Evaluating the Effectiveness of Calcium Silicate in Enhancing Soybean Growth and Yield
Abstract
1. Introduction
2. Results
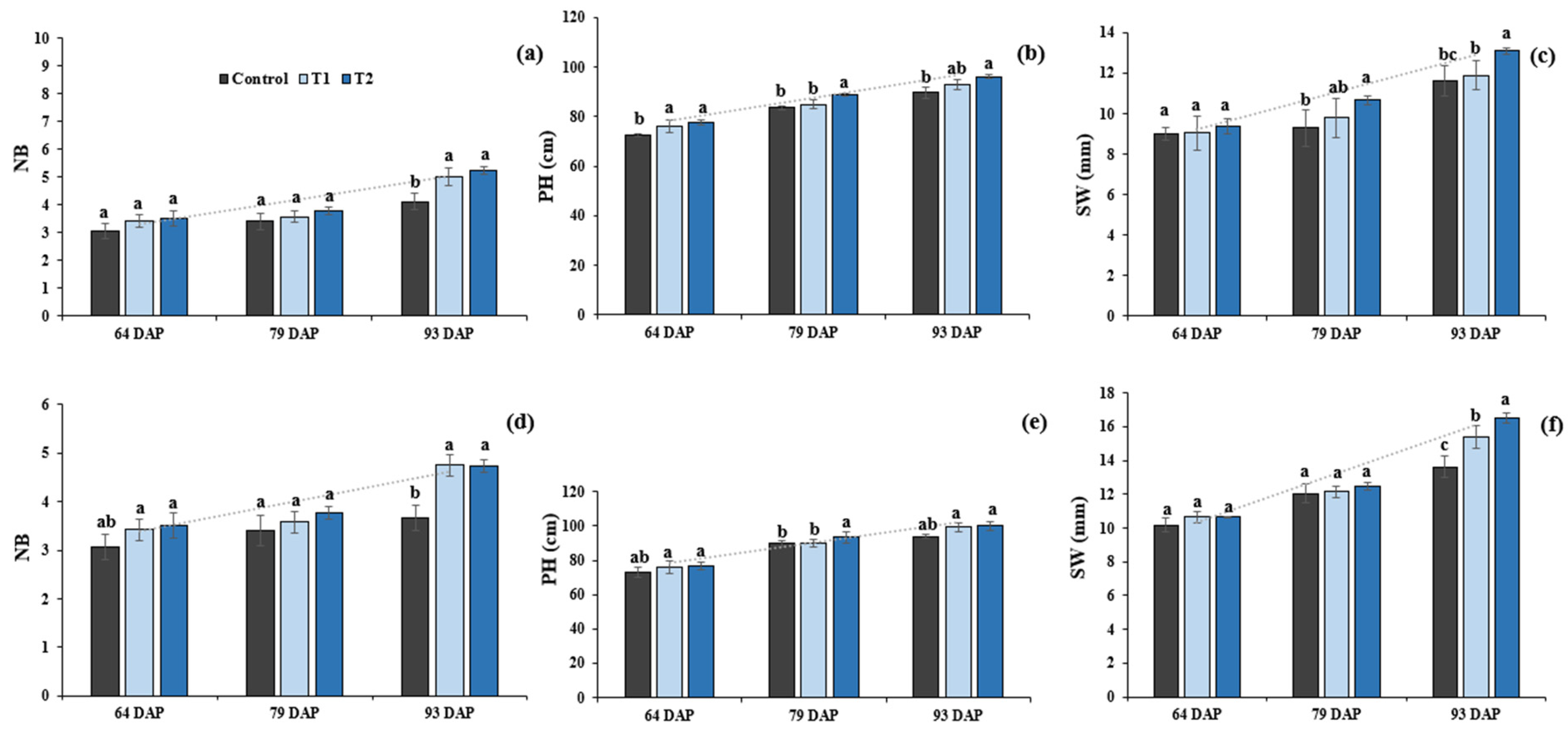
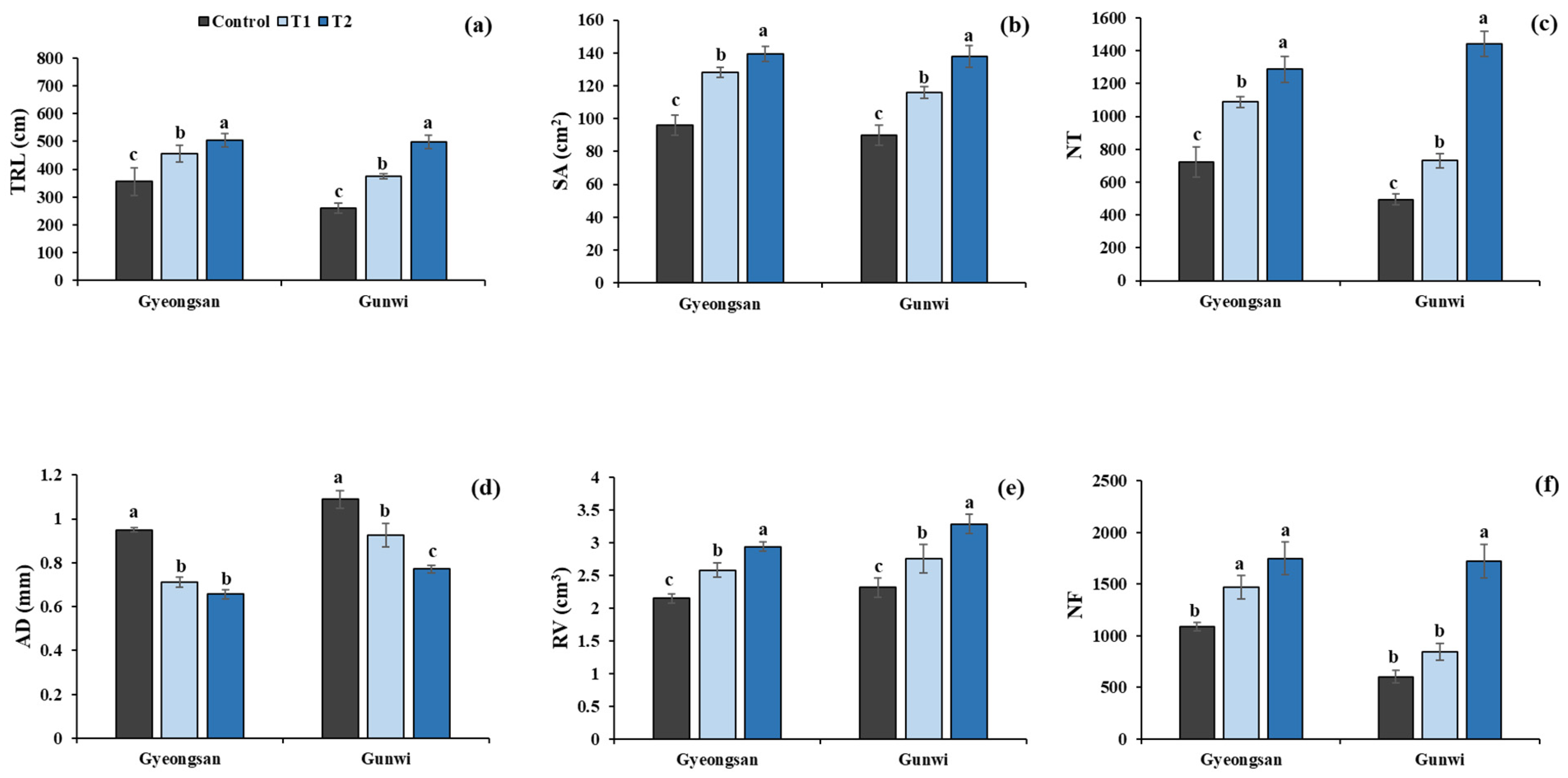
2.1. Effects of Si Treatment on Soybean Plant Attributes
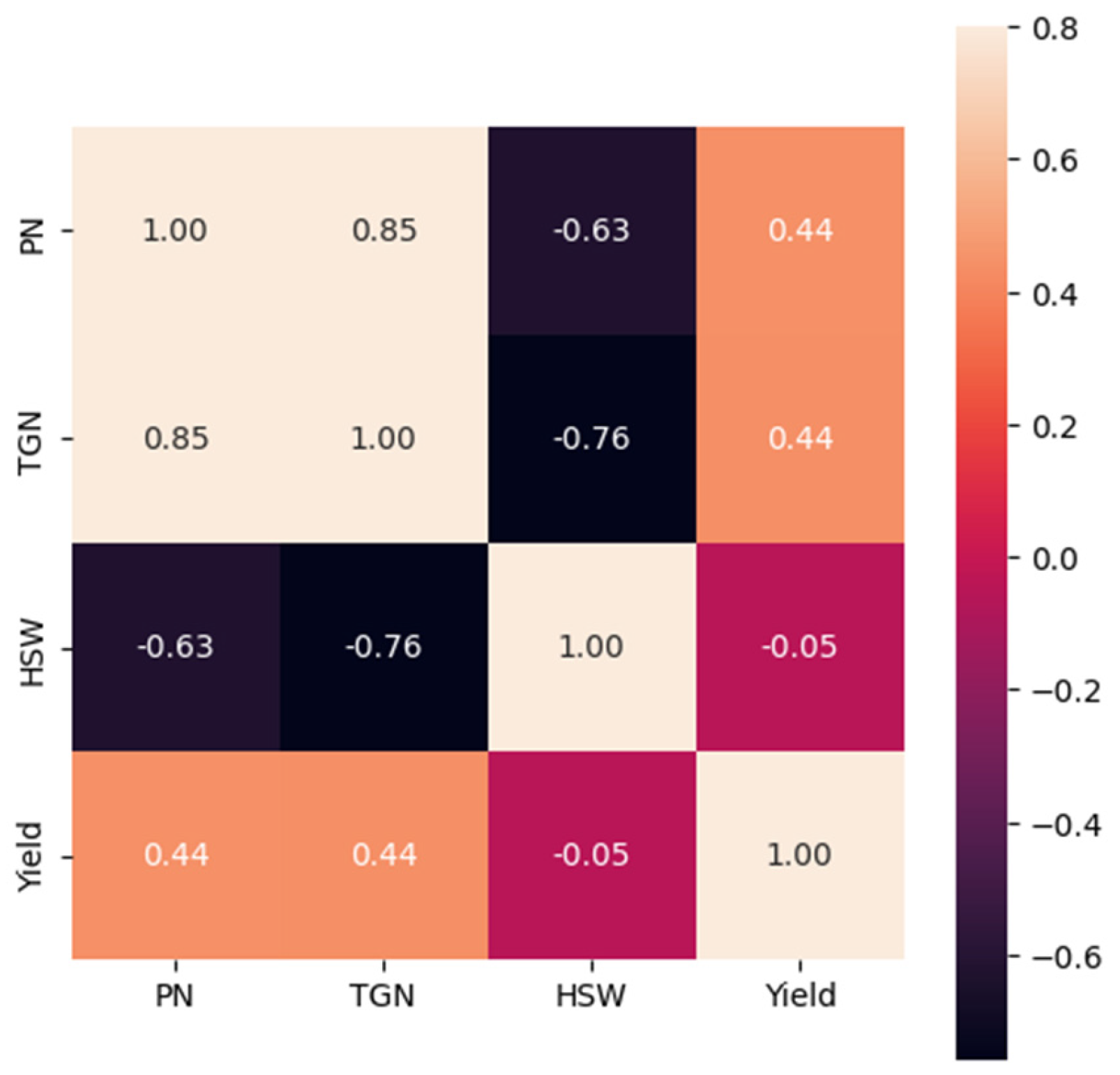
2.2. Correlations between Yield and Root and Shoot Traits
3. Discussion
4. Materials and Methods
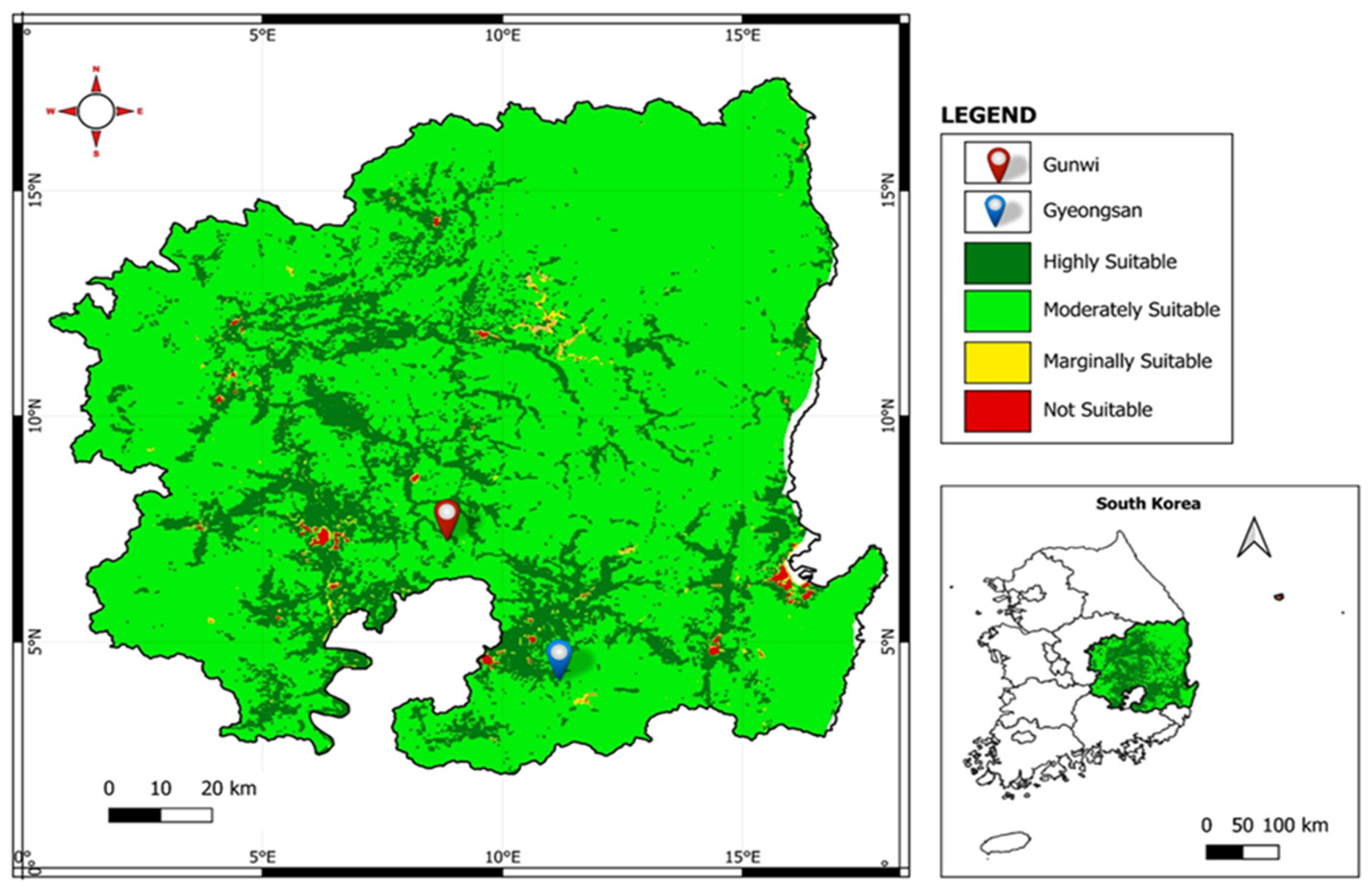
4.1. Land Suitability Analysis
4.2. Experimental Design and Treatments
4.3. Measurement of Agronomic Traits
4.3.1. Measurement of Shoot Characteristics (PH, Stem Width [SW], and the Number of Lateral Branches)
4.3.2. Measurement of Photosynthetic Parameters (Vegetative Indices and Chlorophyll Content)
4.3.3. Determination of Soybean Root and Yield Traits
4.3.4. Analysis of Root Morphological Traits
4.3.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, P.; Na, C.-I.; Kim, Y.-H. Effect of silicon fertilizer treatment on nodule formation and yield in soybean (Glycine max L.). Eur. J. Agron. 2021, 122, 126172. [Google Scholar] [CrossRef]
- Vogel, J.T.; Liu, W.; Olhoft, P.; Crafts-Brandner, S.J.; Pennycooke, J.C.; Christiansen, N. Soybean Yield Formation Physiology—A Foundation for Precision Breeding Based Improvement. Front. Plant Sci. 2021, 12, 719706. [Google Scholar] [CrossRef]
- Uppalige, S.; Nagabovanalli, P. Effect of Foliar Application of Silicic Acid on Soybean Yield and Seed Quality under Field Conditions. J. Indian Soc. Soil Sci. 2018, 66, 406. [Google Scholar] [CrossRef]
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic Improvement of U.S. Soybean in Maturity Groups II, III, and IV. Crop Sci. 2014, 54, 1419–1432. [Google Scholar] [CrossRef]
- Joshi-Paneri, J.; Sharma, S.; Guruprasad, K.N.; Kataria, S. Enhancing the Yield Potential of Soybean after Magneto-Priming: Detailed Study on Its Relation to Underlying Physiological Processes. Seeds 2023, 2, 6. [Google Scholar] [CrossRef]
- Shamshiripour, M.; Motesharezadeh, B.; Rahmani, H.A.; Alikhani, H.A.; Etesami, H. Optimal Concentrations of Silicon Enhance the Growth of Soybean (Glycine Max L.) Cultivars by Improving Nodulation, Root System Architecture, and Soil Biological Properties. Silicon 2022, 14, 5333–5345. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Lang, D.; Cui, J.; Zhang, X. Beneficial effects of silicon on abiotic stress tolerance in legumes. J. Plant Nutr. 2017, 40, 2224–2236. [Google Scholar] [CrossRef]
- Brahma, R.N.; Ahmed, P.; Choudhury, M.K. Silicon nutrition for alleviation of abiotic stress in plants: A review. J. Pharmacogn. Phytochem. 2020, 9, 1374–1381. [Google Scholar]
