Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats
Abstract
:1. Introduction
2. Results
2.1. Differences in the Concentrations of HCO3− in Three Simulated Habitats under Different Drought Periods
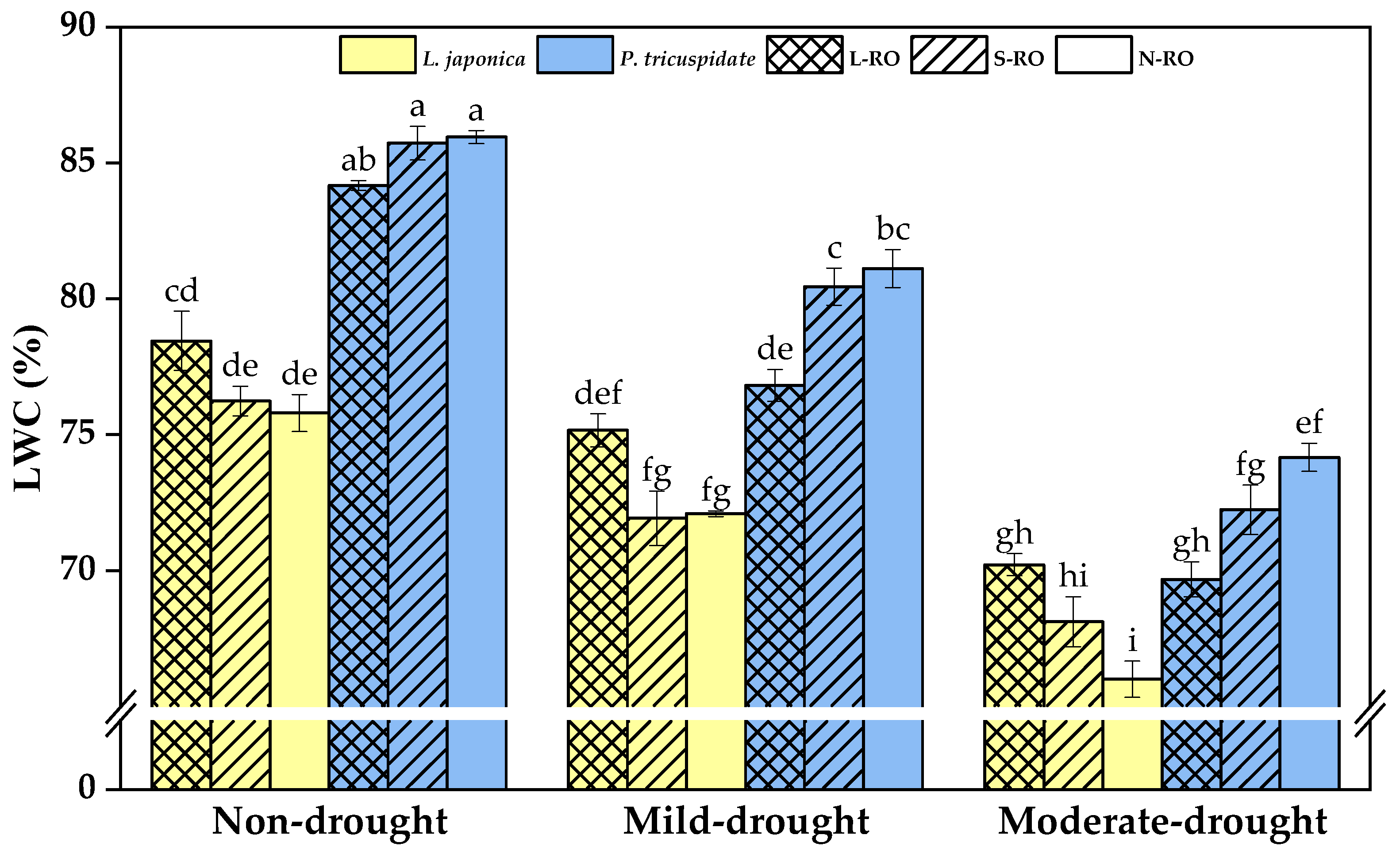
2.2. Differential Analysis of Water Content and Electrophysiological Parameters of L. japonica and P. quinquefolia Leaves in Three Simulated Habitats under Different Drought Periods
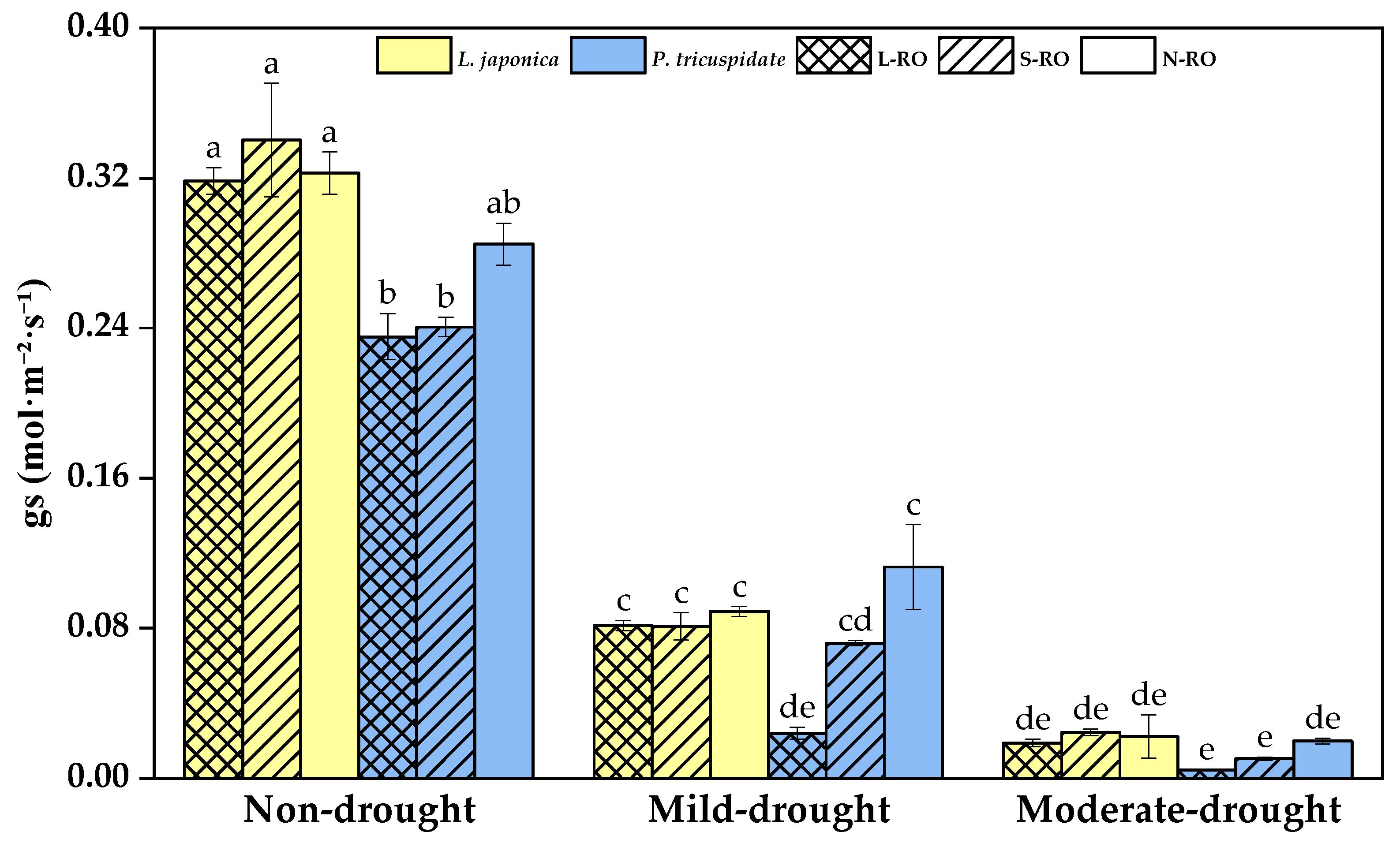
2.3. Differential Analysis of Photosynthetic Parameters of L. japonica and P. quinquefolia Leaves in Three Simulated Habitats under Different Drought Periods
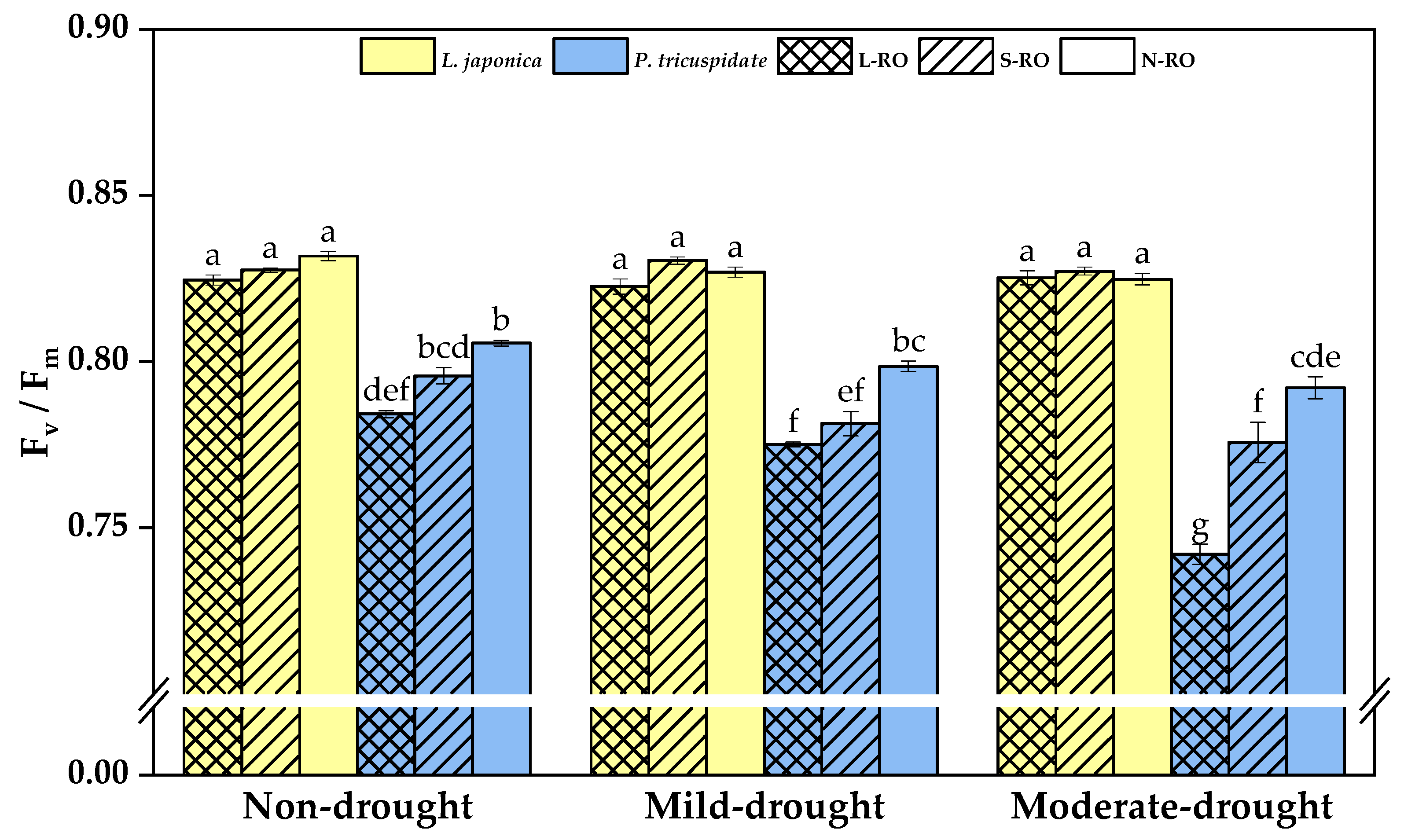
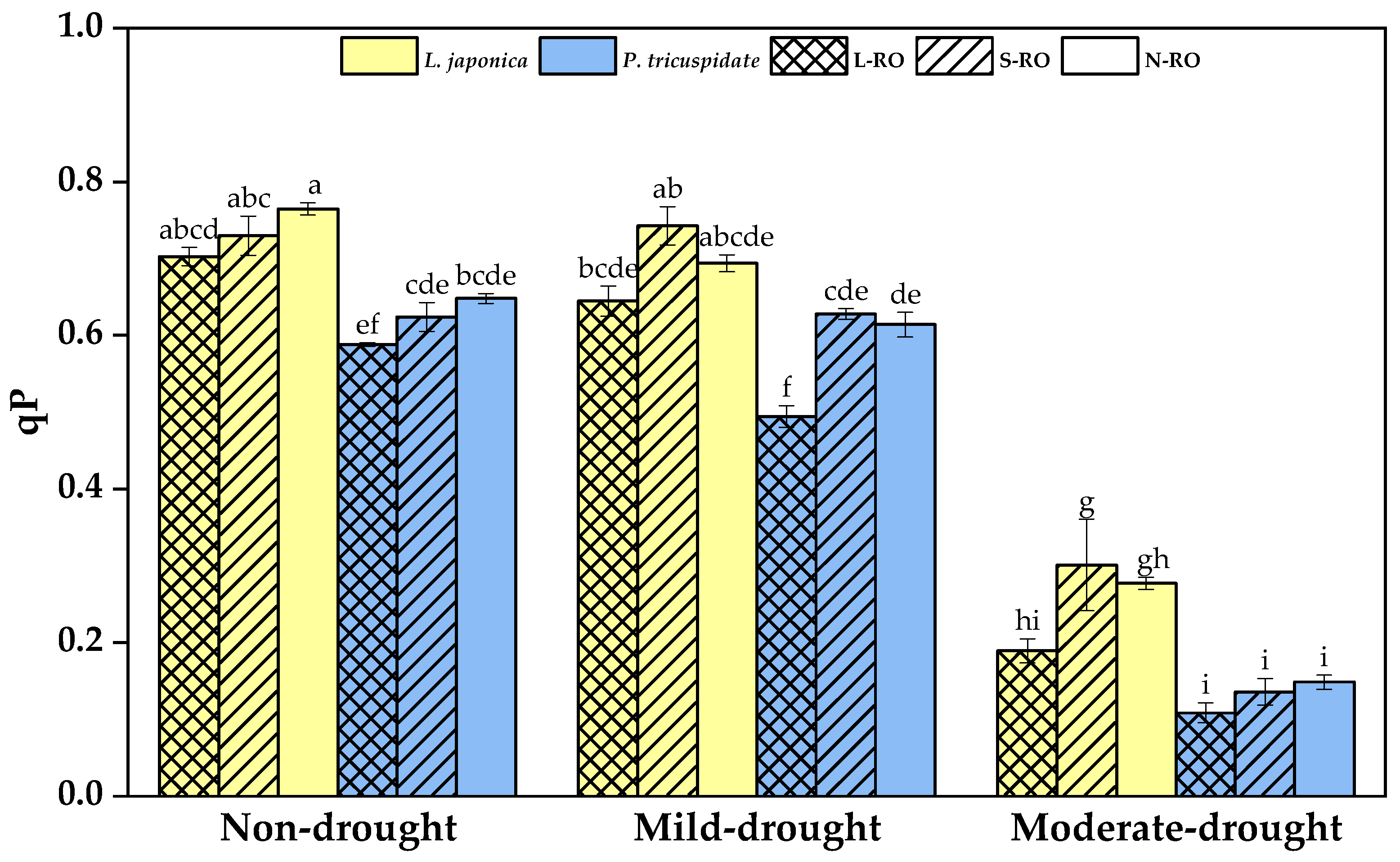
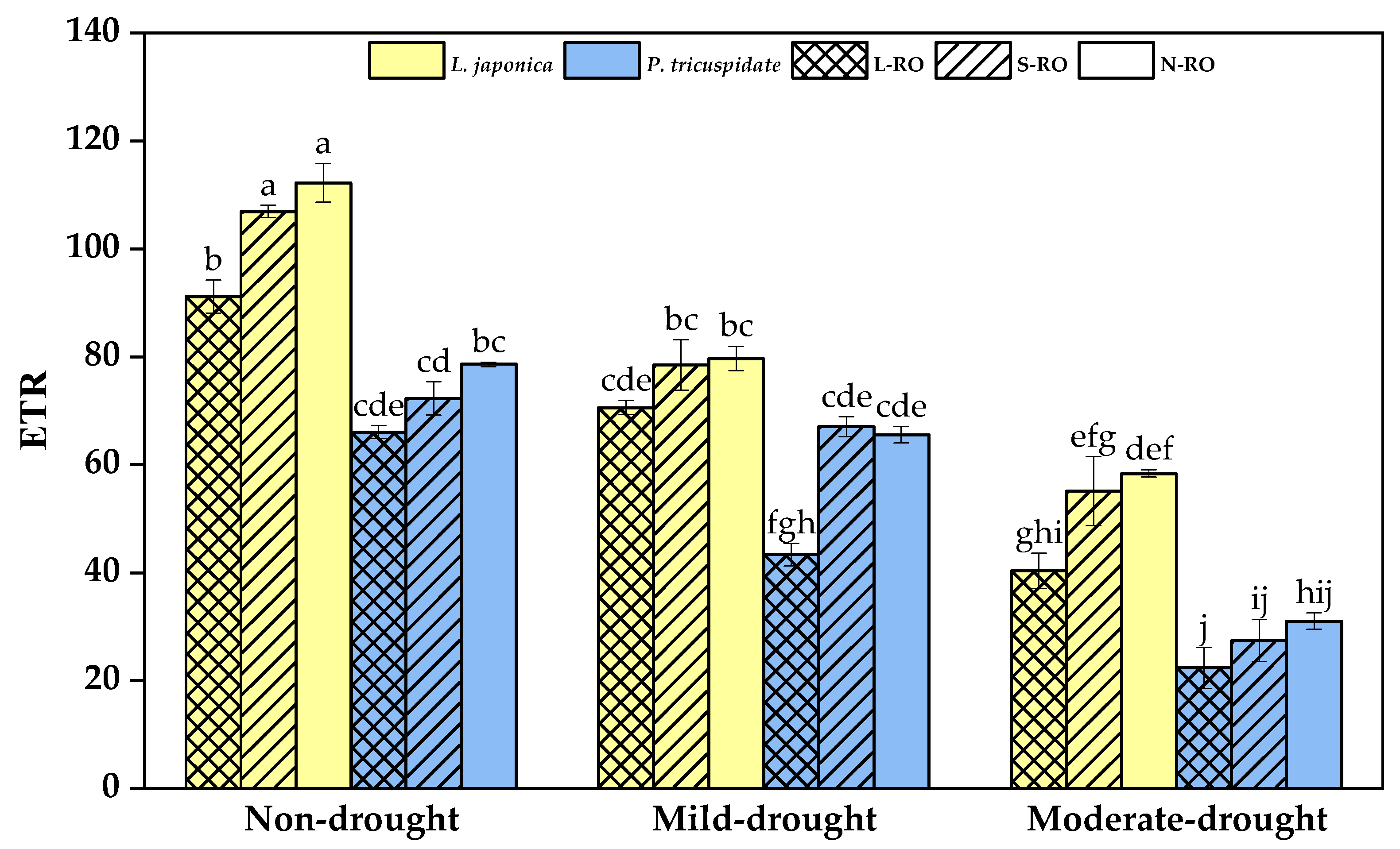
2.4. Differential Analysis of Fluorescence Parameters of L. japonica and P. quinquefolia Leaves in Three Simulated Habitats under Different Drought Periods
3. Discussion
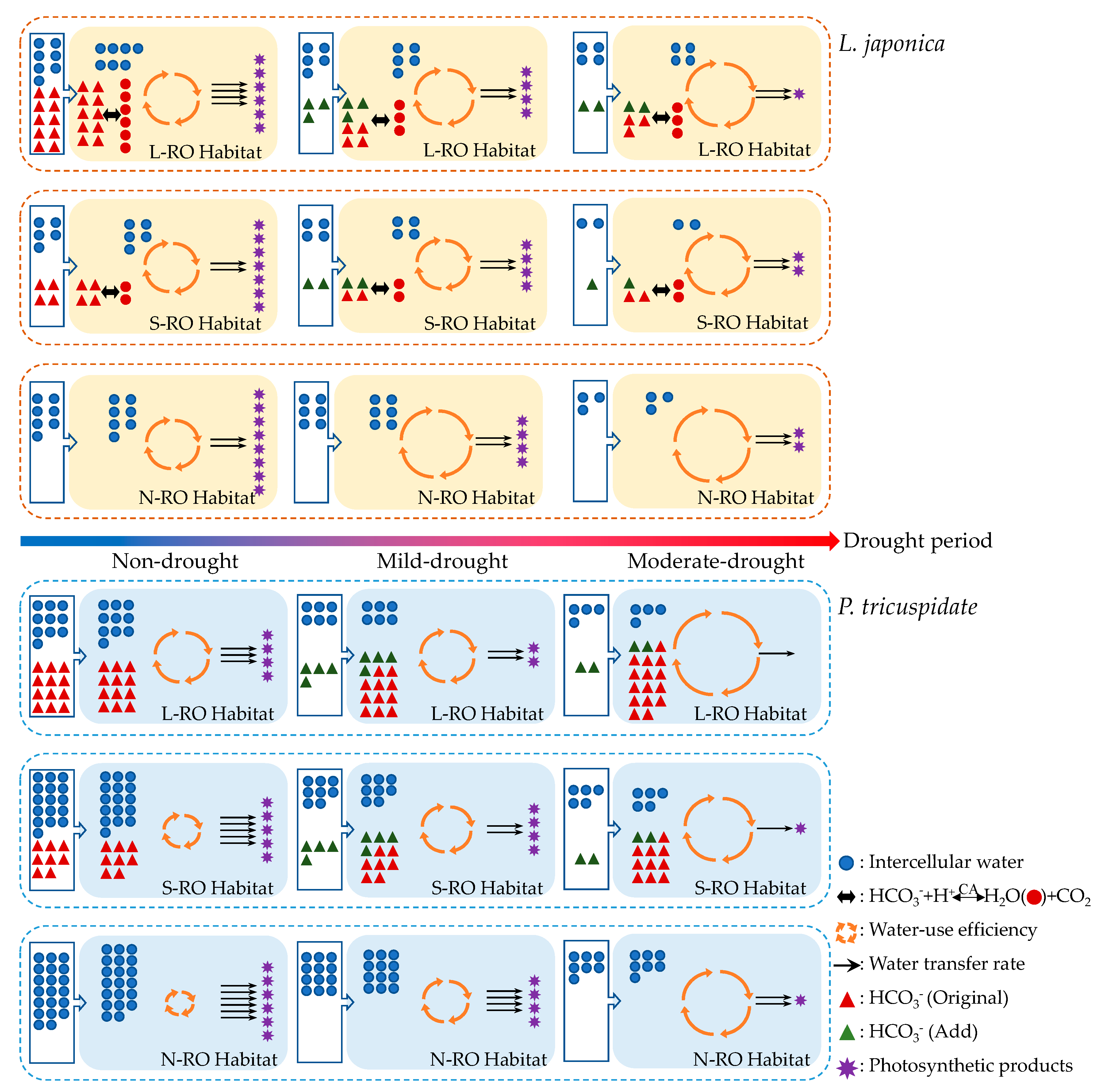
3.1. Analysis of the Intra- and Intercellular Water Metabolic Response Mechanisms of L. japonica Leaves in Heterogeneous RO Habitats
3.2. Analysis of the Intra- and Intercellular Water Metabolic Response Mechanisms of P. quinquefolia Leaves in Heterogeneous RO Habitats
4. Materials and Methods
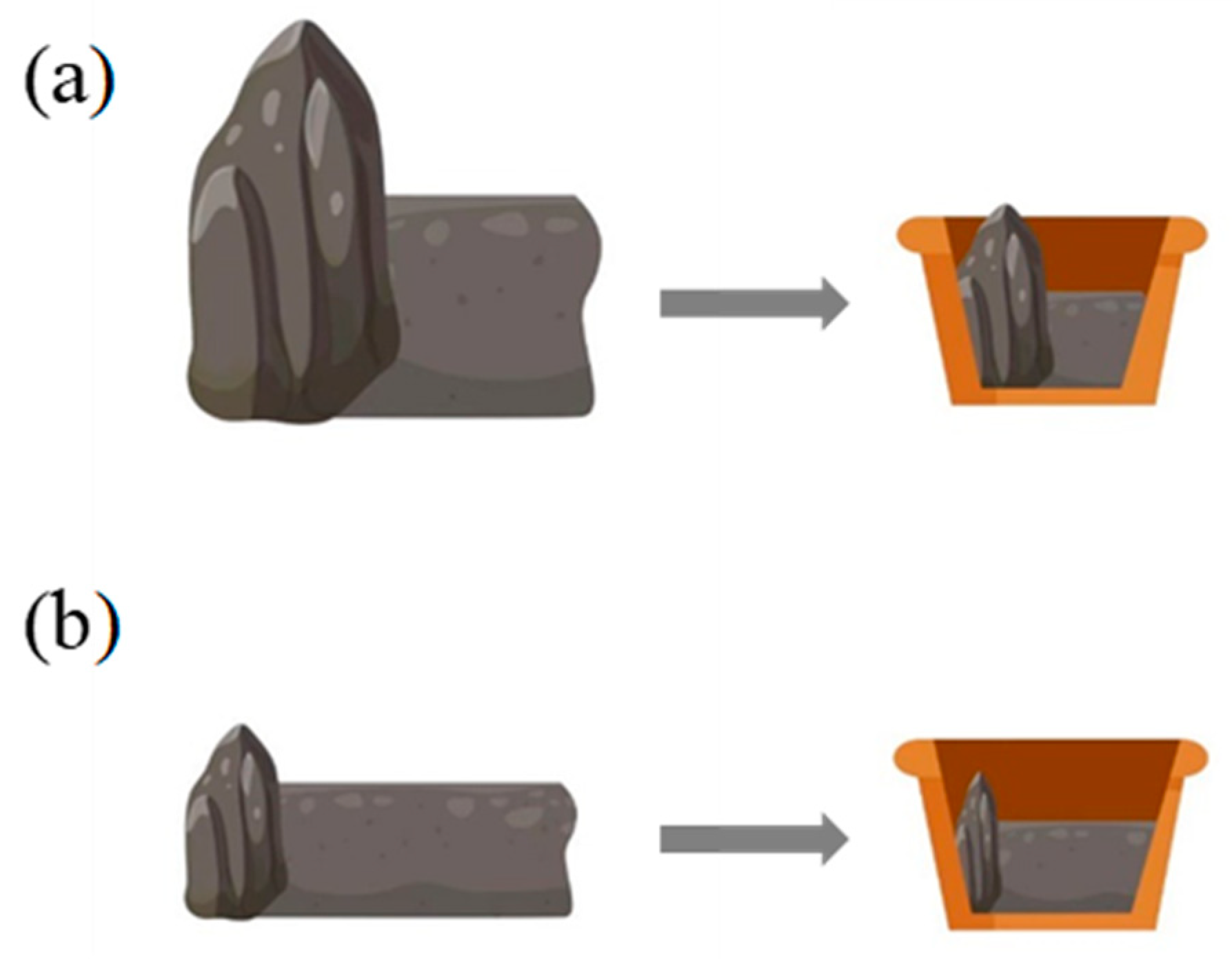
4.1. Simulation of Heterogeneous RO Habitats in Natural Environment
4.2. Plant Material and Water Control
4.3. Theory and Methods for Obtaining Index Parameters
4.3.1. Selection of Plant Leaf Materials and Data Collection Time
4.3.2. The Obtaining of Photosynthetic Parameters
4.3.3. The Obtaining of Fluorescence Parameters
4.3.4. Calculation of the Water Content of Plant Leaves
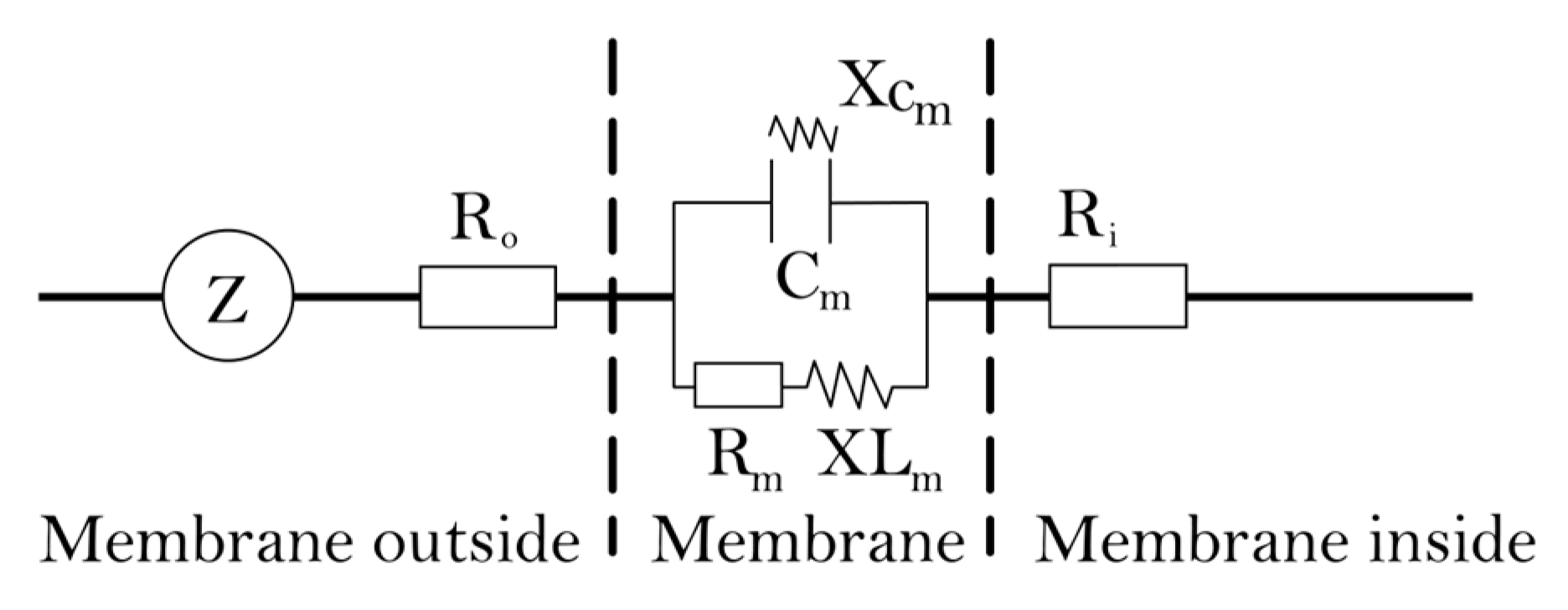
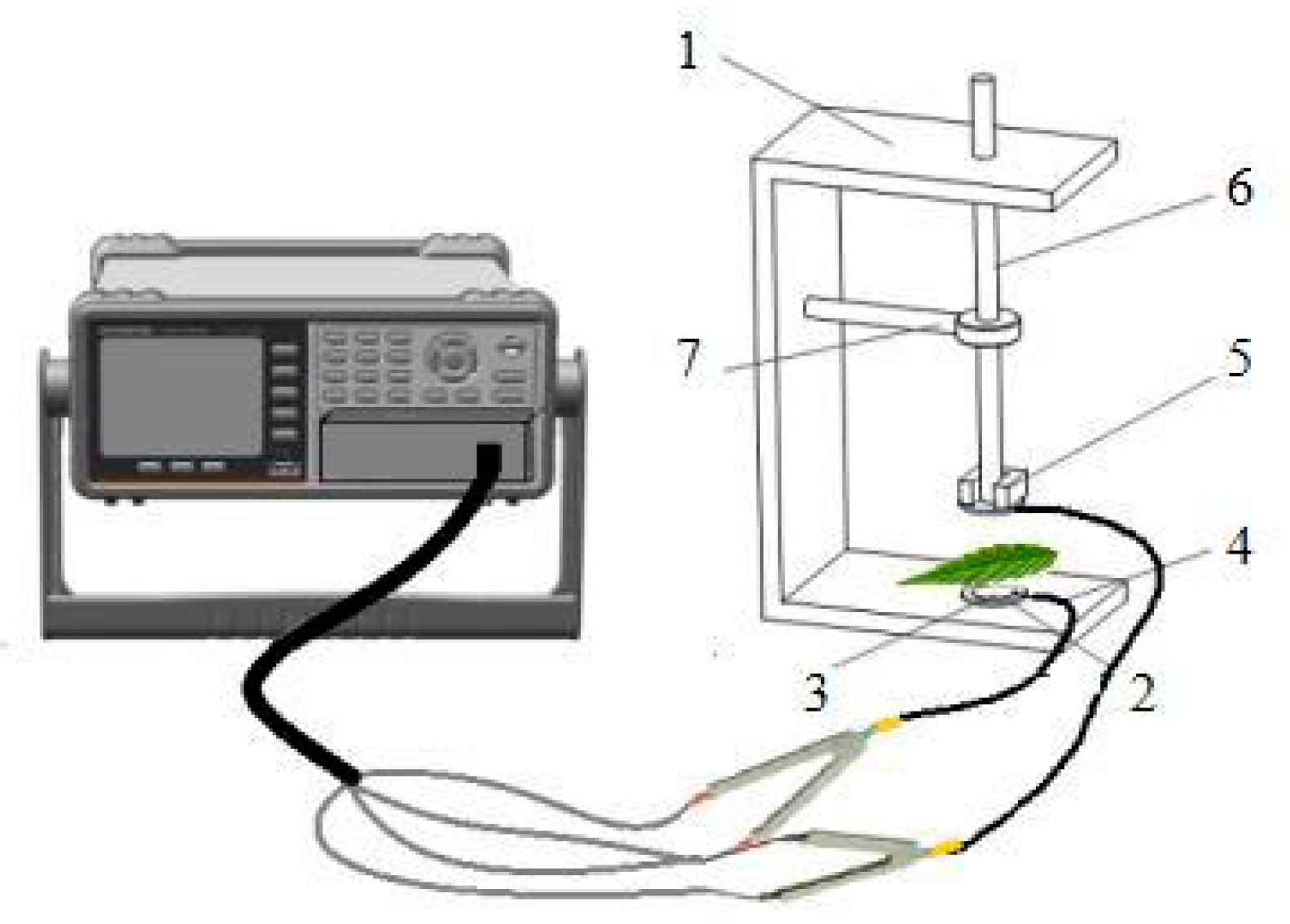
4.3.5. The Theory and Modeling of Electrophysiological Parameters
