Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction
Abstract
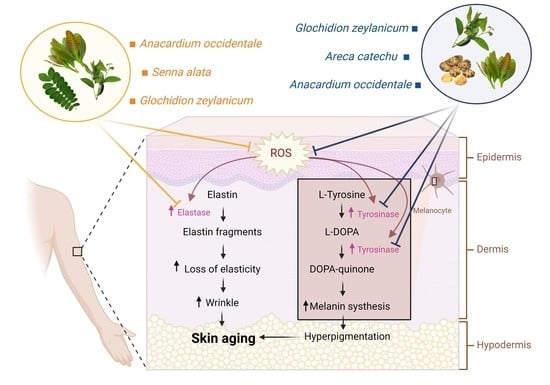
:1. Introduction
2. Results
2.1. Extraction Yields
2.2. Total Phenolic Content of Thai Plants
2.3. Total Flavonoid Content of Thai Plants
2.4. DPPH Radical Scavenging Activity of Thai Plants
2.5. ABTS Radical Scavenging Activity of Thai Plants
2.6. Anti-Elastase Activity of Thai Plants
2.7. Anti-Tyrosinase Activity of Thai Plants
2.8. Correlation Analysis
2.9. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials and Extraction
4.3. Determination of Total Phenolic Content
4.4. Determination of Total Flavonoid Content
4.5. Determination of DPPH Radical Scavenging Activity
4.6. Determination of ABTS Radical Scavenging Activity
4.7. Determination of Anti-Elastase Activity
4.8. Determination of Anti-Tyrosinase Activity
4.9. Molecular Docking
4.9.1. Ligand Preparation
4.9.2. Protein Preparation
4.9.3. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kligman, A.M. What is the ‘true’ function of skin? Exp. Dermatol. 2002, 11, 159. [Google Scholar]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. (Eds.) Degenerative Changes in Aging Skin. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–30. [Google Scholar]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Rinnerhaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef] [Green Version]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Lin, C.-C.; Wang, H.-Y.; Shih, Y.; Chou, S.-T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Park, J.H.; Ku, H.J.; Lee, J.H.; Park, J.-W. IDH2 deficiency accelerates skin pigmentation in mice via enhancing melanogenesis. Redox Biol. 2018, 17, 16–24. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, K.; Alzate, F.; Osorio, E. Fruits of selected wild and cultivated Andean plants as sources of potential compounds with antioxidant and anti-aging activity. Ind. Crops Prod. 2016, 85, 341–352. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Shrestha, K.P.; Bussmann, R.W. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomedicine 2010, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Passsari, A.K.; Leo, V.V.; Mishra, V.K.; Subbarayan, S.; Singh, B.P.; Kumar, B.; Kumar, S.; Gupta, V.K.; Lalhlenmawia, H. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front. Plant Sci. 2016, 7, 407. [Google Scholar] [CrossRef] [Green Version]
- Byun, N.-y.; Heo, M.-R.; Yim, S.-H. Correlation of anti-wrinkling and free radical antioxidant activities of Areca nut with phenolic and flavonoid contents. Food Sci. Technol. 2021, 41, 1041–1049. [Google Scholar] [CrossRef]
- Sellar, T.; Arulrajah, A.A.; Lanka, V. The Role of Social Support on Job Burnout in the Apparel Firm. Int. Bus. Res. 2019, 12, 110–118. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.-Y. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from Helichrysum teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M.N. Anti–elastase, anti–tyrosinase and matrix metalloproteinase–1 inhibitory activity of earthworm extracts as potential new anti–aging agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Konan, N.A.; Bacchi, E.M. Antiulcerogenic effect and acute toxicity of a hydroethanolic extract from the cashew (Anacardium occidentale L.) leaves. J. Ethnopharmacol. 2007, 112, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ajileye, O.; Obuotor, E.; Akinkunmi, E.; Aderogba, M. Isolation and characterization of antioxidant and antimicrobial compounds from Anacardium occidentale L.(Anacardiaceae) leaf extract. J. King Saud Univ. Sci. 2015, 27, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Chotphruethipong, L.; Benjakul, S.; Kijroongrojana, K. Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale L.) leaves using response surface methodology. J. Food Biochem. 2017, 41, e12379. [Google Scholar] [CrossRef]