- Thakral, V.; Bhat, J.A.; Kumar, N.; Myaka, B.; Sudhakaran, S.; Patil, G.; Sonah, H.; Shivaraj, S.M.; Deshmukh, R. Role of silicon under contrasting biotic and abiotic stress conditions provides benefits for climate smart cropping. Environ. Exp. Bot. 2021, 189, 104545. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Sakurai, G.; Satake, A.; Yamaji, N.; Mitani-Ueno, N.; Yokozawa, M.; Feugier, F.G.; Ma, J.F. In silico simulation modeling reveals the importance of the Casparian strip for efficient silicon uptake in rice roots. Plant Cell Physiol. 2015, 56, 631–639. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar]
- Keeping, M.G.; Kvedaras, O.L.; Bruton, A.G. Epidermal silicon in sugarcane: Cultivar differences and role in resistance to sugarcane borer Eldana saccharina. Environ. Exp. Bot. 2009, 66, 54–60. [Google Scholar] [CrossRef]
- Han, Y.; Lei, W.; Wen, L.; Hou, M. Silicon-Mediated Resistance in a Susceptible Rice Variety to the Rice Leaf Folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0120557. [Google Scholar] [CrossRef]
- Haynes, R.J.; Belyaeva, O.N.; Kingston, G. Evaluation of industrial wastes as sources of fertilizer silicon using chemical extractions and plant uptake. J. Plant Nutr. Soil Sci. 2013, 176, 238–248. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Mauad, M.; Crusciol, C.A.C.; Nascente, A.S.; Grassi Filho, H.; Lima, G.P.P. Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars. Rev. Ciência Agronômica 2016, 47, 532–539. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon Improves Water Use Efficiency in Maize Plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Metalloid transporters and their regulation in plants. Plant Physiol. 2021, 187, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Bélanger, R.R. Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2016, 30, 1277–1285. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Chen, D.; Yin, L.; Li, H.; Deng, X. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front. Plant Sci. 2015, 6, 759. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.; Goyette, M.H.; Labbé, C.; Laur, J.; Gaudreau, L.; Gosselin, A.; Dorais, M.; Deshmukh, R.K.; Bélanger, R.R. Silicon Transporters and Effects of Silicon Amendments in Strawberry under High Tunnel and Field Conditions. Front. Plant Sci. 2017, 8, 949. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Jugdaohsingh, R.; Vivancos, J.; Marron, A.; Deshmukh, R.; Ma, J.F.; Mitani-Ueno, N.; Robertson, J.; Wills, J.; Boekschoten, M.V.; et al. Identification of a mammalian silicon transporter. Am. J. Physiol. Cell Physiol. 2017, 312, C550–C561. [Google Scholar] [CrossRef]