4.4. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.L.; Zhang, C.H.; Chen, H.S.; Yue, Y.M.; Zhang, W.; Zhang, M.Y.; Qi, X.K.; Fu, Z.Y. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.M.; Shen, Y.X.; Jiang, R.H.; Wang, Q.H. Rock outcrops change infiltrability and water flow behavior in a karst soil. Vadose Zone J. 2020, 19, e20002. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.X.; Yu, Y.; Lucas-Borja, M.E.; Chen, F.J.; Chen, Q.Q.; Tang, Y.Y. Change of soil K, N and P following forest restoration in rock outcrop rich karst area. Catena 2020, 186, 104395. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.S.; Lian, J.J.; Fu, Z.Y.; Nie, Y.P. Hydrological response of karst stream to precipitation variation recognized through the quantitative separation of runoff components. Sci. Total Environ. 2020, 748, 142483. [Google Scholar] [CrossRef]
- Nico, G.; Zhao, C.; Augusto, S.A.; Michel, B.; Stefan, B.; David, D.; Jens, H.; Jiang, G.H.; Nils, M.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar]
- Zhang, C. Carbonate rock dissolution rates in different landuses and their carbon sink effect. Chin. Sci. Bull. 2011, 56, 3759–3765. [Google Scholar] [CrossRef] [Green Version]
- McCray, J.M.; Matocha, J.E. Effects of soil water levels on solution bicarbonate, chlorosis and growth of sorghum. J. Plant Nutr. 1992, 15, 1877–1890. [Google Scholar] [CrossRef]
- Shen, Y.X.; Wang, D.J.; Chen, Q.Q.; Tang, Y.Y.; Chen, F.J. Large heterogeneity of water and nutrient supply derived from runoff of nearby rock outcrops in karst ecosystems in SW China. Catena 2019, 172, 125–131. [Google Scholar] [CrossRef]
- Sun, J.K.; He, L.; Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE 2019, 14, e0220340. [Google Scholar] [CrossRef] [Green Version]
