Performance of Hop Cultivars Grown with Artificial Lighting under Subtropical Conditions
Abstract
1. Introduction
2. Results and Discussion
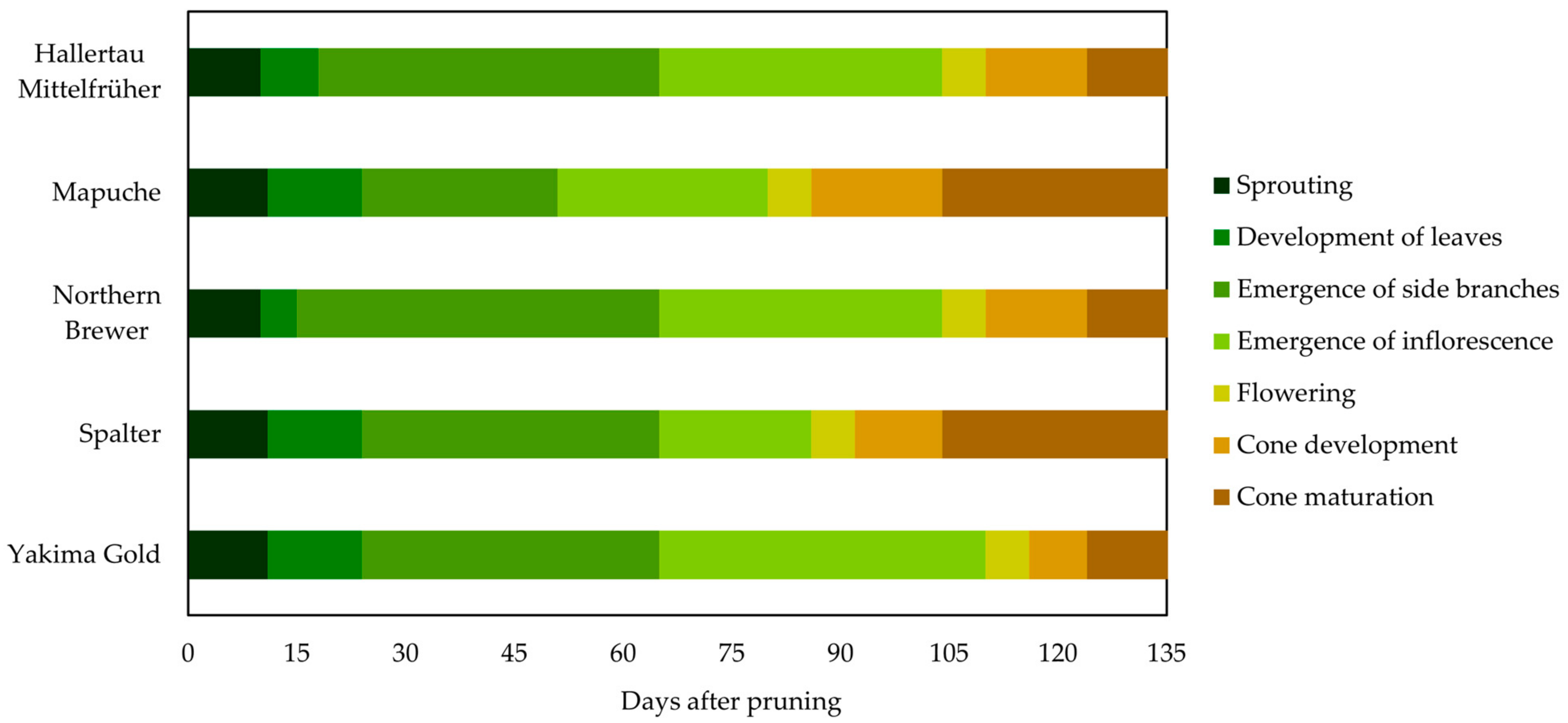
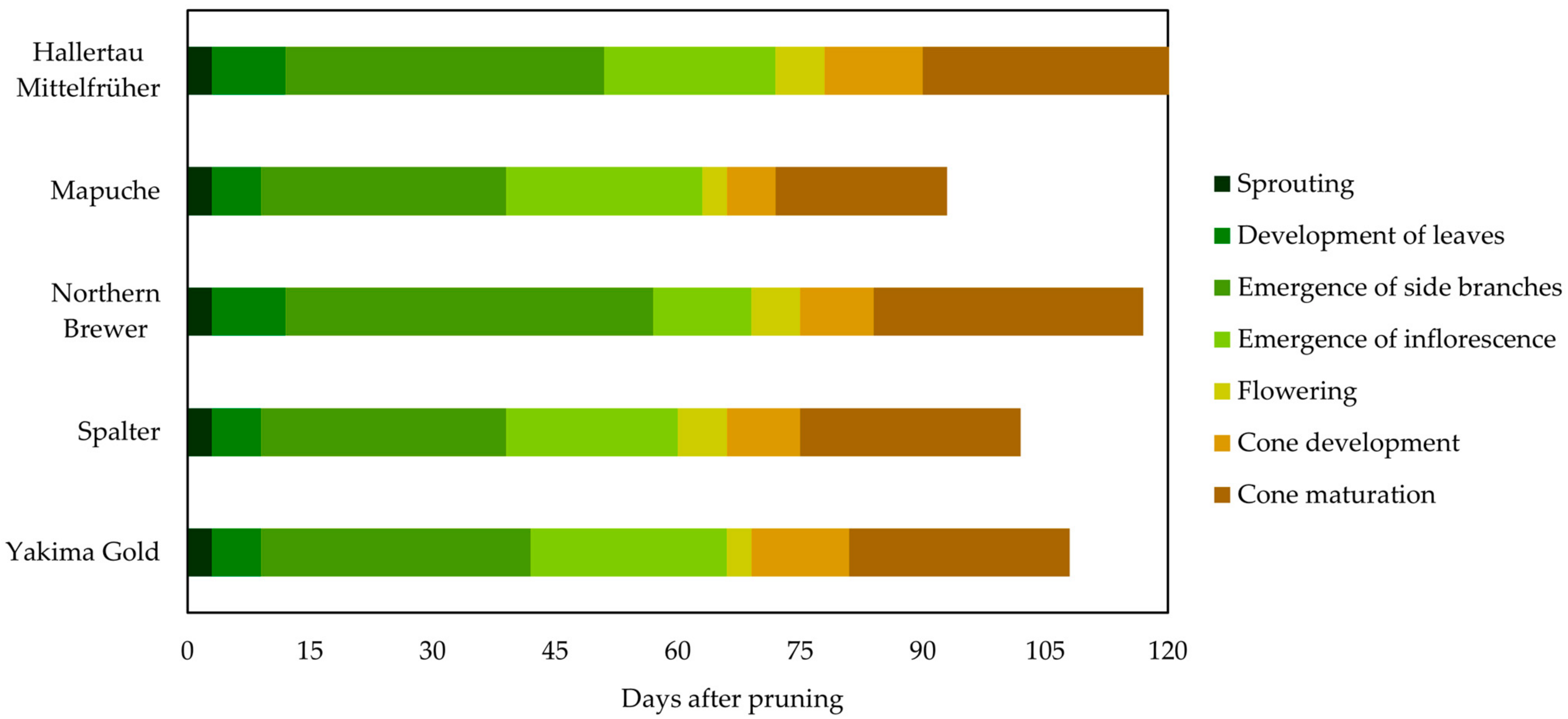
2.1. Hop Cultivar Phenology
2.1.1. Season of 2021
2.1.2. Season of 2022
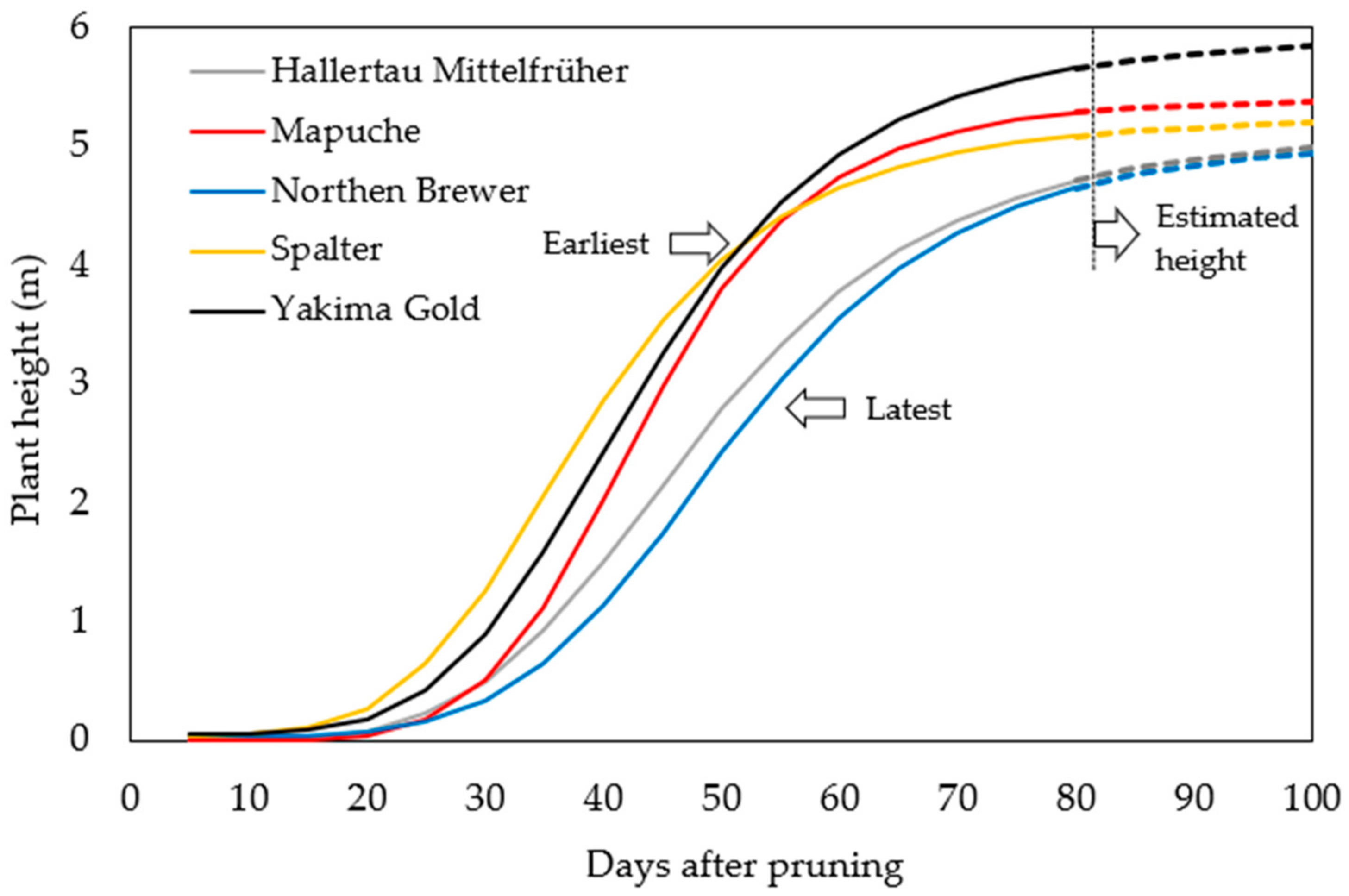
2.2. Hop Growth Development
2.2.1. Season of 2021
2.2.2. Season of 2022
2.3. Yield Components
2.3.1. Season of 2021
2.3.2. Season of 2022
2.4. Chemical Components of Cones
2.4.1. Season of 2021
2.4.2. Season of 2022
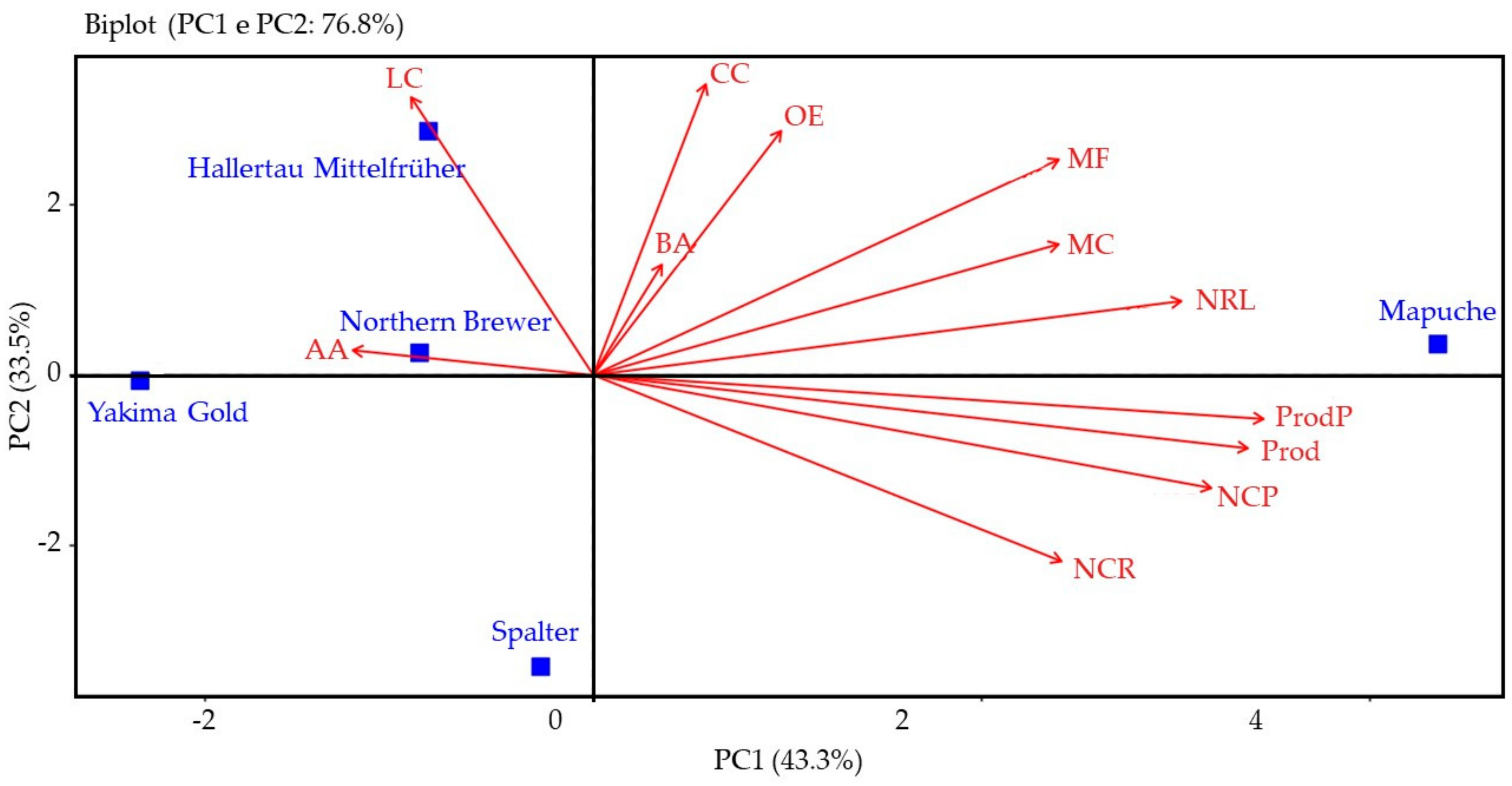
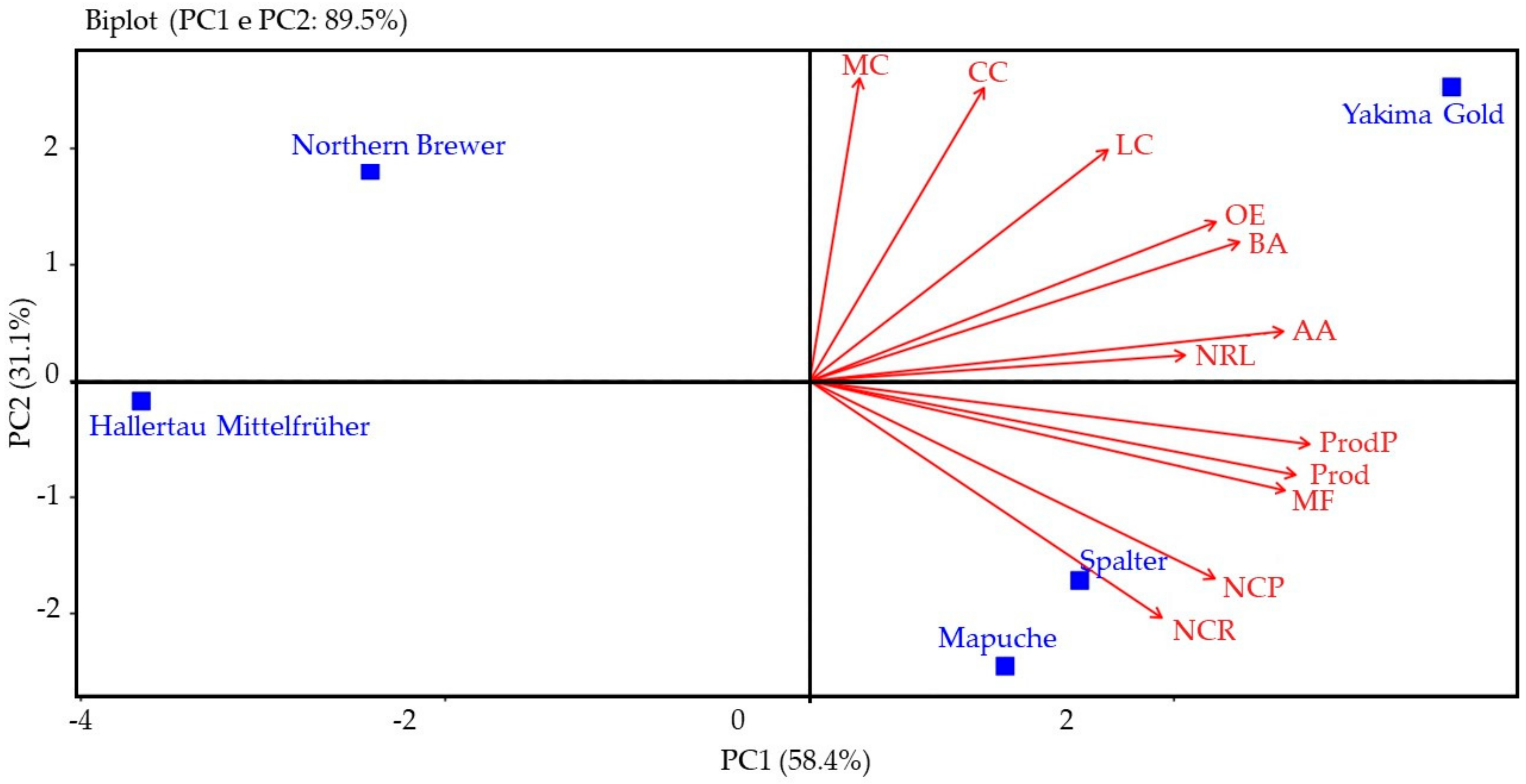
2.5. Multivariate Analysis
2.5.1. Season of 2021
2.5.2. Season of 2022
3. Materials and Methods
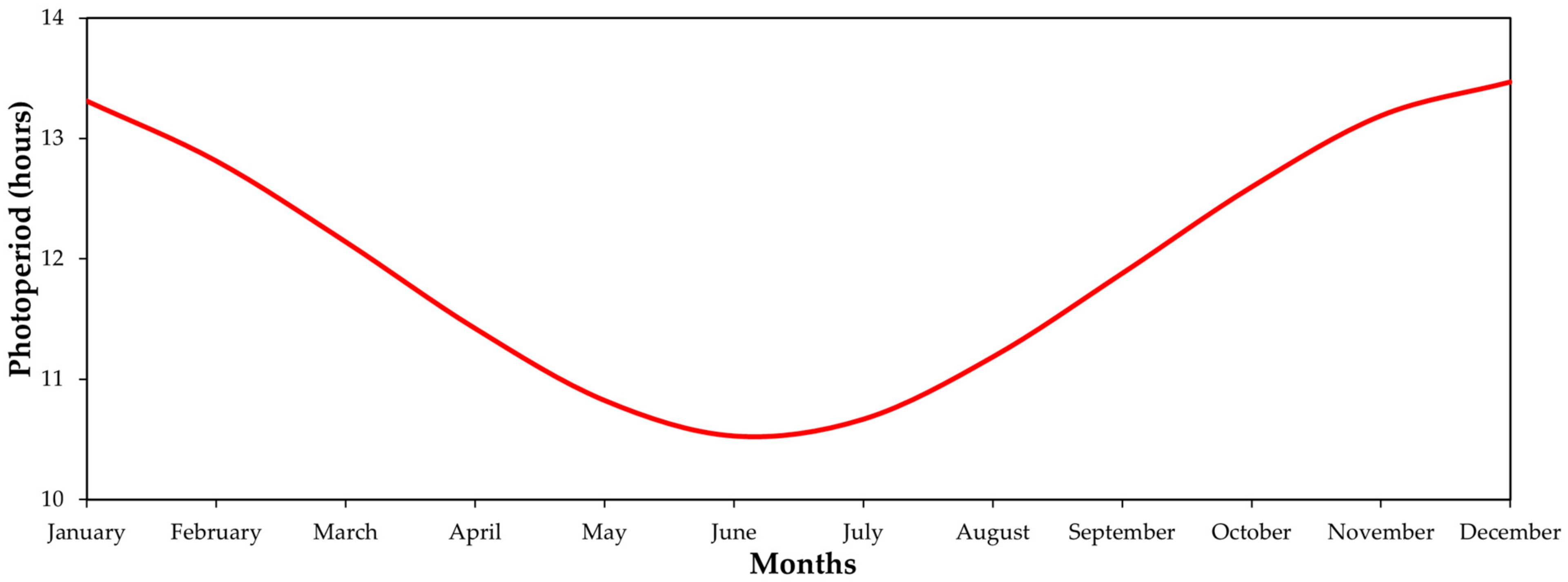
3.1. Experimental Area Description
3.2. Experimental Design and Assessments
3.2.1. Phenology of Hop Cultivars
3.2.2. Hop Growth Development
3.2.3. Yield Components
3.2.4. Chemical Components of Cones
3.3. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acosta-Rangel, A.; Rechcigl, J.; Bollin, S.; Deng, Z.; Agehara, S. Hop (Humulus lupulus L.) phenology, growth, and yield under subtropical climatic conditions: Effects of cultivars and crop management. Aust. J. Crop Sci. 2021, 15, 764–772. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a Novel Multipurpose Crop for the Mediterranean Region of Europe: Challenges and Opportunities of Their Cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Almaguer, C.; Gastl, M.; Arendt, E.K.; Becker, T. Comparative study of the contribution of hop (Humulus lupulus L.) hard resins extracted from different hop varieties to beer quality parameters. J. Am. Soc. Brew. Chem. 2015, 73, 115–123. [Google Scholar] [CrossRef]
- Jastrombek, J.M.; Faguerazzi, M.M.; Pierezan, H.C.; Rufato, L.; Sato, A.J.; RICCE, W.S.; Marques, V.V.; Leles, N.R.; Roberto, S.R. Hop: An Emerging Crop in Subtropical Areas in Brazil. Horticulturae 2022, 8, 393. [Google Scholar] [CrossRef]
- Sirrine, R. Growing Hops; Michigan State University Extension: East Lansing, MI, USA, 2014; p. E3210. [Google Scholar]