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Law, C.; Exley, C. New insight into silica deposition in horsetail (Equisetum arvense). BMC Plant Biol. 2011, 11, 112. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Labbé, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Salim, B.; Abou El-Yazied, A.; Salama, Y.; Raza, A.; Osman, H.S. Impact of silicon foliar application in enhancing antioxidants, growth, flowering and yield of squash plants under deficit irrigation condition. Ann. Agric. Sci. 2021, 66, 176–183. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Santos, M.R.d.; Martinez, M.A.; Donato, S.L.; Coelho, E.F. ‘Tommy Atkins’ mango yield and photosynthesis under water deficit in semiarid region of Bahia. Rev. Bras. Eng. Agrícola Ambient. 2014, 18, 899–907. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Is plant ecology more siliceous than we realise? Trends Plant Sci. 2011, 16, 61–68. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Gou, T.; Chen, X.; Han, R.; Liu, J.; Zhu, Y.; Gong, H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.P.; Prasad, S.M.; Sharma, S.; Ramawat, N.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K. Interactive effect of silicon (Si) and salicylic acid (SA) in maize seedlings and their mechanisms of cadmium (Cd) toxicity alleviation. J. Plant Growth Regul. 2019, 38, 1587–1597. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.P.; Giehl, R.F.H.; Pitann, B.; Mühling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Ahmad, P.; Chauhan, D.K.; Prasad, S.M. Silicon in Plants: Advances and Future Prospects; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Alqarawi, A.A.; Hashem, A.; Ahmad Malik, J.; Abd Allah, E.F. Silicon supplementation modulates antioxidant system and osmolyte accumulation to balance salt stress in Acacia gerrardii Benth. Saudi J. Biol. Sci. 2019, 26, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Janicka, M.; Wróbel, B. Effect of Silicon-Containing Fertilizers on the Nutritional Value of Grass–Legume Mixtures on Temporary Grasslands. Agriculture 2022, 12, 145. [Google Scholar]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Padula, M.P.; Zeng, R.; Gurr, G.M. Silicon: Potential to Promote Direct and Indirect Effects on Plant Defense Against Arthropod Pests in Agriculture. Front. Plant Sci. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Bueno, A.M.; Flores, R.A.; de Brito Ferreira, E.P.; de Andrade, A.F.; de Lima, F.R.S.; de Souza Junior, J.P.; de Oliveira Abdala, K.; Mesquita, M.; de Mello Prado, R. Effects of Foliar Silicon Application, Seed Inoculation and Splitting of N Fertilization on Yield, Physiological Quality, and Economic Viability of the Common Bean. Silicon 2022, 14, 4169–4181. [Google Scholar] [CrossRef]
