Antioxidant, Whitening, Antiwrinkle, and Anti-Inflammatory Effect of Ajuga spectabilis Nakai Extract
Abstract
1. Introduction
2. Results
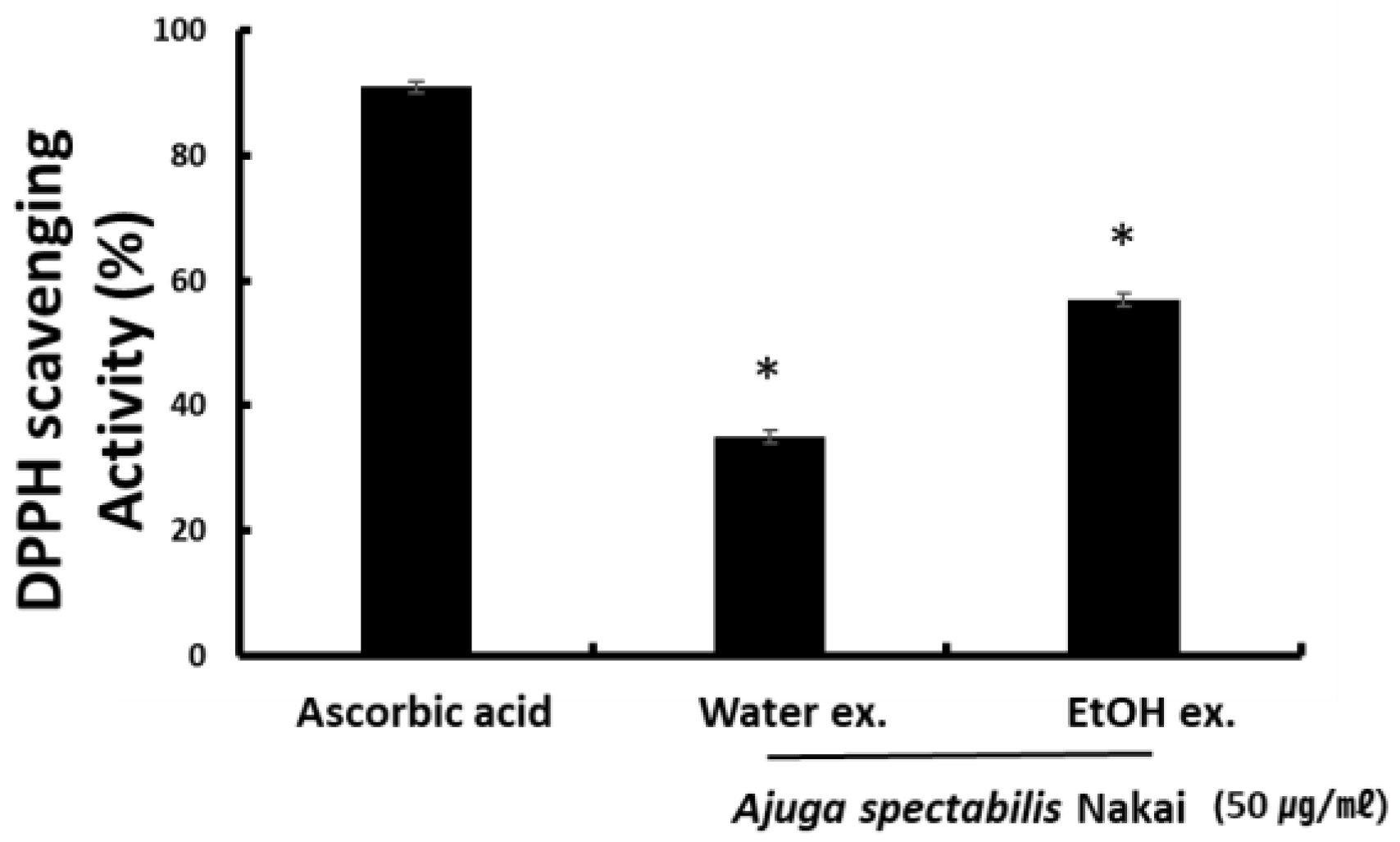
2.1. DPPH Radical Scavenging Activity
2.2. Tyrosinase Inhibitory Activity
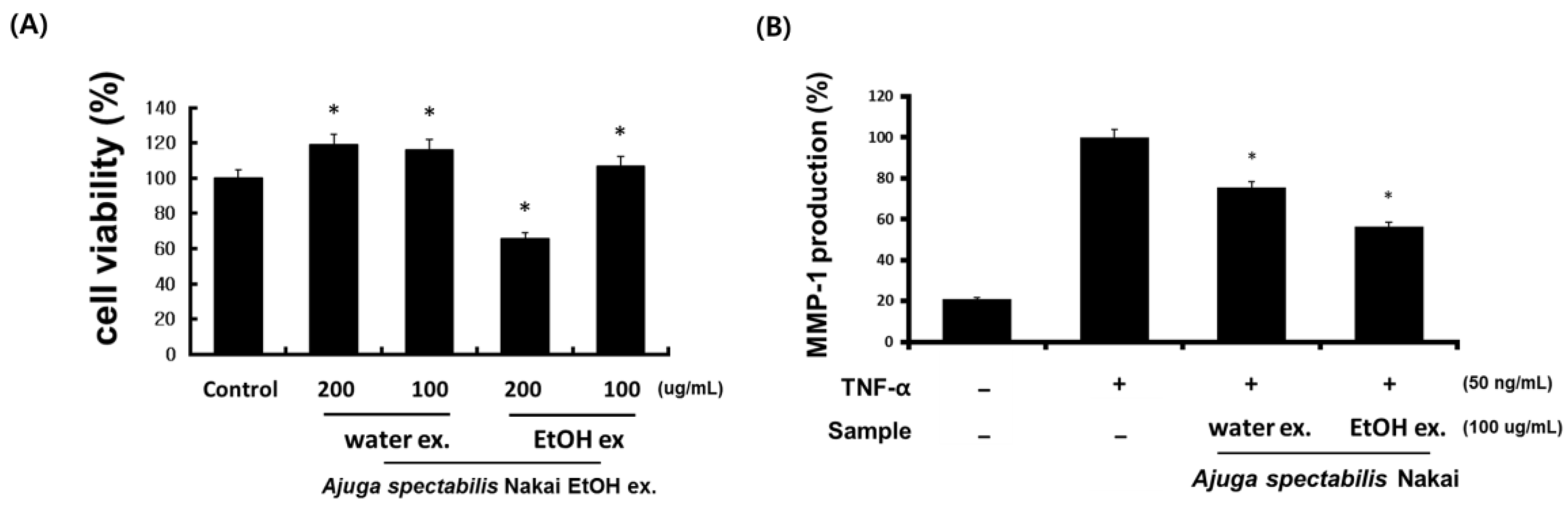
2.3. Cell Viability and MMP-1 Expression Inhibitory Activity (CCD-986sK)
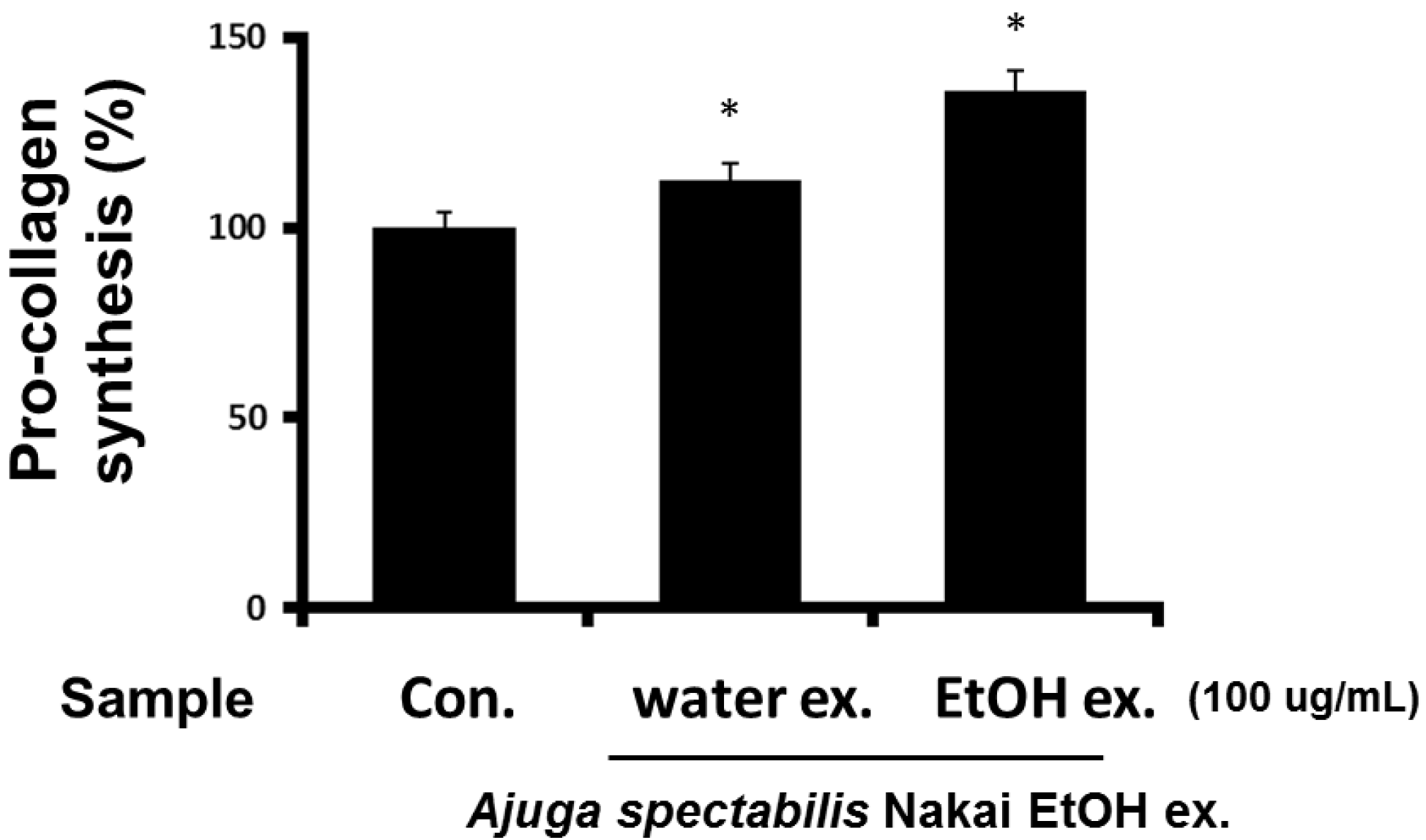
2.4. Measurement of Pro-Collagen Synthesis in Fibroblasts (CCD-986sK)
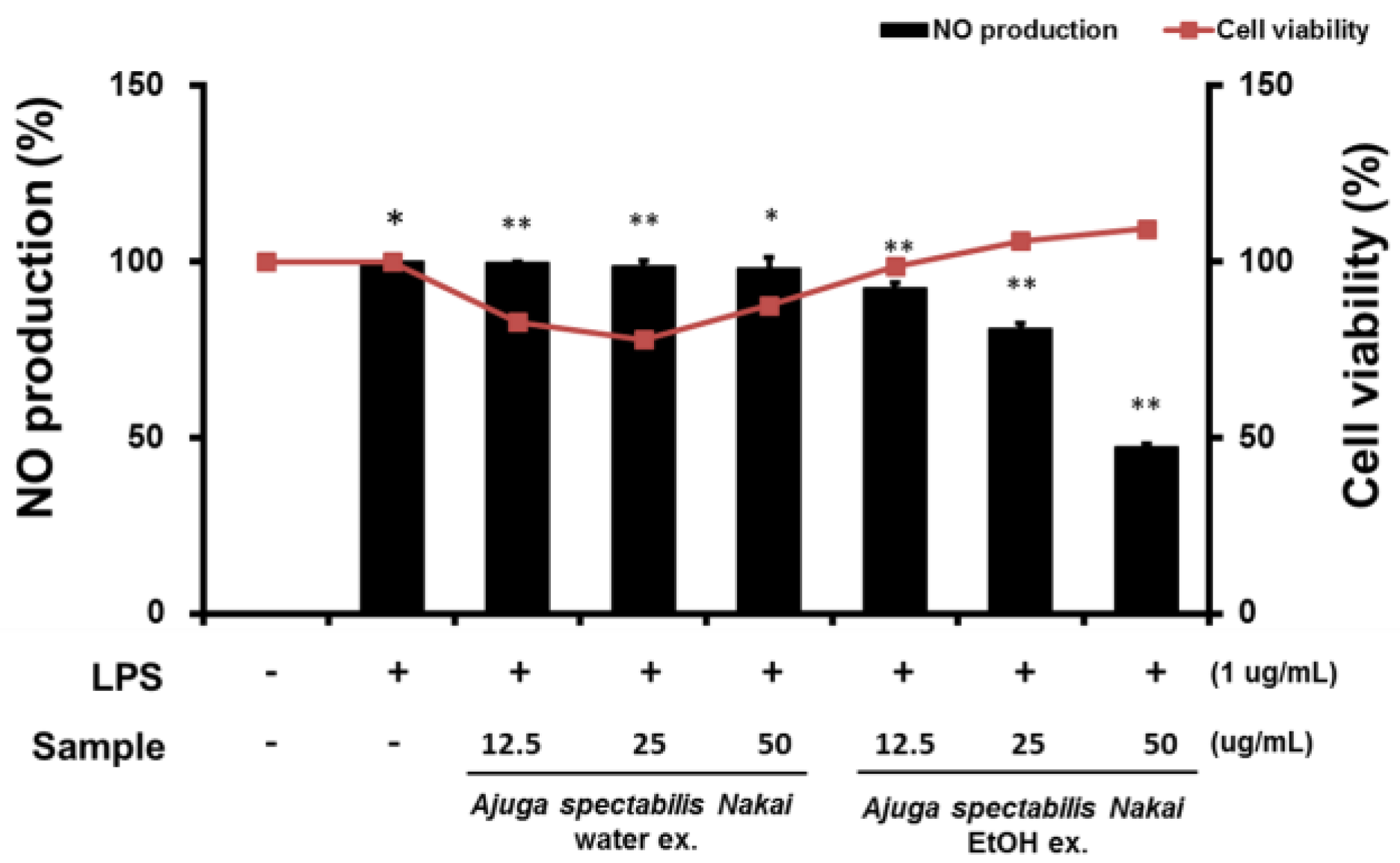
2.5. Cell Viability and NO Production of Macrophages RAW264.7
2.6. Inhibition of Inflammatory Cytokine (IL-6, TNF-α)
3. Discussions
4. Materials and Methods
4.1. Chemical Reagents
4.2. Sample Preparation
4.3. Cell Culture
4.4. DPPH Radical Scavenging Activity
4.5. Tyrosinase Inhibitory Activity
4.6. MMP-1 Expression Inhibition Activity