- Simieli, B.M.; Gazola, R.P.D.; Pagliarini, M.K.; Vargas, P.F.; Castilho, R.M.M. Development and production of hop in a high temperature region. Res. Soc. Dev. 2021, 10, e127101320863. [Google Scholar] [CrossRef]
- Dodds, K. Hops, a Guide for New Growers; NSW Department of Primary Industries: Orange, NSW, Australia, 2017. [Google Scholar]
- Agehara, S. Using Supplemental Lighting to Control Flowering of Hops in Florida. EDIS 2020, 2020, hs1365. [Google Scholar] [CrossRef]
- Mapa-Ministério da Agricultura, Pecuária e Abastecimento. Anuário da Cerveja 2020; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2021. [Google Scholar]
- Ibge—Instituto Brasileiro de Geografia e Estatística. Available online: Sidra.ibge.gov.br/ (accessed on 20 March 2023).
- Comex—Portal do Comércio Exterior do Brasil. Available online: http://comexstat.mdic.gov.br/pt/geral (accessed on 7 March 2023).
- Müller, C.V.; Marcusso, E.F. Mapa Informa: As Cervejarias Continuam a Crescer; Divisão de Inspeção de Produtos de Origem Vegetal: Brasília, Brazil, 2018. [Google Scholar]
- Durello, R.S.; Silva, L.M.; Bogusz Junior, S. Química do Lúpulo. Quim. Nova 2019, 42, 900–919. [Google Scholar] [CrossRef]
- Rodolfi, M.; Chiancone, B.; Liberatore, C.M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in chemical profile of Cascade hop cones according to the growing area. J. Sci. Food Agric. 2019, 99, 6011–6019. [Google Scholar] [CrossRef]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016, 11, 180–187. [Google Scholar] [CrossRef]
- Lizotte, E.; Sirrine, R. Michigan Hop Management Guide 2020; Michigan State University Extension: East Lansing, MI, USA, 2020. [Google Scholar]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic Flowering Response of Essential Oil, Grain, and Fiber Hemp (Cannabis sativa L.) Cultivars. Front. Plant Sci. 2021, 12, 694153. [Google Scholar] [CrossRef]