- Dakora, F.D.; Nelwamondo, A. Silicon nutrition promotes root growth and tissue mechanical strength in symbiotic cowpea. Funct. Plant Biol. 2003, 30, 947–953. [Google Scholar] [CrossRef]
- Nelwamondo, A.; Jaffer, M.A.; Dakora, F.D. Subcellular organization of N2-fixing nodules of cowpea (Vigna unguiculata) supplied with silicon. Protoplasma 2001, 216, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nelwamondo, A.; Dakora, F.D. Silicon Promotes Nodule Formation and Nodule Function in Symbiotic Cowpea (Vigna unguiculata). New Phytol. 1999, 142, 463–467. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Flores, R.A.; Arruda, E.M.; Souza Junior, J.P.D.; de Mello Prado, R.; Santos, A.C.A.D.; Aragão, A.S.; Pedreira, N.G.; da Costa, C.F. Nutrition and production of Helianthus annuus in a function of application of leaf silicon. J. Plant Nutr. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Ji, X.; Liu, S.; Juan, H.; Bocharnikova, E.A.; Matichenkov, V.V. Effect of silicon fertilizers on cadmium in rice (Oryza sativa) tissue at tillering stage. Environ. Sci. Pollut. Res. Int. 2017, 24, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, K.R.; Bansal, A.; Rout, G.R. Silicon amendment induces synergistic plant defense mechanism against pink stem borer (Sesamia inferens Walker.) in finger millet (Eleusine coracana Gaertn.). Sci. Rep. 2020, 10, 4229. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Song, F.; Xu, H.; Shao, H.; Song, R. Effects of Silicon on Photosynthetic Characteristics of Maize (Zea mays L.) on Alluvial Soil. Sci. World J. 2014, 2014, 718716. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.; Chu, G. Applying silicate fertilizer increases both yield and quality of table grape (Vitis vinifera L.) grown on calcareous grey desert soil. Sci. Hortic. 2017, 225, 757–763. [Google Scholar] [CrossRef]
- Parecido, R.J.; Soratto, R.P.; Guidorizzi, F.V.C.; Perdoná, M.J.; Gitari, H.I. Soil application of silicon enhances initial growth and nitrogen use efficiency of Arabica coffee plants. J. Plant Nutr. 2022, 45, 1061–1071. [Google Scholar] [CrossRef]