- Hitti, Y.; MacPherson, S.; Lefsrud, M. Separate Effects of sodium on germination in saline–sodic and alkaline forms at different concentrations. Plants 2023, 12, 1234. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Du, G.H.; Yang, F.; Deng, G.; Yang, Y.; Liu, F.H. Fiber and seed type of hemp (Cannabis sativa L.) responded differently to salt-alkali stress in seedling growth and physiological indices. Ind. Crops Prod. 2019, 129, 624–630. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Wang, K. Physiological responses of Leymus chinensis to long-term salt, alkali and mixed salt-alkali stresses. J. Plant Nutr. 2015, 38, 526–540. [Google Scholar] [CrossRef]
- Sagervanshi, A.; Geilfus, G.M.; Kaiser, H.; Mühling, K.H. Alkali salt stress causes fast leaf apoplastic alkalinization together with shifts in ion and metabolite composition and transcription of key genes during the early adaptive response of Vicia faba L. Plant Sci. 2022, 319, 111253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Lu, X.Y.; Tao, Y.F.; Guo, H.J.; Min, W. Comparative ionomics and metabolic responses and adaptive strategies of cotton to salt and alkali stress. Front. Plant Sci. 2022, 13, 871387. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Li, Y.X.; Zhang, X.Z.; Liu, C.L.; Liang, W.; Li, C.; Ma, F.W.; Li, C.Y. Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Shevela, D.; Do, H.N.; Fantuzzi, A.; Rutherford, A.W.; Messinger, J. Bicarbonate-mediated CO2 formation on both sides of Photosystem II. Biochemistry 2020, 59, 2442–2449. [Google Scholar] [CrossRef]
- Koroidov, S.; Shevela, D.; Shutova, T.; Samuelsson, G.; Messinger, J. Mobile hydrogen carbonate acts as proton acceptor in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 6299–6304. [Google Scholar] [CrossRef] [Green Version]
- Lü, X.F.; He, Q.F.; Wang, Z.J.; Cao, M.; Zhao, J.Y.; Jiang, J.J.; Zhao, R.Y.; Zhang, H. Calcium carbonate precipitation mediated by bacterial carbonic anhydrase in a karst cave: Crystal morphology and stable isotopic fractionation. Chem. Geol. 2019, 530, 119331. [Google Scholar] [CrossRef]
