The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants
Abstract
1. Introduction
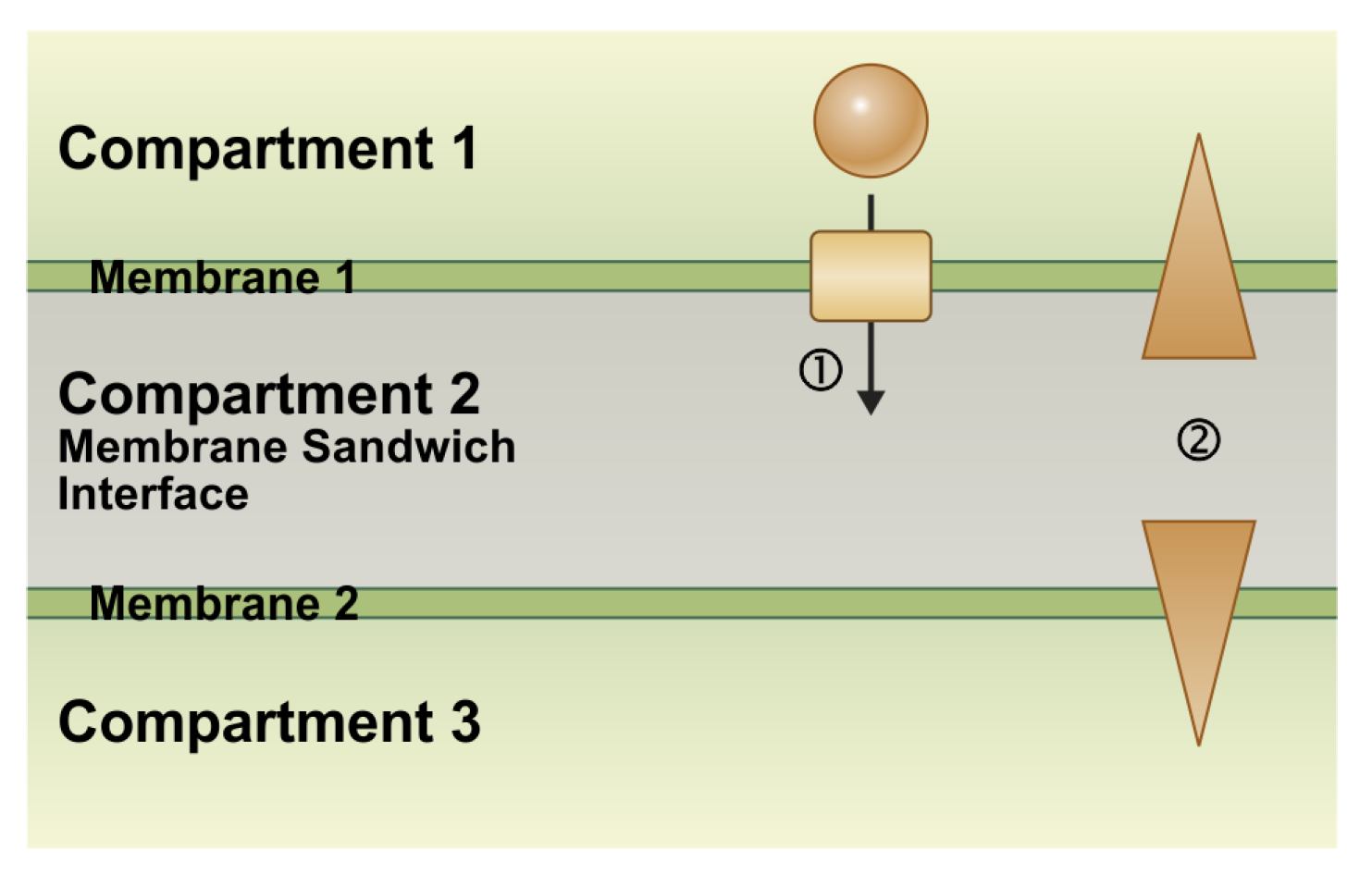
2. Fundamental Biophysical Properties of Membrane Sandwiches in Plants
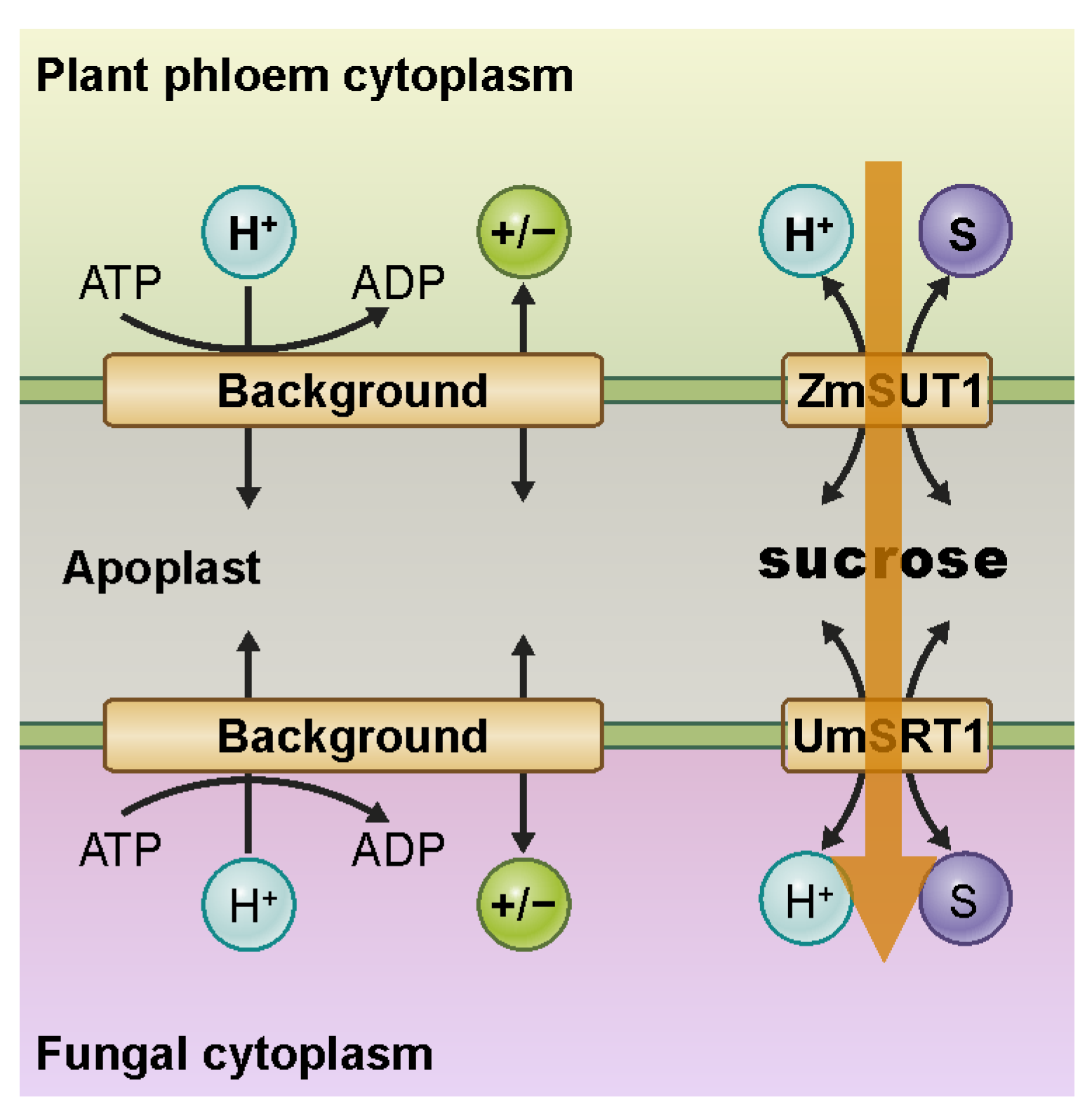
3. Example 1: Remote Control of Phloem (Re-)Loading
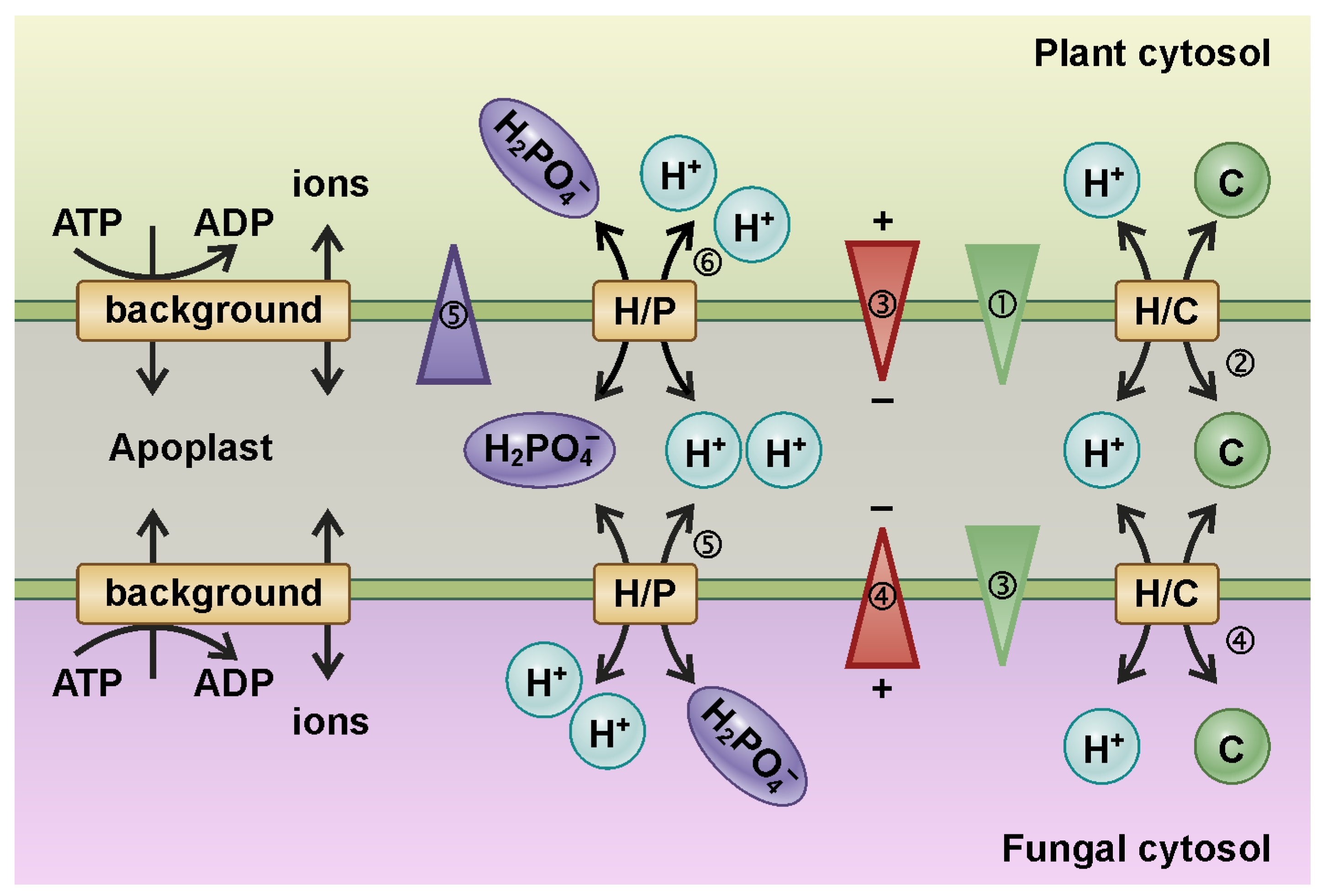
4. Example 2: Self-Regulatory Nutrient Trading in Mycorrhizal Symbioses
5. Example 3: A Small Step from Mutualism to Parasitism in Plant–Fungus Interactions
6. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirel, Y.; Gerbaud, V. Thermodynamics and Biological Systems. In Nonequilibrium Thermodynamics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 489–571. ISBN 978-0-444-64112-0. [Google Scholar]
- Burgess, J. Introduction to Plant Cell Development, 1st ed.; Cambridge University Press: Cambridge, UK, 1985; ISBN 0521316111. [Google Scholar]
- Cain, N.E.; Starr, D.A. SUN proteins and nuclear envelope spacing. Nucleus 2015, 6, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Conklin, P.L. Stromules, functional extensions of plastids within the plant cell. Curr. Opin. Plant Biol. 2020, 58, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, P.; Halkier, B.A.; Schulz, A. Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development. J. Exp. Bot. 2019, 70, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Peng, Y.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for Apoplasmic Phloem Unloading in Developing Apple Fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef]