4.7. Procollagen Synthesis Activity
4.8. Measurement of NO Production Inhibition
4.9. Measurement of Cell Viability
4.10. Inflammation Cytokine Inhibitory Measurement
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, J.W.; Ryu, M.S.; Yang, H.J.; Jeong, S.J.; Jeong, D.Y. The antioxidant and skin-whitening effects of Saccharomyces cerevisiae FT4-4 isolated from berries grown in Sunchang. J. Life Sci. 2021, 31, 175–182. [Google Scholar]
- Kim, B.H.; Park, K.C.; Park, J.H.; Lee, C.G.; Ye, S.K.; Park, J.Y. Inhibition of tyrosinase activity and melanin production by the chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl)-propenone. Biochem. Biophys. Res. Commun. 2016, 480, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.C.; Grossman, D. Role of melanin in melanocyte dysregulation of reactive oxygen species. BioMed Res. Int. 2013, 2013, 908797. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Ahn, J.Y.; Lee, H.Y.; Ha, T.Y. Various properties and phenolic acid contents of rices and rice brans with different milling fractions. Kor. J. Food Sci. Technol. 2004, 36, 930–936. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought stress responses in plants, oxidative stress, and antioxidant defense. In Climate Change and Plant Abiotic Stress Tolerance; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 161–165. [Google Scholar] [CrossRef]
- Bai, J.; Rodriguez, A.M.; Melendez, J.A.; Cederbaum, A.I. Over-expression of catalase in cytosolic or mitochondrial compartment protests Hep G2 cells against oxidative injury. J. Biol. Chem. 1999, 274, 26217–26224. [Google Scholar] [CrossRef]
- Tome, M.E.; Baker, A.F.; Powis, G.; Payne, C.M.; Briehl, M.M. Catalase-overexpressing thymocytes are resistant to glucocorticoid-induced apoptosis and exhibit increased net tumor growth. Cancer Res. 2001, 61, 2766–2773. [Google Scholar]
- Kim, J.R.; Kim, M.M. Effect of Rhynchosia Nulubilis ethanolic extract on DOPA oxidation and melanin synthesis. J. Life Sci. 2018, 28, 331–338. [Google Scholar]
- Le, K.T.; Schachter, J.; Quigley, M.G. The role of melanin in protecting the skin and the retina from light damage: A comparative biological framework for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 627. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol. 1994, 14, 7996–8006. [Google Scholar] [PubMed]
- Korea Health Compendium Research Society. Functional Test Guide for Health Functional Foods (2); Korea Health Compendium Research Society: Cheongju-si, Korea, 2004; pp. 821–858. Available online: https://impfood.mfds.go.kr/CFBDD06F02/getCntntsDetail?cntntsSn=288167 (accessed on 17 November 2022).