- Wu, Y.Y. Combined effect of bicarbonate and water in photosynthetic oxygen evolution and carbon neutrality. Acta Geochim. 2023, 42, 77–88. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.Y.; Xing, D.K.; Hang, H.T.; Xie, X.L.; Yang, X.Q.; Zhang, K.Y.; Rao, S. Biomass production of three biofuel energy plants’ use of a new carbon resource by carbonic anhydrase in simulated karst soils: Mechanism and capacity. Energies 2017, 10, 1370. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Sun, K.; Zhang, X.H.; Zhou, J.; Yang, Q.; Liu, Q. Microstructure changes of red clay during its loss and leakage in the karst rocky desertification area. Environ. Earth Sci. 2016, 75, 537. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, Y.G.; Guo, K.; Zheng, Y.R.; Li, G.Q.; Yu, L.F.; Yang, R. Influence of drought intensity on the response of six woody karst species subjected to successive cycles of drought and rewatering. Physiol. Plant. 2010, 139, 39–54. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-González, L.; Alarcón, N.; Bastías, R.; Pérez, C.; Sanz, R.; Peña-Neira, Á.; Pastenes, C. Photoprotection is achieved by photorespiration and modification of the leaf incident light, and their extent is modulated by the stomatal sensitivity to water deficit in grapevines. Plants 2022, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G. Plant Electrophysiology: Signaling and Responses; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Zhang, M.M.; Wu, Y.Y.; Xing, D.K.; Zhao, K.; Yu, R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Trans. ASABE 2015, 58, 1441–1446. [Google Scholar]
- Sinyukhin, A.M.; Britikov, E.A. Action potentials in the reproductive system of plants. Nature 1967, 215, 1278–1280. [Google Scholar] [CrossRef]