- Trancoso, I.; Souza, G.A.R.; Santos, P.R.; Santos, K.D.; Miranda, R.M.S.N.; SILVA, A.L.P.M.; Zsolt Santos, D.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L.: Crop Management and Abiotic Factors That Affect Phytocannabinoid Production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Yield, Potency, and Leaf Photosynthesis Respond Differently to Increasing Light Levels in an Indoor Environment. Front. Plant Sci. 2021, 12, 646020. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. The Effects of Photoperiod on Phenological Development and Yields of Industrial Hemp. J. Nat. Fibers 2014, 11, 87–106. [Google Scholar] [CrossRef]
- Dang, M.; Arachchige, N.M.; Campbell, L.G. Optimizing Photoperiod Switch to Maximize Floral Biomass and Cannabinoid Yield in Cannabis sativa L.: A Meta-Analytic Quantile Regression Approach. Front. Plant Sci. 2022, 12, 797425. [Google Scholar] [CrossRef]
- Mozny, M.; Tolasz, R.; Nekovar, J.; Sparks, T.; Trnka, M.; Zalud, Z. The impact of climate change on the yield and quality of Saaz hops in the Czech Republic. Agric. For. Meteorol. 2009, 149, 913–919. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Review of flowering control in industrial hemp. J. Nat. Fibers 2012, 9, 23–36. [Google Scholar] [CrossRef]
- Ruggeri, R.; Loreti, P.; Rossini, F. Exploring the potential of hop as a dual-purpose crop in the Mediterranean environment: Shoot and cone yield from nine commercial cultivars. Eur. J. Agron. 2018, 93, 11–17. [Google Scholar] [CrossRef]
- Skomra, U.; Bocianowski, J.; Agacka, M. Agro-morphological differentiation between European hop (Humulus lupulus L.) cultivars in relation to their origin. J. Food Agric. Environ. 2013, 11, 1123–1128. [Google Scholar]
- Spósito, M.B.; Ismael, R.V.; Barbosa, C.M.A.; Tagliaferro, A.L. A Cultura do Lúpulo; ESALQ Divisão de Biblioteca: Piracicaba, Brazil, 2019. [Google Scholar]
- Thomas, G.G.; Schwabe, W.W. Factors Controlling Flowering in the Hop (Humulus lupulus L.). Ann. Bot. 1969, 33, 781–793. [Google Scholar] [CrossRef]
- Mcadam, E.L.; Vaillancourt, R.E.; Koutoulis, A.; Whittock, S.P. Quantitative genetic parameters for yield, plant growth and cone chemical traits in hop (Humulus lupulus L.). BMC Genet. 2014, 15, 22. [Google Scholar] [CrossRef]