- Abd-Alkarim, E.; Bayoumi, Y.; Elmahdy, M.; Rakha, M. Silicon supplements affect yield and fruit quality of cucumber (Cucumis sativus L.) grown in net houses. Afr. J. Agric. Res. 2017, 12, 2518–2523. [Google Scholar] [CrossRef]
- Santos, A.; Teixeira, G.; Campos, C.; Baio, F.; Prado, R.; Teodoro, L.; Vilela, R.; Paiva Neto, V.; Teodoro, P. Silicon increases chlorophyll and photosynthesis and improves height and NDVI of cotton (Gossypium hirsutum L. r. latifolium Hutch). Res. Soc. Dev. 2020, 9, 548973826. [Google Scholar] [CrossRef]
- Araújo, W.B.S.; Teixeira, G.C.M.; de Mello Prado, R.; Rocha, A.M.S. Silicon mitigates nutritional stress of nitrogen, phosphorus, and calcium deficiency in two forages plants. Sci. Rep. 2022, 12, 6611. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Helal, N.M.; Khattab, H.I.; Emam, M.M.; Niedbała, G.; Wojciechowski, T.; Hammami, I.; Alabdallah, N.M.; Darwish, D.B.E.; El-Mogy, M.M.; Hassan, H.M. Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants 2022, 11, 1819. [Google Scholar] [CrossRef]
- Song, A.; Li, Z.; Wang, E.; Xu, D.; Wang, S.; Bi, J.; Wang, H.; Jeyakumar, P.; Li, Z.; Fan, F. Supplying silicon alters microbial community and reduces soil cadmium bioavailability to promote health wheat growth and yield. Sci. Total Environ. 2021, 796, 148797. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Alayafi, A.H.; Al-Solaimani, S.G.M.; Abd El-Wahed, M.H.; Alghabari, F.M.; Sabagh, A.E. Silicon supplementation enhances productivity, water use efficiency and salinity tolerance in maize. Front. Plant Sci. 2022, 13, 953451. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.S.; Pagliari, P.H.; Rodrigues, W.L.; Fernandes, G.C.; Boleta, E.H.M.; Santini, J.M.K.; Jalal, A.; Buzetti, S.; Lavres, J.; Teixeira Filho, M.C.M. Silicon Amendment Enhances Agronomic Efficiency of Nitrogen Fertilization in Maize and Wheat Crops under Tropical Conditions. Plants 2021, 10, 1329. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Saharan, V.; Kumari, S.; Chandra Choudhary, R.; Pal, A.; Sharma, S.S.; Rakshit, S.; Raliya, R.; Biswas, P. Chitosan-silicon nanofertilizer to enhance plant growth and yield in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 53–66. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Guo, D.J.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Wei, K.J.; Sharma, A.; Li, D.P.; et al. Foliar application of silicon boosts growth, photosynthetic leaf gas exchange, antioxidative response and resistance to limited water irrigation in sugarcane (Saccharum officinarum L.). Plant Physiol. Biochem. 2021, 166, 582–592. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; Dos Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Dotaniya, M.; Coumar, V.; Rajendiran, S.; Ajay; Kundu, S.; Rao, A. A Case for Silicon Fertilization to Improve Crop Yields in Tropical Soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef]