- Jamaludin, D.; Abd Aziz, S.; Ahmad, D.; Jaafar, H.Z. Impedance analysis of Labisia pumila plant water status. Inf. Process. Agric. 2015, 2, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Wu, Y.Y.; Xing, D.K. Can electrophysiological parameters substitute for growth, and photosynthetic parameters to characterize the response of mulberry and paper mulberry to drought? Plants 2021, 10, 1772. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Li, H.T.; Fang, L.; Xing, D.K. Plant’s electrophysiological information manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Xing, D.K.; Dai, Y.; Wu, Y.S.; Fang, L. A plant’s electrical parameters indicate its physiological state: A study of intracellular water metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Gromova, E.; Grinberg, M.; Popova, A.; Sukhova, E.; Sukhov, V. Influence of burning-induced electrical signals on photosynthesis in pea can be modified by soil water shortage. Plants 2022, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Dutoit, F.; Najdenovska, E.; Wallbridge, N.; Plummer, C.; Mazza, M.; Raileanu, L.E.; Camps, C. Electrophysiological assessment of plant status outside a Faraday cage using supervised machine learning. Sci. Rep. 2019, 9, 17073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.J.; Xing, D.K.; Wu, Y.Y.; Wang, W.X.; Li, M.Q.; Solangi, K. Diurnal variation in transport and use of intracellular leaf water and related photosynthesis in three karst plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Wu, D.D.; Cheng, G.; Li, H.Y.; Zhou, S.H.; Yao, N.; Zhang, J.; Xie, L.J. The cultivation techniques and quality characteristics of a new germplasm of Vitis adenoclada Hand.-Mazz grape. Agronomy 2020, 10, 1851. [Google Scholar] [CrossRef]
