Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection
Abstract
1. Introduction
2. Results and Discussion
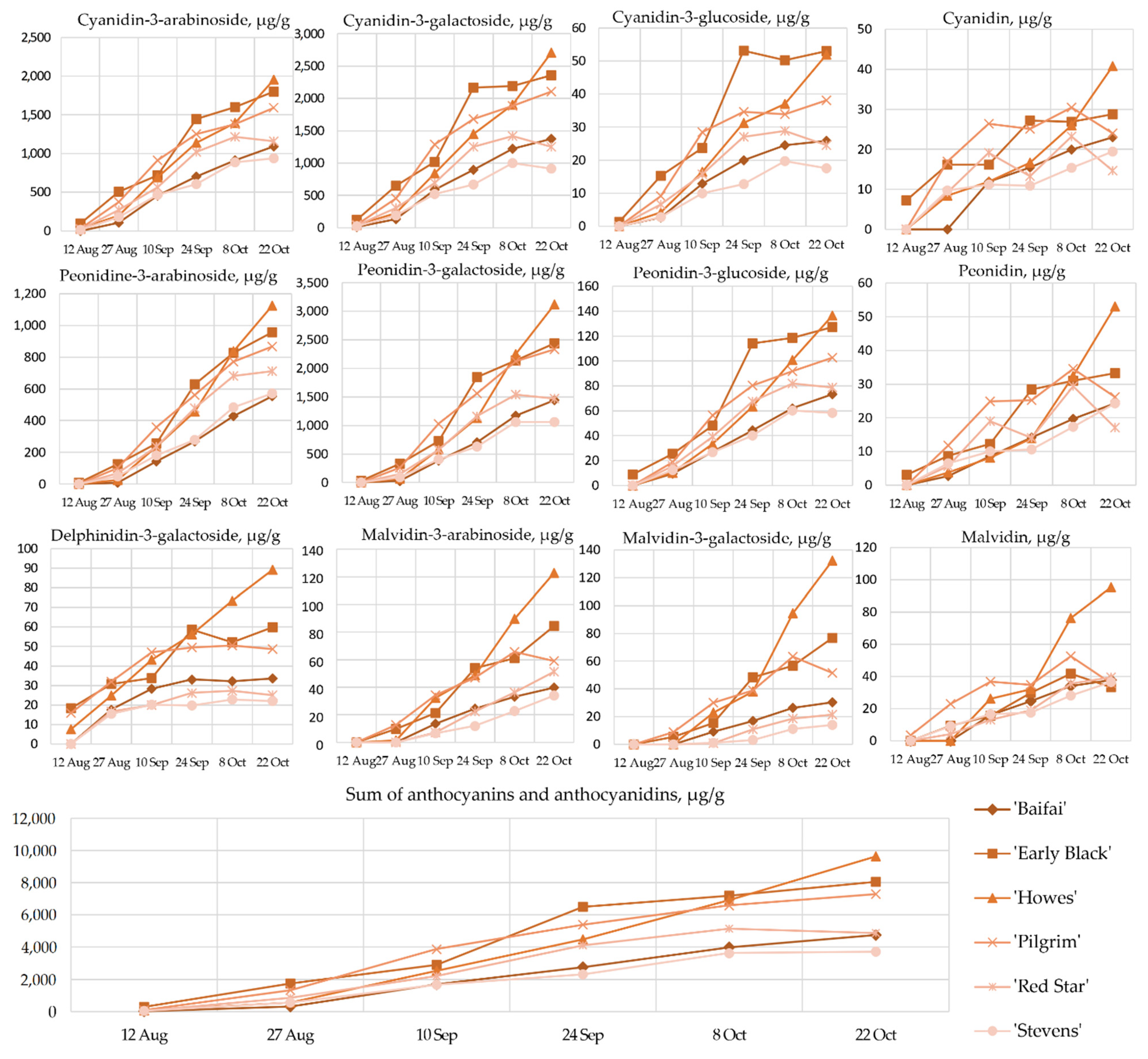
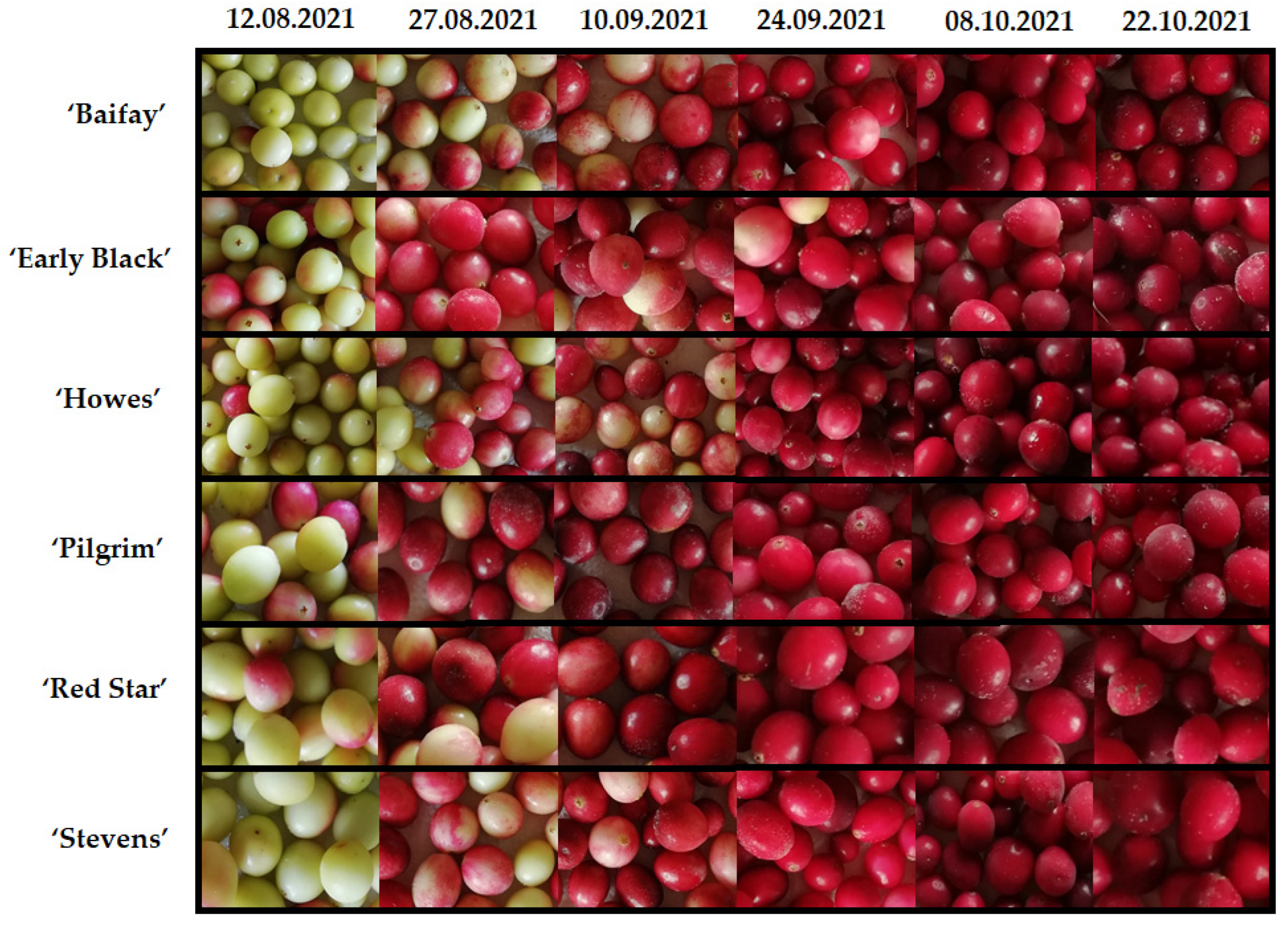
2.1. Content of Anthocyanins and Anthocyanidins
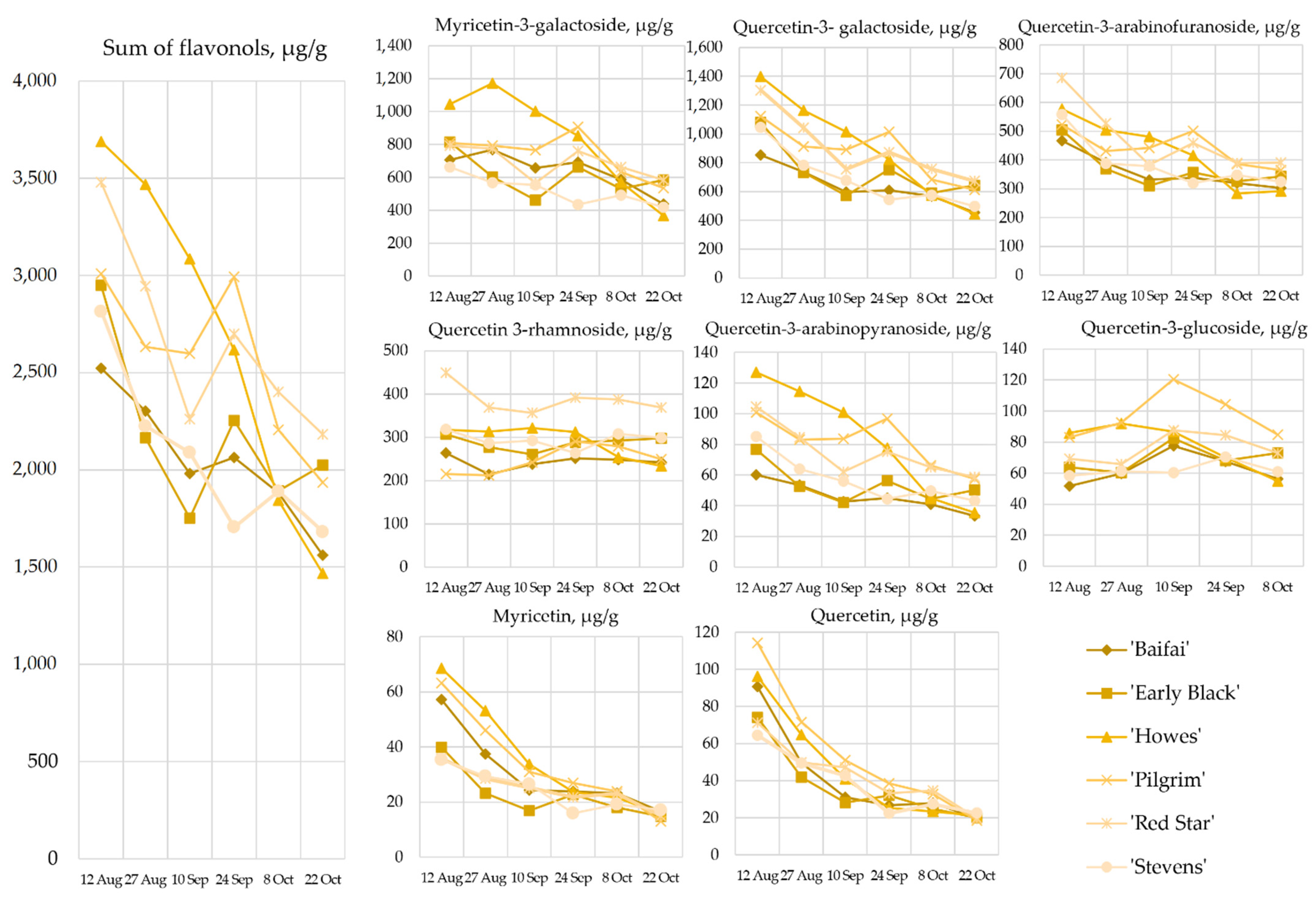
2.2. Content of Flavonols