- Hunziker, P.; Schulz, A. Transmission Electron Microscopy of the Phloem with Minimal Artifacts. In Phloem. Methods in Molecular Biology; Liesche, J., Ed.; Humana Press Inc.: New York, NY, USA, 2019; Volume 2014, pp. 17–27. [Google Scholar]
- Balestrini, R.; Bonfante, P. The interface compartment in arbuscular mycorrhizae: A special type of plant cell wall? Plant Biosyst. 2005, 139, 8–15. [Google Scholar] [CrossRef]
- Boorer, K.J.; Loo, D.D.F.; Frommer, W.B.; Wright, E.M. Transport Mechanism of the Cloned Potato H+/Sucrose Cotransporter StSUT1. J. Biol. Chem. 1996, 271, 25139–25144. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, L.; Dietrich, P.; Dreyer, I.; Hedrich, R. Pronounced differences between the native K+ channels and KAT1 and KST1 alpha-subunit homomers of guard cells. Planta 1999, 207, 370–376. [Google Scholar] [CrossRef]
- Derrer, C.; Wittek, A.; Bamberg, E.; Carpaneto, A.; Dreyer, I.; Geiger, D. Conformational changes represent the rate-limiting step in the transport cycle of maize sucrose transporter1. Plant Cell 2013, 25, 3010–3021. [Google Scholar] [CrossRef][Green Version]
- Babst, B.A.; Braun, D.M.; Karve, A.A.; Frank Baker, R.; Tran, T.M.; Kenny, D.J.; Rohlhill, J.; Knoblauch, J.; Knoblauch, M.; Lohaus, G.; et al. Sugar loading is not required for phloem sap flow in maize plants. Nat. Plants 2022, 8, 171–180. [Google Scholar] [CrossRef]
- Van Bel, A.J.E. The plant axis as the command centre for (re)distribution of sucrose and amino acids. J. Plant Physiol. 2021, 265, 153488. [Google Scholar] [CrossRef]
