, Y., Li, Z., & Cui, B. (2023). Response of Soil Microenvironment and Crop Growth to Cyclic Irrigation Using Reclaimed Water and Brackish Water. Plants, 12(12), 2285. https://doi.org/10.3390/plants12122285