2.3. Quantification of Proanthocyanidins and Chlorogenic Acid
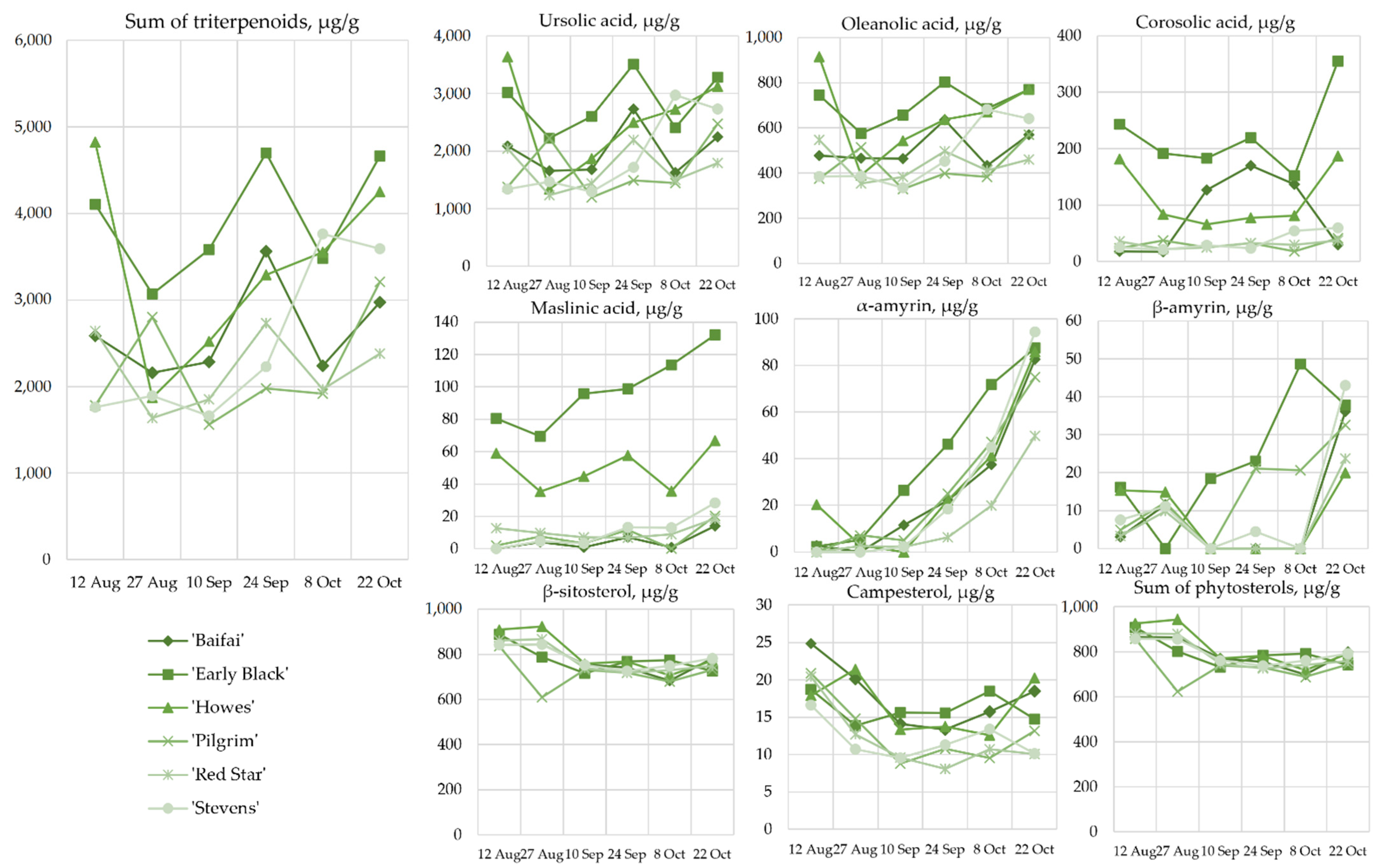
2.4. Content of Triterpenoids and Phytosterols
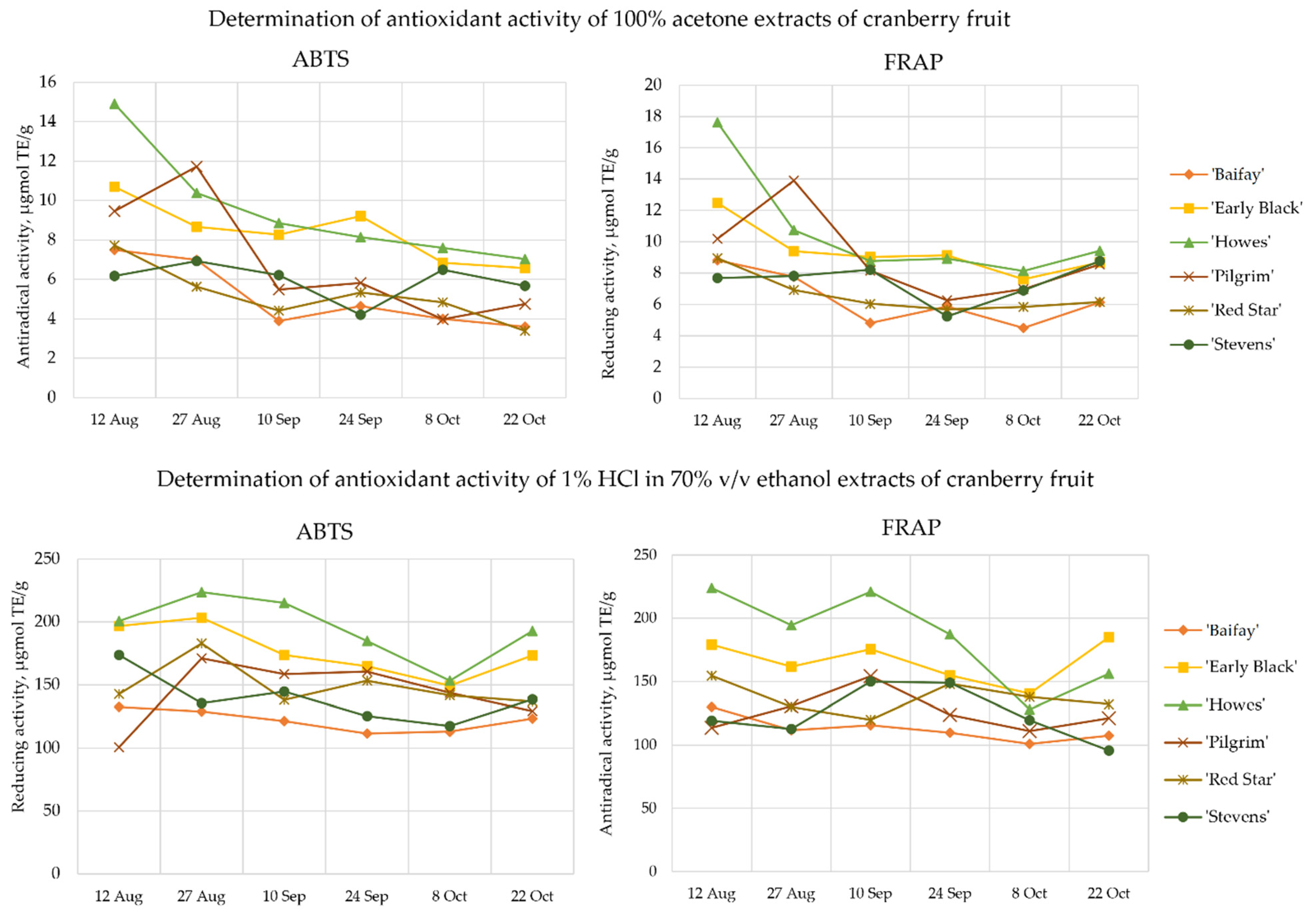
2.5. Antioxidant Activity
3. Materials and Methods
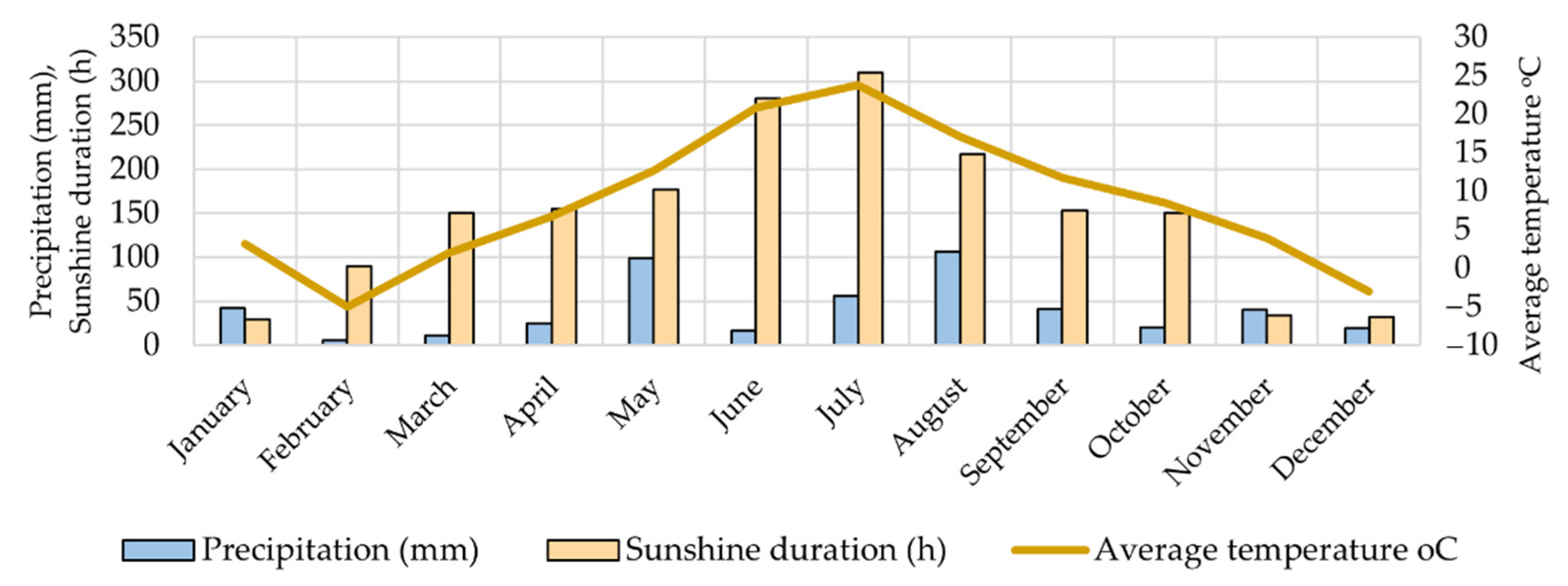
3.1. Raw Material
3.2. Preparation of Cranberry Extracts