- Moriyama, H.; Iizuka, T.; Nagai, M.; Miyataka, H.; Satoh, T. Antiinflammatory activity of heat-treated Cassia alata leaf extract and its flavonoid glycoside. Yakugaku Zasshi 2003, 123, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Chatatikun, M.; Yamauchi, T.; Yamasaki, K.; Aiba, S.; Chiabchalard, A. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in α-MSH stimulated B16F10 cells. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar] [CrossRef]
- Wang, R.; Pan, F.; He, R.; Kuang, F.; Wang, L.; Lin, X. Arecanut (Areca catechu L.) seed extracts extracted by conventional and eco-friendly solvents: Relation between phytochemical compositions and biological activities by multivariate analysis. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100336. [Google Scholar] [CrossRef]
- Nonaka, G.-i.; Hsu, F.-L.; Nishioka, I. Structures of dimeric, trimeric, and tetrameric procyanidins from Areca catechu L. J. Chem. Soc. Chem. Commun. 1981, 15, 781–783. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Wink, M.; Tencomnao, T. Anacardium Occidentale, L. Leaf Extracts Protect Against Glutamate/H2O2-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein. Front. Pharmacol. 2021, 12, 627738. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical description and biological activities of Senna alata. Evid. Based Complement. Alternat. Med. 2020, 2020, 2580259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Liu, Y.-J.; Wu, N.; Sun, T.; He, X.-Y.; Gao, Y.-X.; Wu, C.-J. Areca catechu L.(Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2015, 164, 340–356. [Google Scholar] [CrossRef]
- Salehi, B.; Konovalov, D.A.; Fru, P.; Kapewangolo, P.; Peron, G.; Ksenija, M.S.; Cardoso, S.M.; Pereira, O.R.; Nigam, M.; Nicola, S. Areca catechu—From farm to food and biomedical applications. Phytother. Res. 2020, 34, 2140–2158. [Google Scholar] [CrossRef]
- Verma, D.K.; Bharat, M.; Nayak, D.; Shanbhag, T.; Shanbhag, V.; Rajput, R.S. Areca catechu: Effect of topical ethanolic extract on burn wound healing in albino rats. Int. J. Pharmacol. Clin. Sci. 2012, 1, 74–78. [Google Scholar]
- Pithayanukul, P.; Nithitanakool, S.; Bavovada, R. Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules 2009, 14, 4987–5000. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Silva, T.G.d.; Coutinho, H.D.M.; Amina, B. Antioxidant, antimicrobial, and anticancer effects of anacardium plants: An ethnopharmacological perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Gu, X.; Wink, M.; Tencomnao, T. Neuroprotective Effects of Glochidion zeylanicum Leaf Extract against H2O2/Glutamate-Induced Toxicity in Cultured Neuronal Cells and Aβ-Induced Toxicity in Caenorhabditis elegans. Biology 2021, 10, 800. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 9012396. [Google Scholar] [CrossRef] [Green Version]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Data on the effects of Glochidion zeylanicum leaf extracts in Caenorhabditis elegans. Data Brief 2019, 26, 104461. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, M.; Doss, D.V.A.; Maddisetty, P.P. Hepato-renal protective effects of hydroethanolic extract of Senna alata on enzymatic and nonenzymatic antioxidant systems in streptozotocin induced diabetic rats. Integr. Med. Res. 2016, 5, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazeddinova, D.; Rahman, M.; Hamdan, S.B.; Matin, M.M.; Bin Bakri, M.K.; Rahman, M.M. Plant Based Polyphenol Associations with Protein: A Prospective Review. BioResources 2022, 17, 1–25. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Tencomnao, T. Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate. BMC Complement. Altern. Med. 2018, 18, 278. [Google Scholar] [CrossRef] [Green Version]
- Abhijit, S.; Manjushree, D. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot. 2010, 6, 299–303. [Google Scholar]
- Widowati, W.; Rani, A.P.; Hamzah, R.A.; Arumwardana, S.; Afifah, E.; Kusuma, H.S.W.; Rihibiha, D.D.; Nufus, H.; Amalia, A. Antioxidant and antiaging assays of Hibiscus sabdariffa extract and its compounds. Nat. Prod. Sci. 2017, 23, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Fernand, V.E.; Dinh, D.T.; Washington, S.J.; Fakayode, S.O.; Losso, J.N.; van Ravenswaay, R.O.; Warner, I.M. Determination of pharmacologically active compounds in root extracts of Cassia alata L. by use of high performance liquid chromatography. Talanta 2008, 74, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Garg, A.; Parascandola, M.; Chaturvedi, P.; Khariwala, S.S.; Stepanov, I. Analysis of alkaloids in areca nut-containing products by liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 1977–1983. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Xu, L.; Zou, Z.; Yang, S. Studies on chemical constituents from leaves of Cassia alata. Zhongguo Zhong Yao Za Zhi 2009, 34, 861–863. [Google Scholar] [PubMed]
- Ming-Yue, W.; Jin-Hui, L.; Jian-Guo, L. Determination of Polyphenols in Areca catechu by HPLC. Nat. Prod. Res. Dev. 2011, 23, 101. [Google Scholar]
- Mohammed, A.R.; Ali, A.; Aboul-Enein, S.; Mohamed, F.; Abou, E.; Magdy, M.; Mohammed, A. Phytochemical, cytotoxicity and antioxidant investigation of Cassia alata leaves growing in Egypt. J. Innov. Pharm. Biol. Sci 2017, 4, 97–105. [Google Scholar]
- Okpuzor, J.; Ogbunugafor, H.; Kareem, G.; Igwo-Ezikpe, M. In vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res. J. Phytochem. 2009, 3, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Pongnimitprasert, N.; Wadkhien, K.; Chinpaisal, C.; Satiraphan, M.; Wetwitayaklung, P. Anti-inflammatory effects of rhein and crude extracts from Cassia alata L. in HaCaT cells. Sci. Eng. Health Stud. 2018, 12, 19–32. [Google Scholar]
- Promgool, T.; Pancharoen, O.; Deachathai, S. Antibacterial and antioxidative compounds from Cassia alata Linn. Songklanakarin J. Sci. Technol. 2014, 36, 459–463. [Google Scholar]
- Singh, B.; Nadkarni, J.R.; Vishwakarma, R.A.; Bharate, S.B.; Nivsarkar, M.; Anandjiwala, S. The hydroalcoholic extract of Cassia alata (Linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. J. Ethnopharmacol. 2012, 141, 469–473. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Simon, J.E. Qualitative and quantitative HPLC/MS determination of proanthocyanidins in areca nut (Areca catechu). Chem. Biodivers. 2007, 4, 2817–2826. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Huang, W.-Y.; Chen, W.-X.; Han, L.; Zhang, H.-D. Optimization of extraction conditions of areca seed polyphenols and evaluation of their antioxidant activities. Molecules 2014, 19, 16416–16427. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, W.; Zeng, Y.; Liu, J. Phenolic constituents from the fruits of Areca catechu and their anti-bacterial activities. J. Trop. Subtrop. Bot. 2009, 17, 74–76. [Google Scholar]