- Cernea, S.; Vâtcă, S. Phenotypic correlations between some quantitative characters of the hop. Hop Med. Plants 2009, 17, 20–23. [Google Scholar]
- Gonsaga, R.F. Desenvolvimento de Híbridos de Lúpulo Adaptados às Condições Tropicais. Ph.D. Thesis, Universidade Estadual Paulista (Unesp), Jaboticabal, Brazil, 2021. [Google Scholar]
- Fagherazzi, M.M. Adaptabilidade de Cultivares de Lúpulo na Região do Planalto Sul Catarinense. Ph.D. Thesis, Universidade Estadual de Santa Catarina, Lages, Brazil, 2020. [Google Scholar]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Fagherazzi, M.M.; Rufato, L.; Kretzschmar, A.A.; Arruda, A.L.; Soares dos Santos, M.F.; Camargo, S.S. A cultura do lúpulo: Botânica e cultivares. Rev. Agron. Bras. 2017, 1, rab201712. [Google Scholar] [CrossRef]
- Testa, H.; Trochine, A.; Bergamini, H. Overview on Mapuche, Traful and Nahuel, local hop varieties cultivated in Patagonia, Argentina. In Proceedings of the Scientific-Technical Commission IHGC (2019), Alsace, France, 7–11 July 2019; pp. 96–100. [Google Scholar]
- Healey, J. The Hops List: 265 Beer Hop Varieties from around the World, 1st ed.; Julian Healey: Melbourne, Australia, 2016; 293p. [Google Scholar]
- Woodske, D. Hop Variety Handbook: Learn More about Hops...Craft Better Beer; CreateSpace: London, UK, 2012. [Google Scholar]
- Marceddu, R.; Carrubba, A.; Sarno, M. Cultivation trials of hop (Humulus lupulus L.) in semiarid environments. Heliyon 2020, 6, e05114. [Google Scholar] [CrossRef]
- Haunold, A.; Nickerson, G.B.; Likens, S.T. Yield and Quality Potential of Hop, Humulus lupulus L. J. Am. Soc. Brew. Chem. 1983, 41, 60–63. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Rybacek, V. Hop Production, 1st ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; 286p. [Google Scholar]
- Guimarães, J.J. Comportamento Agronômico do Lúpulo (Humulus lupulus L.) em Cultivo Protegido Submetido a Irrigações com Diferentes Faixas de pH da Água na Região de Botucatu—SP. Master’s Thesis, Universidade Estadual Paulista (Unesp), Botucatu, Brazil, 2020. [Google Scholar]