- Gajdanowicz, P.; Michard, E.; Sandmann, M.; Rocha, M.; Corrêa, L.G.G.; Ramírez-Aguilar, S.J.; Gomez-Porras, J.L.; González, W.; Thibaud, J.-B.; van Dongen, J.T.; et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, M.; Skłodowski, K.; Gajdanowicz, P.; Michard, E.; Rocha, M.; Gomez-Porras, J.L.; González, W.; Corrêa, L.G.G.; Ramírez-Aguilar, S.J.; Cuin, T.A.; et al. The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal. Behav. 2011, 6, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Gomez-Porras, J.L.; Riedelsberger, J. The potassium battery: A mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017, 216, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Michard, E.; Lacombe, B.; Thibaud, J.B. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001, 505, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Pilot, G.; Michard, E.; Gaymard, F.; Sentenac, H.; Thibaud, J.B. A Shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000, 12, 837–851. [Google Scholar] [CrossRef]
- Marten, I.; Hoth, S.; Deeken, R.; Ache, P.; Ketchum, K.A.; Hoshi, T.; Hedrich, R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. USA 1999, 96, 7581–7586. [Google Scholar] [CrossRef]
- Ache, P.; Becker, D.; Deeken, R.; Dreyer, I.; Weber, H.; Fromm, J.; Hedrich, R. VFK1, a Vicia faba K+ channel involved in phloem unloading. Plant J. 2001, 27, 571–580. [Google Scholar] [CrossRef]
- Michard, E.; Lacombe, B.; Porée, F.; Mueller-Roeber, B.; Sentenac, H.; Thibaud, J.-B.; Dreyer, I. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J. Gen. Physiol. 2005, 126, 605–617. [Google Scholar] [CrossRef]
- Michard, E.; Dreyer, I.; Lacombe, B.; Sentenac, H.; Thibaud, J.B. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005, 44, 783–797. [Google Scholar] [CrossRef]