- Tamada, T.; Kinoshita, T.; Kurihara, K.; Adachi, M.; Ohhara, T.; Imai, K.; Kuroki, R.; Tada, T. Combined high-resolution neutron and X-ray analysis of inhibited elastase confirms the active-site oxyanion hole but rules against a low-barrier hydrogen bond. J. Am. Chem. Soc. 2009, 131, 11033–11040. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]





| Scientific Name | Part Used | Source | Voucher Number | Extraction Method/Solvent | %Yield (w/w) |
|---|---|---|---|---|---|
| Anacardium occidentale L. | Leaf | Songkhla, Thailand | 015863 (BCU) | Soxhlet/Methanol | 16.0 |
| Areca catechu L. | Fruit | Surat Thani, Thailand | 016434 (BCU) | Soxhlet/Ethanol | 10.5 |
| Carissa carandas L. | Fruit | Chachoengsao, Thailand | 016531 (BCU) | Soxhlet/Ethanol | 36.3 |
| Centella asiatica (L.) Urb. | Leaf | Bangkok, Thailand | 016426 (BCU) | Maceration/Ethanol | 4.1 |
| Clitoria macrophylla Wall. | Flower | Bangkok, Thailand | - a | Soxhlet/Ethanol | 11.9 |
| Clitoria ternatea L. | Flower | Chonburi, Thailand | - a | Soxhlet/Methanol | 12.8 |
| Eleutherine americana (Aubl.) Merr. | Rhizome | Ubon Ratchathani, Thailand | 016530 (BCU) | Maceration/Ethanol | 3.3 |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | Songkhla, Thailand | 016061 (BCU) | Soxhlet/Methanol | 11.8 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | Nonthaburi, Thailand | 016446 (BCU) | Soxhlet/Ethanol | 2.0 |
| Mangifera caloneura Kurz. | Leaf | Songkhla, Thailand | 016445 (BCU) | Soxhlet/Ethanol | 18.2 |
| Piper nigrum L. | Seed | Bangkok, Thailand | 016428 (BCU) | Maceration/Ethanol | 5.0 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | Chachoengsao, Thailand | 017139 (BCU) | Soxhlet/Ethanol | 6.4 |
| Senna alata (L.) Roxb. | Leaf | Ubon Ratchathani, Thailand | 016298 (BCU) | Maceration/Ethanol | 10.5 |
| Streblus asper Lour. | Bark | Rayong, Thailand | 013419(BCU) | Maceration/Ethanol | 2.5 |
| Streblus asper Lour. | Leaf | Rayong, Thailand | 013419(BCU) | Maceration/Ethanol | 4.0 |
| Zingiber cassumunar Roxb. | Rhizome | Rayong, Thailand | 013701 (BCU) | Maceration/Ethanol | 6.4 |
| Zingiber officinale Roscoe. | Rhizome | Bangkok, Thailand | 016425 (BCU) | Maceration/Ethanol | 5.2 |
| Scientific Name | Part Used | Total Phenolic Content (mg GAE/g Dry Weight Extract) | Total Flavonoid Content (mg QE/g Dry Weight Extract) |
|---|---|---|---|
| Anacardium occidentale L. | Leaf | 173.86 ± 4.75 | 25.34 ± 2.88 |
| Areca catechu L. | Fruit | 295.79 ± 11.97 | 2.62 ± 0.36 |
| Carissa carandas L. | Fruit | 14.06 ± 1.55 | 2.83 ± 0.78 |
| Centella asiatica (L.) Urb. | Leaf | 15.26 ± 0.76 | 11.25 ± 2.87 |
| Clitoria macrophylla Wall. | Flower | 64.85 ± 2.81 | 14.74 ± 2.71 |
| Clitoria ternatea L. | Flower | 25.60 ± 2.19 | 10.33 ± 2.31 |
| Eleutherine americana (Aubl.) Merr. | Rhizome | 73.73 ± 1.87 | 2.61 ± 0.42 |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | 320.14 ± 7.95 | 52.54 ± 7.25 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | 70.39 ± 4.57 | 22.20 ± 3.20 |
| Mangifera caloneura Kurz. | Leaf | 210.99 ± 10.40 | 84.48 ± 18.32 |
| Piper nigrum L. | Seed | 47.86 ± 2.27 | 3.46 ± 0.70 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | 61.82 ± 0.61 | 28.01 ± 4.39 |
| Senna alata (L.) Roxb. | Leaf | 45.36 ± 1.15 | 10.24 ± 2.52 |