- Donner, P.; Pokorný, J.; Ježek, J.; Krofta, K.; Patzak, J.; Pulkrábek, J. Influence of weather conditions, irrigation and plant age on yield and alpha-acids content of Czech hop (Humulus lupulus L.) cultivars. Plant Soil Environ. 2020, 66, 41–46. [Google Scholar] [CrossRef]
- Fagherazzi, M.M.; Santos, M.F.S.; Santos, K.V.T.; Rufato, L. Análise de custo de implantação de lúpulo na região do Planalto Sul Catarinense. Congrega 2018, 15, 721–730. [Google Scholar]
- Forster, A.; Schüll, F. The impact of climate change on hops. Brauwelt Int. 2020, 3, 174–178. [Google Scholar]
- Klimczak, K.; Cioch-Skoneczny, M. Changes in beer bitterness level during the beer production process. Eur. Food Res. Technol. 2023, 249, 13–22. [Google Scholar] [CrossRef]
- Rutnik, K.; Hrnčič, M.K.; Košir, I.J. Hop Essential Oil: Chemical Composition, Extraction, Analysis, and Applications. Food Rev. Int. 2021, 1, 529–551. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.-P. 22-Hop Essential Oil: Analysis, Chemical Composition and Odor Characteristics. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 239–254. [Google Scholar] [CrossRef]
- Mcadam, E.L.; Freeman, J.S.; Whittock, S.P.; Buck, E.J.; Jakse, J.; Cerenak, A.; Koutoulis, A. Quantitative trait loci in the hop (Humulus lupulus L.) reveal complex genetic architecture underlying variation in sex, yield and cone chemistry. BMC Genom. 2013, 14, 360. [Google Scholar] [CrossRef]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A new perspective of a well-recognized raw material: Phenolic content, antioxidant and antimicrobial activities and α- and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. LWT—Food Sci. Technol. 2021, 141, 110905. [Google Scholar] [CrossRef]
- Santos, F.C.; Santos, M.; Huezsmann, R.D.; Ceola, D.; Souza, E.M.D.; Santos Junior, C.F.; Guidolin, A.F.; Coimbra, J.L.M. Phenotypic Variability in the Induction of Alpha Acids in Hops (Humulus lupulus L.) in Brazil. J. Agric. Sci. 2022, 14, 198–205. [Google Scholar] [CrossRef]