- He, W.J.; Yan, K.; Zhang, Y.; Bian, L.X.; Mei, H.M.; Han, G.X. Contrasting photosynthesis, photoinhibition and oxidative damage in honeysuckle (Lonicera japonica Thunb.) under iso-osmotic salt and drought stresses. Environ. Exp. Bot. 2021, 182, 104313. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.E.; Leontaridou, K.; Kanellis, A.K.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The effect of water deficit on two Greek Vitis vinifera L. cultivars: Physiology, grape composition and gene expression during berry development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.J.; Xiao, H.; Xu, W.N.; Xia, Z.Y. Selection and configuration of plants in high-and steep-cutting rock slope greening in a subtropical region: Case study of the first-phase urban expressway of Xiazhou Avenue in Yichang. J. Highw. Transp. Res. Dev. 2019, 13, 102–110. [Google Scholar] [CrossRef]
- Javed, Q.; Azeem, A.; Sun, J.; Ullah, I.; Jabran, K.; Anandkumar, A.; Prabakaran, K.; Buttar, N.A.; Du, D. Impacts of salt stress on the physiology of plants and opportunity to rewater the stressed plants with diluted water: A Review. Appl. Ecol. Environ. Res. 2019, 17, 12583–12604. [Google Scholar] [CrossRef]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; Leoni, B.; Crecchio, C. Photosynthetic responses of durum wheat to chemical/microbiological fertilization management under salt and drought stresses. Acta Physiol. Plant. 2021, 43, 123. [Google Scholar] [CrossRef]
- Xing, D.K.; Wu, Y.Y. Photosynthetic response of three climber plant species to osmotic stress induced by polyethylene glycol (PEG) 6000. Acta Physiol. Plant 2012, 34, 1659–1668. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Zhao, P.; Tan, Z.; Peng, Y.; Xu, L.; Jin, Y.; Wei, F.; Guo, L.; Yao, X. Combining Physio-Biochemical Characterization and Transcriptome Analysis Reveal the Responses to Varying Degrees of Drought Stress in Brassica napus L. Int. J. Mol. Sci. 2022, 23, 8555. [Google Scholar] [CrossRef]