| Streblus asper Lour. | Bark | 18.02 ± 0.30 | 2.72 ± 0.89 |
| Streblus asper Lour. | Leaf | 43.58 ± 2.40 | 3.30 ± 1.50 |
| Zingiber cassumunar Roxb. | Rhizome | 44.63 ± 0.61 | 4.85 ± 0.98 |
| Zingiber officinale Roscoe. | Rhizome | 139.94 ± 2.27 | 2.86 ± 0.74 |
| Scientific Name | Part Used | DPPH Radical Scavenging | ||
|---|---|---|---|---|
| Scavenging Activity (%) | mg VCEAC/g Dry Weight Extract | IC50 (µg/mL) | ||
| Anacardium occidentale L. | Leaf | 89.01 ± 1.51 | 387.43 ± 13.97 | 18.68 ± 0.59 |
| Areca catechu L. | Fruit | 88.12 ± 5.04 | 627.64 ± 8.94 | 9.85 ± 0.91 |
| Carissa carandas L. | Fruit | 12.76 ± 1.13 | 16.63 ± 2.32 | >100 |
| Centella asiatica (L.) Urb. | Leaf | 15.38 ± 0.93 | 19.52 ± 1.48 | >100 |
| Clitoria macrophylla Wall. | Flower | 9.01 ± 1.43 | 10.03 ± 1.50 | >100 |
| Clitoria ternatea L. | Flower | 6.40 ± 0.45 | 7.49 ± 0.29 | >100 |
| Eleutherine americana (Aubl.) Merr. | Rhizome | 45.58 ± 7.14 | 53.49 ± 6.97 | 188.05 ± 43.01 |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | 93.18 ± 0.64 | 1154.54 ± 36.19 | 6.56 ± 0.46 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | 26.57 ± 2.03 | 33.91 ± 2.07 | >100 |
| Mangifera caloneura Kurz. | Leaf | 90.61 ± 3.27 | 289.44 ± 10.68 | 20.89 ± 2.27 |
| Piper nigrum L. | Seed | 14.71 ± 1.72 | 18.74 ± 2.78 | >100 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | 46.84 ± 3.68 | 55.09 ± 2.72 | 120.84 ± 25.33 |
| Senna alata (L.) Roxb. | Leaf | 27.16 ± 4.56 | 33.12 ± 4.28 | >100 |
| Streblus asper Lour. | Bark | 11.93 ± 2.26 | 13.14 ± 1.76 | >100 |
| Streblus asper Lour. | Leaf | 28.53 ± 2.63 | 30.94 ± 2.05 | >100 |
| Zingiber cassumunar Roxb. | Rhizome | 33.37 ± 4.70 | 40.61 ± 4.99 | 253.63 ± 24.05 |
| Zingiber officinale Roscoe. | Rhizome | 71.73 ± 5.29 | 82.22 ± 6.68 | 67.21 ± 13.31 |
| Scientific Name | Part Used | ABTS Radical Scavenging | ||
|---|---|---|---|---|
| Scavenging Activity (%) | mg VCEAC/g Dry Weight Extract | IC50 (µg/mL) | ||
| Anacardium occidentale L. | Leaf | 99.23 ± 0.29 | 675.44 ± 65.66 | 8.64 ± 0.66 |
| Areca catechu L. | Fruit | 99.31 ± 0.32 | 837.47 ± 44.16 | 5.14 ± 1.42 |
| Carissa carandas L. | Fruit | 13.93 ± 1.03 | 12.41 ± 1.97 | >100 |
| Centella asiatica (L.) Urb. | Leaf | 18.86 ± 4.81 | 16.98 ± 4.73 | 324.22 ± 46.00 |
| Clitoria macrophylla Wall. | Flower | 38.27 ± 7.24 | 34.78 ± 5.01 | >100 |
| Clitoria ternatea L. | Flower | 26.48 ± 2.24 | 26.35 ± 3.28 | >100 |
| Eleutherine americana (Aubl.) Merr. | Rhizome | 95.62 ± 6.13 | 213.63 ± 9.12 | 20.23 ± 4.72 |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | 99.37 ± 0.21 | 1184.59 ± 51.41 | 3.76 ± 0.79 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | 82.16 ± 2.17 | 81.73 ± 4.78 | 44.23 ± 5.13 |
| Mangifera caloneura Kurz. | Leaf | 98.88 ± 1.55 | 531.29 ± 26.11 | 9.31 ± 0.85 |
| Piper nigrum L. | Seed | 33.28 ± 4.72 | 30.93 ± 4.13 | 150.35 ± 34.82 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | 83.99 ± 6.01 | 79.65 ± 4.36 | 49.52 ± 7.01 |
| Senna alata (L.) Roxb. | Leaf | 64.95 ± 7.32 | 61.92 ± 5.38 | 52.20 ± 2.94 |
| Streblus asper Lour. | Bark | 23.55 ± 2.92 | 23.29 ± 1.33 | >100 |
| Streblus asper Lour. | Leaf | 65.60 ± 6.47 | 59.33 ± 6.67 | 66.36 ± 7.89 |
| Zingiber cassumunar Roxb. | Rhizome | 73.36 ± 5.89 | 69.88 ± 5.88 | 43.60 ± 5.49 |
| Zingiber officinale Roscoe. | Rhizome | 94.07 ± 3.44 | 175.47 ± 16.28 | 22.76 ± 9.79 |
| Scientific Name | Part Used | Elastase Inhibition (%) | ||
|---|---|---|---|---|
| 0.5 mg/mL | 0.1 mg/mL | IC50 (µg/mL) | ||