- Hieronymus, S. For the Love of Hops: The Practical Guide to Aroma, Bitterness and the Culture of Hops; Brewers Publications: Boulder, CO, USA, 2012. [Google Scholar]
- Borém, A.; Miranda, G.V.; Fritsche-Neto, R. Melhoramento de Plantas; Editora UFV: Viçosa, Brazil, 2013. [Google Scholar]
- Santos, F.C. Variabilidade Fenotípica de Alfa Ácido de Lúpulo (Humulus lupulus L.) Cultivados nas Regiões do Brasil. Master’s Dissertation, Universidade do Estado de Santa Catarina, Lages, Brazil, 2020. [Google Scholar]
- Gomes, J.B.V.; Wrege, M.S. Municípios Formadores da Bacia do Paraná 3 e Palotina: Estudos de Clima, Solos e Aptidão das Terras Para o Cultivo do Eucalipto; Embrapa Florestas: Colombo, Brazil, 2020. [Google Scholar]
- Varejão-Silva, M.A. Meteorologia e Climatologia, Versão Digital 2; INMET: Recife, Brazil, 2006. [Google Scholar]
- Robbauer, V.G.; Buhr, L.; Hack, H.; Hauptmann, S.; Klose, R.; Meier, U.; Staub, R.; Weber, E. Phanologische Entwicklungsstadien von Kultur-Hopfen (Humulus lupulus L.). Nachrichtenbl. Deut. Pflanzenschutzd. 1995, 47, 249–253. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Egts, H.; Durben, D.J.; Dixson, J.A.; Zehfus, M.H. A Multicomponent UV Analysis of α- and β-Acids in Hops. J. Chem. Educ. 2012, 89, 117–120. [Google Scholar] [CrossRef]
- Santos, A.S.; Alves, S.M.; Figueirêdo, F.J.C.; Rocha Neto, O.G. Descrição de Sistema e de Métodos de Extração de Óleos Essenciais e Determinação de Umidade de Biomassa em Laboratório; Ministério da Agricultura, Pecuária e Abastecimento: Belém, Brazil, 2004. [Google Scholar]




| Cultivars | Plant Fresh Mass (kg) | N° of Side Branches per Plant |
|---|---|---|
| Hallertau Mittelfrüher | 1.7 | 71.9 b |
| Mapuche | 1.9 | 84.3 a |
| Northern Brewer | 1.5 | 83.5 a |
| Spalter | 1.2 | 56.8 c |
| Yakima Gold | 1.4 | 64.7 bc |
| CV% | 35.8 | 6.3 |
| F | 1.1 ns | 27.7 ** |
| Cultivars | Fresh Mass of Plants (kg) | N° of Side Branches per Plant |
|---|---|---|
| Hallertau Mittelfrüher | 1.3 b | 91.7 d |
| Mapuche | 2.3 a | 104.8 cd |