- Ligaba-Osena, A.; Guo, W.; Choi, S.C.; Limmer, M.A.; Seyfferth, A.L.; Hankoua, B.B. Silicon Enhances Biomass and Grain Yield in an Ancient Crop Tef [Eragrostis tef (Zucc.) Trotter]. Front. Plant Sci. 2020, 11, 608503. [Google Scholar] [CrossRef] [PubMed]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y. Silicon as a Smart Fertilizer for Sustainability and Crop Improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Duangpan, S.; Tongchu, Y.; Hussain, T.; Eksomtramage, T.; Onthong, J. Beneficial Effects of Silicon Fertilizer on Growth and Physiological Responses in Oil Palm. Agronomy 2022, 12, 413. [Google Scholar] [CrossRef]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Bokor, B.; Soukup, M.; Vaculík, M.; Vd’ačný, P.; Weidinger, M.; Lichtscheidl, I.; Vávrová, S.; Šoltys, K.; Sonah, H.; Deshmukh, R.; et al. Silicon Uptake and Localisation in Date Palm (Phoenix dactylifera)—A Unique Association With Sclerenchyma. Front. Plant Sci. 2019, 10, 988. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Lepsch, I. Chapter 7: Effect of silicon on plant growth and crop yield. In Studies in Plant Science; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 133–147. [Google Scholar]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality-A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Li, N.; Feng, A.; Liu, N.; Jiang, Z.; Wei, S. Silicon application improved the yield and nutritional quality while reduced cadmium concentration in rice. Environ. Sci. Pollut. Res. 2020, 27, 20370–20379. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Effect of Silicon on Crop Growth, Yield and Quality. In Silicon in Agriculture: From Theory to Practice; Liang, Y., Nikolic, M., Bélanger, R., Gong, H., Song, A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 209–223. [Google Scholar]
- Sousa, L.; Poggio, L.; Batjes, N.; Heuvelink, G.; Kempen, B.; Riberio, E.; Rossiter, D. SoilGrids 2.0: Producing quality-assessed soil information for the globe. Soil Discuss. 2020, 2020, 1–37. [Google Scholar]
- Huffman, G.J.; Stocker, E.F.; Bolvin, D.T.; Nelkin, E.J.; Jackson, T. GPM IMERG Late Precipitation L3 1 day 0.1 degree × 0.1 degree V06. Goddard Earth Sciences Data and Information Services Center. 2019. Available online: https://disc.gsfc.nasa.gov/datasets/GPM_3IMERGDL_06/summary (accessed on 29 May 2023).