- Shin, D.C.; Kim, G.C.; Song, S.Y.; Kim, H.J.; Yang, J.C.; Lee, Y.H.; Kim, B.A. Antiaging activity of mixed extracts from Korean medicinal herbs on HS68 skin fibroblast. Korea J. Herbol. 2014, 29, 39. [Google Scholar] [CrossRef][Green Version]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (-)epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287. [Google Scholar] [CrossRef]
- Chung, T.H. Korean Flora, Vol. II, Herbaceous Plants; Shinjisa Publishing Co.: Seoul, Korea, 1972; pp. 1–589. [Google Scholar]
- Lichti, H.; Wartburg, A.V. Structure of scillaglaucoside. 56. Section on cardiac glycosides. Helv. Chi. Acta 1996, 49, 1552. [Google Scholar] [CrossRef]
- Woo, K.W.; Sim, M.O.; Kim, H.A.; Kang, B.M.; Jung, H.K.; An, B.; Cho, J.H.; Cho, H.W. Quantitative Analysis of luteolin 5-glucoside in Ajuga spectabilis and Their neuroprotective Effects. Kor. J. Pharm. 2016, 47, 211–216. [Google Scholar]
- Chung, B.S.; Lee, H.K.; Kim, J.W. Iridoid Glycoside (I)-Studies on the Iridoid Glycoside of Ajuga spectabilis Nakai. Korean J. Pharmacogn. 1980, 11, 15–23. [Google Scholar]
- Yang, Y.X.; Lee, J.A. Anti-inflammatory, anti-wrinkle and astringent activities of the Euphorbia thymifolia extract: An in vitro study for anti-aging applications. J. Korean Soc. Cosmetol. 2022, 28, 525–530. [Google Scholar] [CrossRef]
- Lee, T.B.; So, Y.K.; Kim, S.Y.; Hwang, J.Y. Biological activities of cosmetic material from ten kinds of flower ethanol extracts. Korean J. Med. Crop Sci. 2020, 28, 260–275. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic functional ingredients from botanical sources for anti-pollution skincare products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Ahn, J.; Gammon, M.D.; Santella, R.M.; Gaudet, M.M.; Britton, J.A.; Teitelbaum, S.L.; Terry, M.B.; Nowell, S.; Davis, W.; Garza, C.; et al. Associations between breast cancer risk and the catalase genotype, fruit and vegetable consumption, and supplement use. Am. J. Epidemiol. 2005, 162, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Suh, S.J.; Lee, K.H.; Yang, J.B.; Choi, S.U.; Park, S.S. Anti-inflammatory effect of peel extracts from citrus fruits. J. Food Hyg. S. Afr. 2013, 28, 342–348. [Google Scholar]
- Kim, K.B.; Yoo, K.H.; Park, H.Y.; Jeong, J.M. Antioxidative activities of commercial edible plant extracts distributed in Korea. J. Korean Soc. Appl. Biol. Chem. 2006, 49, 328–333. [Google Scholar]
- Ji, Y.J.; Lee, E.Y.; Lee, J.Y.; Lee, Y.J.; Lee, S.E.; Seo, K.H.; Kim, H.D. Antioxidant and anti-diabetic effects of Agastache rugosa extract. J. East Asian Soc. Diet Life 2020, 30, 297–305. [Google Scholar] [CrossRef]
- Kameyama, K. Pigment production in murine melanoma cells is regulated protein, dopachrome tautomerase(TRP2) and a melanogenic inhibitor. J. Investig. Dermatol. 1993, 141, 20–29. [Google Scholar]
- Lee, S.H.; Kim, S.Y.; Kim, J.J.; Jang, T.S.; Chung, S.R. The isolation of the inhibitory constitutents on melanin polymer formation from the leaves of Cercis chinensis. Korean J. Pharm. 1999, 30, 397–403. [Google Scholar]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma; a review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Lee, Y.R. Biological Activities of Extracts from Leaf of Angelica gigas Nakai. Korean J. Food Nutr. 2021, 34, 181–186. [Google Scholar]
- Boissy, R.E.; Manga, P. On the etiology of contact/occu- pational vitiligo. Pigment Cell Res. 2004, 17, 208–214. [Google Scholar] [CrossRef]
- Kondo, S. The roles of cytokines in photoaging. J. Dermatol. Sci. 2000, 23 (Suppl. 1), S30–S36. [Google Scholar] [CrossRef]