| Northern Brewer | 1.4 b | 111.5 bc |
| Spalter | 2.1 a | 138.1 a |
| Yakima Gold | 2.2 a | 126.6 ab |
| CV% | 17.7 | 7.0 |
| F | 8.7 ** | 20.4 ** |
| Cultivars | N° of Cones per Side Branch | N° of Cones per Plant | Mass of Cones (g) |
|---|---|---|---|
| Hallertau Mittelfrüher | 5.9 c | 422.8 c | 0.4 a |
| Mapuche | 24.0 a | 2008.5 a | 0.4 a |
| Northern Brewer | 8.2 c | 599.2 c | 0.3 ab |
| Spalter | 17.9 b | 1376.1 b | 0.3 ab |
| Yakima Gold | 5.6 c | 513.2 c | 0.2 b |
| CV% | 12.3 | 22.0 | 22.4 |
| F | 117.1 ** | 44.1 ** | 6.0 ** |
| Cultivars | Cone Length (cm) | Cone Width (cm) |
|---|---|---|
| Hallertau Mittelfrüher | 2.5 | 1.9 a |
| Mapuche | 2.2 | 1.2 ab |
| Northern Brewer | 2.0 | 1.5 ab |
| Spalter | 1.6 | 0.9 b |
| Yakima Gold | 2.0 | 1.3 ab |
| CV% | 23.2 | 28.8 |
| F | 1.9 ns | 3.9 * |
| Cultivars | Production of Fresh Cones (kg/Plant) | Yield of Fresh Cones (kg ha−1) |
|---|---|---|
| Hallertau Mittelfrüher | 0.2 b | 579.2 bc |
| Mapuche | 0.9 a | 2861.5 a |
| Northern Brewer | 0.2 b | 639.6 bc |
| Spalter | 0.4 b | 1480.9 b |
| Yakima Gold | 0.1 b | 444.8 c |
| CV% | 37.3 | 38.0 |
| F | 19.4 ** | 19.7 ** |
| Cultivars | N° Of Cones per Side Branch | N° of Cones per Plant | Mass of Cones (g) |
|---|---|---|---|
| Hallertau Mittelfrüher | 7.3 cd | 624.6 c | 0.4 ab |
| Mapuche | 20.1 a | 2120.4 ab | 0.3 b |
| Northern Brewer | 5.4 d | 571.2 c | 0.4 ab |
| Spalter | 18.6 ab | 2192.5 a | 0.3 b |
| Yakima Gold | 12.5 bc | 1557.4 b | 0.5 a |
| CV% | 23.8 | 19.1 | 14.5 |
| F | 18.4 ** | 33.8 ** | 6.1 ** |
| Cultivars | Length of Cones (cm) | Width of Cones (cm) |
|---|---|---|
| Hallertau Mittelfrüher | 1.8 b | 1.2 b |
| Mapuche | 1.8 b | 1.3 ab |
| Northern Brewer | 2.2 a | 1.5 ab |
| Spalter | 1.9 b | 1.4 ab |
| Yakima Gold | 2.3 a | 1.6 a |
| CV% | 5.1 | 11.7 |
| F | 21.3 ** | 4.7 * |
| Cultivars | Production of Fresh Cones (kg/Plant) | Yield of Fresh Cones (kg ha−1) |
|---|---|---|
| Hallertau Mittelfrüher | 0.2 b | 811.1 b |
| Mapuche | 0.6 a | 2051.0 a |
| Northern Brewer | 0.2 b | 657.6 b |
| Spalter | 0.6 a | 2122.9 a |