- Xing, D.K.; Chen, L.; Wu, Y.Y.; Zwiazek, J.J. Leaf physiological impedance and elasticity modulus in Orychophragmus violaceus seedlings subjected to repeated osmotic stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]



| Plant Species | Drought Period | Simulated Habitat | ||
|---|---|---|---|---|
| L-RO | S-RO | N-RO | ||
| L. japonica | Non-drought | 388.73 ± 21.33 b | 207.51 ± 18.82 cdef | 206.70 ± 13.32 cdef |
| Mild | 256.81 ± 31.15 cd | 178.06 ± 25.20 defg | 181.18 ± 1.62 defg | |
| Moderate | 204.25 ± 18.82 cdef | 122.48 ± 16.91 efg | 92.20 ± 6.32 g | |
| P. quinquefolia | Non-drought | 296.87 ± 9.05 de | 502.43 ± 18.42 a | 599.11 ± 43.01 a |
| Mild | 176.80 ± 4.08 defg | 243.16 ± 23.80 cd | 376.03 ± 22.84 b | |
| Moderate | 113.90 ± 5.48 fg | 153.80 ± 19.74 defg | 221.39 ± 15.49 cde | |
| Plant Species | Drought Period | Simulated Habitat | ||
|---|---|---|---|---|
| L-RO | S-RO | N-RO | ||
| L. japonica | Non-drought | 7.92 ± 0.85 bc | 4.07 ± 0.37 efgh | 4.04 ± 0.26 efgh |
| Mild | 4.99 ± 0.55 ef | 3.50 ± 0.48 efghi | 3.48 ± 0.04 efghi | |
| Moderate | 4.02 ± 0.21 efgh | 2.50 ± 0.40 ghi | 1.77 ± 0.12 i | |
| P. quinquefolia | Non-drought | 5.60 ± 0.25 de | 9.64 ± 0.80 ab | 11.70 ± 0.78 a |
| Mild | 3.33 ± 0.06 fghi | 4.64 ± 0.44 efg | 7.21 ± 0.43 cd | |
| Moderate | 2.16 ± 0.10 hi | 2.92 ± 0.37 fghi | 4.23 ± 0.29 efgh | |
| Plant Species | Drought Period | Simulated Habitat | ||
|---|---|---|---|---|
| L-RO | S-RO | N-RO | ||
| L. japonica | Non-drought | 0.12 ± 0.00 bcd | 0.12 ± 0.01 bcde | 0.11 ± 0.01 cde |
| Mild | 0.13 ± 0.01 bcd | 0.12 ± 0.00 bcde | 0.16 ± 0.00 abc | |
| Moderate | 0.15 ± 0.01 abc | 0.14 ± 0.01 abc | 0.17 ± 0.01 ab | |
| P. quinquefolia | Non-drought | 0.12 ± 0.01 bcde | 0.08 ± 0.01 de | 0.06 ± 0.00 e |
| Mild | 0.12 ± 0.01 bcde | 0.12 ± 0.01 bcde | 0.11 ± 0.01 cde | |
| Moderate | 0.19 ± 0.03 a | 0.15 ± 0.02 abc | 0.15 ± 0.02 abc | |