- Kim, N.M.; Koo, B.S.; Lee, S.K.; Hwang, E.I.; So, S.H.; Do, J.H. Effect of Korean red ginseng on collagen biosynthesis and MMP-I activity in human dermal fibroblast. J. Ginseng Res. 2007, 31, 86–92. [Google Scholar]
- Hong, E.S.; Ahn, G.W.; Jo, B.K. The study on the potential antiaging properties of Prunella vulgaris extract in vitro and in vivo. THE FACESHOP R&D Center. Korea J. Soc. Cosmet. Sci. Korea 2008, 34, 129–135. [Google Scholar]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced type I and type III procollagens in photodamaged adult human skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Um, M.S. The evaluation of Anti-oxidation, Anti-inflammation and Antiwrinkle activity of Jeju Native Achyranthes japonica Nakai. J. Korean Appl. Sci. Technol. 2021, 38, 1209–1218. [Google Scholar]
- Furuzawa-Carballeda, J.; Vargas-Rojas, M.I.; Cabral, A.R. Autoimmune inflammation from the Th17 perspective. Autoimmun. Rev. 2007, 6, 169–175. [Google Scholar] [CrossRef]
- Maeng, O.Y.; Kim, Y.C.; Kim, Y.S.; Paik, S.G.; Lee, H.Y. The function and regulation of NO; Identification of a gene that is up-regulated in RAW264.7 macrophage cells by nitric oxide. Boll. Biotechnol. 2002, 2, 1–9. [Google Scholar]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-induced TNFα and IL-6 signaling:the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Oztuurk, A.; Ozbek, H. The anti-inflammatory activity of Eugenia caryophyllata essential oil: An animal model of anti-inflammatory activity. Eur. J. Gen. Med. 2005, 2, 159–163. [Google Scholar]
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008, 110, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Shin, J.H.; Kang, M.J. Antioxidant and anti-inflammatory activities of water extracts and ethanol extracts from Portulaca oleracea L. Korean J. Food Preserv. 2018, 25, 98–106. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Yagi, A.; Kanbara, T.; Morinobu, N. Inhibition of mushroom-tyrosinase by Aloe extract. Planta Med. 1987, 53, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Krupsky, M.; Fine, A.; Berk, J.L.; Goldstein, R.H. Retinoic acid-induced inhibition of type I collagen gene expression by human lung fibroblasts. Biochim. Biophys. Acta 1994, 1219, 335–341. [Google Scholar] [CrossRef]
- Namkoong, S.; Jang, S.A.; Sohn, E.H.; Bak, J.P.; Sohn, E.; Koo, H.J.; Yoon, W.J.; Kwon, J.E.; Jeong, Y.J.; Meng, X.; et al. Comparative study of Litsea japonica leaf and fruit extract on the anti-inflammatory effects. Korean J. Plant Res. 2015, 28, 145–152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.S.; Oh, Y.J.; Kim, J.W.; Han, K.M.; Kim, D.S.; Park, J.W.; Kim, H.M.; Kim, D.W.; Kim, Y.-S. Antioxidant, Whitening, Antiwrinkle, and Anti-Inflammatory Effect of Ajuga spectabilis Nakai Extract. Plants 2023, 12, 79. https://doi.org/10.3390/plants12010079
Lee MS, Oh YJ, Kim JW, Han KM, Kim DS, Park JW, Kim HM, Kim DW, Kim Y-S. Antioxidant, Whitening, Antiwrinkle, and Anti-Inflammatory Effect of Ajuga spectabilis Nakai Extract. Plants. 2023; 12(1):79. https://doi.org/10.3390/plants12010079
Chicago/Turabian StyleLee, Min Sung, Yu Jin Oh, Jae Woo Kim, Kyung Min Han, Da Som Kim, Ji Won Park, Hyeok Mo Kim, Dae Wook Kim, and Yeong-Su Kim. 2023. "Antioxidant, Whitening, Antiwrinkle, and Anti-Inflammatory Effect of Ajuga spectabilis Nakai Extract" Plants 12, no. 1: 79. https://doi.org/10.3390/plants12010079
APA StyleLee, M. S., Oh, Y. J., Kim, J. W., Han, K. M., Kim, D. S., Park, J. W., Kim, H. M., Kim, D. W., & Kim, Y.-S. (2023). Antioxidant, Whitening, Antiwrinkle, and Anti-Inflammatory Effect of Ajuga spectabilis Nakai Extract. Plants, 12(1), 79. https://doi.org/10.3390/plants12010079