- Dreyer, I. Nutrient cycling is an important mechanism for homeostasis in plant cells. Plant Physiol. 2021, 187, 2246–2261. [Google Scholar] [CrossRef]
- Dreyer, I.; Michard, E. High- and low-affinity transport in plants from a thermodynamic point of view. Front. Plant Sci. 2020, 10, 1797. [Google Scholar] [CrossRef]
- Almario, J.; Fabiańska, I.; Saridis, G.; Bucher, M. Unearthing the plant–microbe quid pro quo in root associations with beneficial fungi. New Phytol. 2022, 234, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Balestrini, R.; Pollmann, S.; Gundel, P.E. Environmental interference of plant-microbe interactions. Plant. Cell Environ. 2022, 45, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Galán, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef]
- Nizam, S.; Qiang, X.; Wawra, S.; Nostadt, R.; Getzke, F.; Schwanke, F.; Dreyer, I.; Langen, G.; Zuccaro, A. Serendipita indica E5’NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep. 2019, 20, e47430. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, H.; Gutjahr, C. Functions of Lipids in Development and Reproduction of Arbuscular Mycorrhizal Fungi. Plant Cell Physiol. 2022, 63, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Duckett, J.G.; Carafa, A.; Ligrone, R. A highly differentiated glomeromycotean association with the mucilage-secreting, primitive antipodean liverwort Treubia (Treubiaceae): Clues to the origins of mycorrhizas. Am. J. Bot. 2006, 93, 797–813. [Google Scholar] [CrossRef]