| Yakima Gold | 0.7 a | 2251.0 a |
| CV% | 19.1 | 19.1 |
| F | 26.5 ** | 26.5 ** |
| Cultivars | Alpha-Acid (%) | Beta-Acid (%) | Essential Oils (mL.100 g−1) |
|---|---|---|---|
| Hallertau Mittelfrüher | 5.9 ab | 1.8 ab | 1.1 ab |
| Mapuche | 5.0 ab | 1.9 ab | 1.2 a |
| Northern Brewer | 3.0 b | 1.2 b | 1.0 b |
| Spalter | 5.1 ab | 1.4 ab | 0.6 c |
| Yakima Gold | 8.5 a | 2.1 a | 1.1 ab |
| CV% | 16.5 | 17.9 | 7.2 |
| F | 5.1 * | 5.5 ** | 34.8 ** |
| Cultivars | Alpha-Acid (%) | Beta-Acid (%) | Essential Oils (mL.100 g−1) |
|---|---|---|---|
| Hallertau Mittelfrüher | 2.3 d | 1.4 d | 0.8 b |
| Mapuche | 5.8 b | 2.5 b | 0.8 b |
| Northern Brewer | 3.2 cd | 1.8 c | 0.8 b |
| Spalter | 4.2 c | 1.9 c | 1.0 ab |
| Yakima Gold | 7.4 a | 4.0 a | 1.3 a |
| CV% | 10.2 | 6.3 | 14.3 |
| F | 75.5 ** | 190.2 ** | 12.0 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leles, N.R.; Sato, A.J.; Rufato, L.; Jastrombek, J.M.; Marques, V.V.; Missio, R.F.; Fernandes, N.L.M.; Roberto, S.R. Performance of Hop Cultivars Grown with Artificial Lighting under Subtropical Conditions. Plants 2023, 12, 1971. https://doi.org/10.3390/plants12101971
Leles NR, Sato AJ, Rufato L, Jastrombek JM, Marques VV, Missio RF, Fernandes NLM, Roberto SR. Performance of Hop Cultivars Grown with Artificial Lighting under Subtropical Conditions. Plants. 2023; 12(10):1971. https://doi.org/10.3390/plants12101971
Chicago/Turabian StyleLeles, Nathalia Rodrigues, Alessandro Jefferson Sato, Leo Rufato, Jessiane Mary Jastrombek, Viviani Vieira Marques, Robson Fernando Missio, Nelson Luis Mello Fernandes, and Sergio Ruffo Roberto. 2023. "Performance of Hop Cultivars Grown with Artificial Lighting under Subtropical Conditions" Plants 12, no. 10: 1971. https://doi.org/10.3390/plants12101971
APA StyleLeles, N. R., Sato, A. J., Rufato, L., Jastrombek, J. M., Marques, V. V., Missio, R. F., Fernandes, N. L. M., & Roberto, S. R. (2023). Performance of Hop Cultivars Grown with Artificial Lighting under Subtropical Conditions. Plants, 12(10), 1971. https://doi.org/10.3390/plants12101971