| Anacardium occidentale L. | Leaf | 84.78 ± 2.16 | - | 18.21 ± 4.91 |
| Areca catechu L. | Fruit | 88.31 ± 0.41 | - | 117.07 ± 21.71 |
| Carissa carandas L. | Fruit | nd | - | - |
| Centella asiatica (L.) Urb. | Leaf | nd | - | - |
| Clitoria macrophylla Wall. | Flower | 9.85 ± 2.26 | - | >500 |
| Clitoria ternatea L. | Flower | nd | - | - |
| Eleutherine americana (Aubl.) Merr. | Rhizome | nd | - | - |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | 87.43 ± 3.80 | - | 47.94 ± 24.75 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | nd | - | - |
| Mangifera caloneura Kurz. | Leaf | na | 13.75 ± 1.61 | >100 |
| Piper nigrum L. | Seed | na | 9.05 ± 0.09 | >100 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | 22.87 ± 2.92 | - | >500 |
| Senna alata (L.) Roxb. | Leaf | 73.95 ± 1.46 | - | 82.25 ± 19.99 |
| Streblus asper Lour. | Bark | 1.33 ± 0.89 | - | > 500 |
| Streblus asper Lour. | Leaf | na | 35.74 ± 0.94 | 153.28 ± 2.39 |
| Zingiber cassumunar Roxb. | Rhizome | na | 3.46 ± 1.29 | >100 |
| Zingiber officinale Roscoe. | Rhizome | na | nd | - |
| EGCG (0.1 mg/mL) | - | 45.27 ± 3.36 | - | |
| Scientific Name | Part Used | Tyrosinase Inhibition (%) | ||
|---|---|---|---|---|
| 1 mg/mL | 0.1 mg/mL | IC50 (µg/mL) | ||
| Anacardium occidentale L. | Leaf | 81.01 ± 2.96 | - | 307.66 ± 65.12 |
| Areca catechu L. | Fruit | 75.38 ± 1.57 | - | 85.73 ± 8.26 |
| Carissa carandas L. | Fruit | 15.00 ± 1.21 | - | >1000 |
| Centella asiatica (L.) Urb. | Leaf | 13.57 ± 1.23 | - | >1000 |
| Clitoria macrophylla Wall. | Flower | 27.36 ± 7.95 | - | >1000 |
| Clitoria ternatea L. | Flower | 10.02 ± 1.61 | - | >1000 |
| Eleutherine americana (Aubl.) Merr. | Rhizome | 45.10 ± 1.59 | - | >1000 |
| Glochidion zeylanicum (Gaertn.) A. Juss. | Leaf | 91.51 ± 5.39 | - | 76.00 ± 4.31 |
| Hylocereus undatus (Haw.) Britt. Rose. | Peel | 15.79 ± 0.84 | - | >1000 |
| Mangifera caloneura Kurz. | Leaf | 76.12 ± 3.98 | - | 457.63 ± 71.73 |
| Piper nigrum L. | Seed | 4.80 ± 1.31 | - | >1000 |
| Pithecellobium dulce (Roxb.) Benth. | Peel | nd | - | - |
| Senna alata (L.) Roxb. | Leaf | 12.94 ± 2.73 | - | >1000 |
| Streblus asper Lour. | Bark | 9.85 ± 1.14 | - | >1000 |
| Streblus asper Lour. | Leaf | 5.11 ± 3.88 | - | >1000 |
| Zingiber cassumunar Roxb. | Rhizome | na | 3.98 ± 0.54 | >100 |
| Zingiber officinale Roscoe. | Rhizome | na | 21.28 ± 2.53 | >100 |
| KA (0.02 mg/mL) | - | 68.35 ± 1.22 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaikhong, K.; Chumpolphant, S.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T.; Prasansuklab, A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants 2023, 12, 65. https://doi.org/10.3390/plants12010065
Chaikhong K, Chumpolphant S, Rangsinth P, Sillapachaiyaporn C, Chuchawankul S, Tencomnao T, Prasansuklab A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants. 2023; 12(1):65. https://doi.org/10.3390/plants12010065
Chicago/Turabian StyleChaikhong, Kamonwan, Sawarin Chumpolphant, Panthakarn Rangsinth, Chanin Sillapachaiyaporn, Siriporn Chuchawankul, Tewin Tencomnao, and Anchalee Prasansuklab. 2023. "Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction" Plants 12, no. 1: 65. https://doi.org/10.3390/plants12010065
APA StyleChaikhong, K., Chumpolphant, S., Rangsinth, P., Sillapachaiyaporn, C., Chuchawankul, S., Tencomnao, T., & Prasansuklab, A. (2023). Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants, 12(1), 65. https://doi.org/10.3390/plants12010065