- Ivanov, S.; Austin, J.; Berg, R.H.; Harrison, M.J. Extensive membrane systems at the host–arbuscular mycorrhizal fungus interface. Nat. Plants 2019, 5, 194–203. [Google Scholar] [CrossRef]
- Roth, R.; Hillmer, S.; Funaya, C.; Chiapello, M.; Schumacher, K.; Lo Presti, L.; Kahmann, R.; Paszkowski, U. Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nat. Plants 2019, 5, 204–211. [Google Scholar] [CrossRef]
- Schott, S.; Valdebenito, B.; Bustos, D.; Gomez-Porras, J.L.; Sharma, T.; Dreyer, I. Cooperation through competition—Dynamics and microeconomics of a minimal nutrient trade system in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2016, 7, 912. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Spitz, O.; Kanonenberg, K.; Montag, K.; Handrich, M.R.; Ahmad, S.; Schott-Verdugo, S.; Navarro-Retamal, C.; Rubio-Meléndez, M.E.; Gomez-Porras, J.L.; et al. Nutrient exchange in arbuscular mycorrhizal symbiosis from a thermodynamic point of view. New Phytol. 2019, 222, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Plassard, C.; Becquer, A.; Garcia, K. Phosphorus Transport in Mycorrhiza: How Far Are We? Trends Plant Sci. 2019, 24, 794–801. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Knoblauch, M.; Turgeon, R. The Phloem as an Arena for Plant Pathogens. Annu. Rev. Phytopathol. 2022, 60, 77–96. [Google Scholar] [CrossRef]
- Sabbagh, S.K. Adaptation à la Pénétration Racinaire de Deux Ustilaginaceae Parasites du Mais: Ustilago Maydis et Sporisorium Reilianum: Analyse Microscopique et Transcriptomique. 2008. Available online: http://www.theses.fr/2008TOU30002 (accessed on 20 December 2022).