| Plant Species | Simulated Habitat | Soil Moisture (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | ||
| L japonica | L-RO | 33.53 ± 0.27 | 27.57 ± 0.32 | 23.93 ± 0.03 | 20.03 ± 0.12 | 15.87 ± 0.54 | 12.73 ± 0.30 | 10.67 ± 0.41 | 9.73 ± 0.18 | 7.50 ± 0.21 | 6.10 ± 0.21 |
| S-RO | 35.73 ± 0.19 | 31.33 ± 0.24 | 26.20 ± 0.12 | 21.53 ± 0.19 | 17.03 ± 0.15 | 14.50 ± 0.32 | 11.30 ± 0.36 | 9.33 ± 0.21 | 7.87 ± 0.24 | 6.90 ± 0.06 | |
| N-RO | 35.53 ± 0.12 | 31.70 ± 0.15 | 26.77 ± 0.18 | 24.83 ± 0.63 | 18.53 ± 0.32 | 14.23 ± 0.37 | 10.63 ± 0.32 | 9.03 ± 0.18 | 7.97 ± 0.09 | 7.00 ± 0.12 | |
| P. quinquefolia | L-RO | 32.80 ± 0.40 | 27.07 ± 0.12 | 20.87 ± 0.13 | 14.57 ± 0.43 | 13.53 ± 0.33 | 12.00 ± 0.36 | 9.80 ± 0.43 | 8.43 ± 0.27 | 7.87 ± 0.21 | 6.03 ± 0.15 |
| S-RO | 34.70 ± 0.21 | 31.63 ± 0.26 | 26.93 ± 0.07 | 18.77 ± 0.12 | 14.97 ± 0.18 | 13.73 ± 0.64 | 11.87 ± 0.27 | 8.37 ± 0.27 | 7.60 ± 0.23 | 6.57 ± 0.22 | |
| N-RO | 34.03 ± 0.12 | 30.77 ± 0.19 | 26.1 ± 0.12 | 19.70 ± 0.21 | 16.03 ± 0.38 | 13.30 ± 0.40 | 11.37 ± 0.22 | 9.50 ± 0.35 | 8.10 ± 0.42 | 6.70 ± 0.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wu, Y.; Xing, D.; Li, H.; Zhang, F. Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats. Plants 2023, 12, 2279. https://doi.org/10.3390/plants12122279
Zhao X, Wu Y, Xing D, Li H, Zhang F. Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats. Plants. 2023; 12(12):2279. https://doi.org/10.3390/plants12122279
Chicago/Turabian StyleZhao, Xiaopan, Yanyou Wu, Deke Xing, Haitao Li, and Furong Zhang. 2023. "Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats" Plants 12, no. 12: 2279. https://doi.org/10.3390/plants12122279
APA StyleZhao, X., Wu, Y., Xing, D., Li, H., & Zhang, F. (2023). Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats. Plants, 12(12), 2279. https://doi.org/10.3390/plants12122279