- FAO. ECOCROP-Database of Crop Constraints and Characteristics. FAO NSL Geospatial Unit 2022. Available online: https://gaez.fao.org/pages/ecocrop (accessed on 29 May 2023).
- FAO. A Framework for Land Evaluation. FAO Soils Bulletin No. 32. 1976. Available online: https://www.fao.org/3/x5310e/x5310e00.htm (accessed on 29 May 2023).
- Beaudoing, H.; Rodell, M. NASA/GSFC/HSL (2020), GLDAS Noah Land Surface Model L4 monthly 0.25 x 0.25 degree V2.1, Greenbelt, Maryland, USA, Goddard Earth Sciences Data and Information Services Center (GES DISC). Available online: https://disc.gsfc.nasa.gov/datasets/GLDAS_NOAH025_M_2.1/summary (accessed on 1 March 2023).



| Traits | Source | DF | Type III SS | Mean Square | F Value | p > F |
|---|---|---|---|---|---|---|
| Chl | Tre | 2 | 191,537.704 | 95,768.852 | 18.34 | <0.0001 |
| Rep | 2 | 8088.87 | 4044.435 | 0.77 | 0.4615 | |
| DC | 2 | 2,790,622.344 | 1,395,311.172 | 267.18 | <0.0001 | |
| Env | 2 | 150,359.098 | 75,179.549 | 14.4 | <0.0001 | |
| Tre*Env | 2 | 19,221.88 | 9610.94 | 1.84 | 0.1598 | |
| NDVI | Tre | 2 | 0.2027455 | 0.10137275 | 9.32 | 0.0001 |
| Rep | 2 | 0.02557458 | 0.01278729 | 1.18 | 0.3093 | |
| DC | 2 | 0.19527912 | 0.09763956 | 8.98 | 0.0001 | |
| Env | 1 | 0.0924627 | 0.0924627 | 8.5 | 0.0037 | |
| Tre*Env | 2 | 0.08579846 | 0.04289923 | 3.94 | 0.0199 | |
| PRI | Tre | 2 | 0.00023445 | 0.00011722 | 0.69 | 0.5018 |
| Rep | 2 | 0.0007302 | 0.0003651 | 2.15 | 0.1174 | |
| DC | 2 | 0.02506911 | 0.01253455 | 73.83 | <0.0001 | |
| Env | 1 | 0.00225234 | 0.00225234 | 13.27 | 0.0003 | |
| Tre*Env | 2 | 0.00028028 | 0.00014014 | 0.83 | 0.4386 | |
| PH | Tre | 2 | 2868.03333 | 1434.01667 | 50.82 | <0.0001 |
| Rep | 2 | 846.34444 | 423.17222 | 15 | <0.0001 | |
| DC | 2 | 37,379.74444 | 18,689.87222 | 662.32 | <0.0001 | |
| Env | 1 | 1833.37963 | 1833.37963 | 64.97 | <0.0001 | |
| Tre*Env | 2 | 41.4037 | 20.70185 | 0.73 | 0.4807 | |
| SW | Tre | 2 | 95.763693 | 47.881847 | 9.25 | 0.0001 |
| Rep | 2 | 51.570641 | 25.785321 | 4.98 | 0.0072 | |
| DC | 2 | 1358.75383 | 679.376915 | 131.18 | <0.0001 | |
| Env | 1 | 595.413002 | 595.413002 | 114.97 | <0.0001 | |
| Tre*Env | 2 | 8.454973 | 4.227487 | 0.82 | 0.4426 | |
| NB | Tre | 2 | 41.7148148 | 20.8574074 | 11.94 | <0.0001 |
| Rep | 2 | 0.337037 | 0.1685185 | 0.1 | 0.908 | |
| DC | 2 | 523.937037 | 261.9685185 | 149.99 | <0.0001 | |
| Env | 1 | 63.3796296 | 63.3796296 | 36.29 | <0.0001 | |
| Tre*Env | 2 | 0.6259259 | 0.312963 | 0.18 | 0.836 | |
| TRL | Tre | 2 | 1,208,726 | 604,363.2 | 40.51 | <0.0001 |
| Rep | 2 | 37,639.47 | 18,819.73 | 1.26 | 0.2858 | |
| Env | 1 | 138,238.1 | 138,238.1 | 9.27 | 0.0027 | |
| Tre*Env | 2 | 77,379.17 | 38,689.58 | 2.59 | 0.0777 | |
| SA | Tre | 2 | 70,151.92 | 35,075.96 | 56.26 | <0.0001 |
| Rep | 2 | 3276.528 | 1638.264 | 2.63 | 0.0751 | |
| Env | 1 | 2781.931 | 2781.931 | 4.46 | 0.0361 | |
| Tre*Env | 2 | 457.2975 | 228.6487 | 0.37 | 0.6935 | |
| AD | Tre | 2 | 4.478359 | 2.239179 | 59.67 | <0.0001 |
| Rep | 2 | 0.113299 | 0.05665 | 1.51 | 0.2239 | |
| Env | 1 | 1.410859 | 1.410859 | 37.6 | <0.0001 | |
| Tre*Env | 2 | 0.147526 | 0.073763 | 1.97 | 0.1432 | |
| RV | Tre | 2 | 25.03816 | 12.51908 | 17.8 | <0.0001 |
| Rep | 2 | 0.120214 | 0.060107 | 0.09 | 0.9181 | |
| Env | 1 | 2.811756 | 2.811756 | 4 | 0.0471 | |