- Wittek, A.; Dreyer, I.; Al-Rasheid, K.A.S.K.A.S.; Sauer, N.; Hedrich, R.; Geiger, D. The fungal UmSrt1 and maize ZmSUT1 sucrose transporters battle for plant sugar resources. J. Integr. Plant Biol. 2017, 59, 422–435. [Google Scholar] [CrossRef]
- Milne, R.J.; Dibley, K.E.; Schnippenkoetter, W.; Mascher, M.; Lui, A.C.W.; Wang, L.; Lo, C.; Ashton, A.R.; Ryan, P.R.; Lagudah, E.S. The Wheat Lr67 Gene from the Sugar Transport Protein 13 Family Confers Multipathogen Resistance in Barley. Plant Physiol. 2019, 179, 1285–1297. [Google Scholar] [CrossRef]
- Vincent, T.R.; Avramova, M.; Canham, J.; Higgins, P.; Bilkey, N.; Mugford, S.T.; Pitino, M.; Toyota, M.; Gilroy, S.; Miller, A.J.; et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 2017, 29, 1460–1479. [Google Scholar] [CrossRef]
- Horaruang, W.; Hills, A.; Blatt, M.R. Communication between the Plasma Membrane and Tonoplast Is an Emergent Property of Ion Transport. Plant Physiol. 2020, 182, 1833–1835. [Google Scholar] [CrossRef]
- Slavov, A.; Garnier, C.; Crépeau, M.J.; Durand, S.; Thibault, J.F.; Bonnin, E. Gelation of high methoxy pectin in the presence of pectin methylesterases and calcium. Carbohydr. Polym. 2009, 77, 876–884. [Google Scholar] [CrossRef]
- Höfte, H.; Voxeur, A. Plant cell walls. Curr. Biol. 2017, 27, R865–R870. [Google Scholar] [CrossRef] [PubMed]
- Martinière, A.; Gibrat, R.; Sentenac, H.; Dumont, X.; Gaillard, I.; Paris, N. Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6488–6493. [Google Scholar] [CrossRef] [PubMed]
- Moreau, H.; Gaillard, I.; Paris, N. Genetically encoded fluorescent sensors adapted to acidic pH highlight subdomains within the plant cell apoplast. J. Exp. Bot. 2022, 73, 6744–6757. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreyer, I.; Vergara-Valladares, F.; Mérida-Quesada, F.; Rubio-Meléndez, M.E.; Hernández-Rojas, N.; Riedelsberger, J.; Astola-Mariscal, S.Z.; Heitmüller, C.; Yanez-Chávez, M.; Arrey-Salas, O.; et al. The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants. Plants 2023, 12, 204. https://doi.org/10.3390/plants12010204
Dreyer I, Vergara-Valladares F, Mérida-Quesada F, Rubio-Meléndez ME, Hernández-Rojas N, Riedelsberger J, Astola-Mariscal SZ, Heitmüller C, Yanez-Chávez M, Arrey-Salas O, et al. The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants. Plants. 2023; 12(1):204. https://doi.org/10.3390/plants12010204
Chicago/Turabian StyleDreyer, Ingo, Fernando Vergara-Valladares, Franko Mérida-Quesada, María Eugenia Rubio-Meléndez, Naomí Hernández-Rojas, Janin Riedelsberger, Sadith Zobeida Astola-Mariscal, Charlotte Heitmüller, Mónica Yanez-Chávez, Oscar Arrey-Salas, and et al. 2023. "The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants" Plants 12, no. 1: 204. https://doi.org/10.3390/plants12010204
APA StyleDreyer, I., Vergara-Valladares, F., Mérida-Quesada, F., Rubio-Meléndez, M. E., Hernández-Rojas, N., Riedelsberger, J., Astola-Mariscal, S. Z., Heitmüller, C., Yanez-Chávez, M., Arrey-Salas, O., San Martín-Davison, A., Navarro-Retamal, C., & Michard, E. (2023). The Surprising Dynamics of Electrochemical Coupling at Membrane Sandwiches in Plants. Plants, 12(1), 204. https://doi.org/10.3390/plants12010204