| Tre*Env | 2 | 0.362826 | 0.181413 | 0.26 | 0.7729 | |
| NT | Tre | 2 | 17,371,086 | 8,685,543 | 111.23 | <0.0001 |
| Rep | 2 | 231,728.6 | 115,864.3 | 1.48 | 0.2296 | |
| Env | 1 | 918,502.5 | 918,502.5 | 11.76 | 0.0008 | |
| Tre*Env | 2 | 1,912,638 | 956,318.9 | 12.25 | <0.0001 | |
| NF | Tre | 2 | 25,309,441 | 12,654,721 | 35.68 | <0.0001 |
| Rep | 2 | 986,063.9 | 493,032 | 1.39 | 0.2518 | |
| Env | 1 | 6,172,119 | 6,172,119 | 17.4 | <0.0001 | |
| Tre*Env | 2 | 3,318,264 | 1,659,132 | 4.68 | 0.0105 | |
| HSW | Tre | 2 | 186.2892 | 93.1446 | 19.7 | <0.0001 |
| Rep | 2 | 0.15731 | 0.078655 | 0.02 | 0.9835 | |
| Env | 1 | 613.5846 | 613.5846 | 129.78 | <0.0001 | |
| Tre*Env | 2 | 611.4961 | 305.7481 | 64.67 | <0.0001 | |
| WPG | Tre | 2 | 0.01684 | 0.00842 | 18.53 | <0.0001 |
| Rep | 2 | 0.001639 | 0.000819 | 1.8 | 0.1679 | |
| Env | 1 | 0.050501 | 0.050501 | 111.12 | <0.0001 | |
| Tre*Env | 2 | 0.06271 | 0.031355 | 68.99 | <0.0001 | |
| TGW | Tre | 2 | 6486.6 | 3243.3 | 535.45 | <0.0001 |
| Rep | 2 | 14.55694 | 7.278472 | 1.2 | 0.3032 | |
| Env | 1 | 136.4335 | 136.4335 | 22.52 | <0.0001 | |
| Tre*Env | 2 | 1696.049 | 848.0246 | 140 | <0.0001 | |
| TGN | Tre | 2 | 107,212.3 | 53,606.16 | 2359.42 | <0.0001 |
| Rep | 2 | 15.8111 | 7.9056 | 0.35 | 0.7066 | |
| Env | 1 | 11,139.2 | 11,139.2 | 490.28 | <0.0001 | |
| Tre*Env | 2 | 5703.6 | 2851.8 | 125.52 | <0.0001 | |
| PN | Tre | 2 | 27,033.3 | 13,516.65 | 1159.01 | <0.0001 |
| Rep | 2 | 34.23333 | 17.11667 | 1.47 | 0.2333 | |
| Env | 1 | 4460.089 | 4460.089 | 382.44 | <0.0001 | |
| Tre*Env | 2 | 2826.678 | 1413.339 | 121.19 | <0.0001 | |
| Yield | Tr | 2 | 0.562403 | 0.281202 | 16.11 | 0.0007 |
| Rep | 2 | 0.005328 | 0.002664 | 0.15 | 0.8604 | |
| Env | 1 | 0.006393 | 0.006393 | 0.37 | 0.5585 | |
| Tr*Env | 2 | 0.002967 | 0.001484 | 0.09 | 0.919 |
| Feature | Specification |
|---|---|
| Product dimensions | 109.22 × 66.04 × 35.56 mm |
| Weight | 539.78 g |
| Image sensor | 24.1 Megapixel CMOS (APS-C) |
| Image processor | DIGIC 8 |
| Lens | EF-M 15–45 mm IS STM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attipoe, J.Q.; Khan, W.; Tayade, R.; Steven, S.; Islam, M.S.; Lay, L.; Ghimire, A.; Kim, H.; Sereyvichea, M.; Propey, T.; et al. Evaluating the Effectiveness of Calcium Silicate in Enhancing Soybean Growth and Yield. Plants 2023, 12, 2190. https://doi.org/10.3390/plants12112190
Attipoe JQ, Khan W, Tayade R, Steven S, Islam MS, Lay L, Ghimire A, Kim H, Sereyvichea M, Propey T, et al. Evaluating the Effectiveness of Calcium Silicate in Enhancing Soybean Growth and Yield. Plants. 2023; 12(11):2190. https://doi.org/10.3390/plants12112190
Chicago/Turabian StyleAttipoe, John Quarshie, Waleed Khan, Rupesh Tayade, Senabulya Steven, Mohammad Shafiqul Islam, Liny Lay, Amit Ghimire, Hogyun Kim, Muong Sereyvichea, Then Propey, and et al. 2023. "Evaluating the Effectiveness of Calcium Silicate in Enhancing Soybean Growth and Yield" Plants 12, no. 11: 2190. https://doi.org/10.3390/plants12112190
APA StyleAttipoe, J. Q., Khan, W., Tayade, R., Steven, S., Islam, M. S., Lay, L., Ghimire, A., Kim, H., Sereyvichea, M., Propey, T., Rana, Y. B., & Kim, Y. (2023). Evaluating the Effectiveness of Calcium Silicate in Enhancing Soybean Growth and Yield. Plants, 12(11), 2190. https://doi.org/10.3390/plants12112190