3.3. Chromatographic Analysis
3.4. Spectrophotometric Studies
3.4.1. Determination of Proanthocyanidin Content
3.4.2. Determination of Antioxidant Activity
3.5. Reagents
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, P.N.; Murch, S.J.; Shipley, P. Phytochemical Diversity of Cranberry (Vaccinium macrocarpon Aiton) Cultivars by Anthocyanin Determination and Metabolomic Profiling with Chemometric Analysis. J. Agric. Food Chem. 2012, 60, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bonilla, L.; Williams, K.A.; Rodríguez Bonilla, F.; Matusinec, D.; Maule, A.; Coe, K.; Wiesman, E.; Diaz-Garcia, L.; Zalapa, J. The Genetic Diversity of Cranberry Crop Wild Relatives, Vaccinium macrocarpon Aiton and V. oxycoccos L., in the US, with Special Emphasis on National Forests. Plants 2020, 9, 1446. [Google Scholar] [CrossRef]
- Vorsa, N.; Zalapa, J. Domestication, genetics, and genomics of the American cranberry. Plant Breed. Rev. 2019, 43, 279–315. [Google Scholar]
- Česonienė, L.; Daubaras, R.; Jasutienė, I.; Miliauskienė, I. Stambiauogės ir paprastosios spanguolės uogos—Biologiškai aktyvių medžiagų šaltinis. Miest. Zeld. Formav. 2016, 13, 25–30. [Google Scholar]
- Karlsons, A.; Osvalde, A.; Čekstere, G.; Pormale, J. Research on the mineral composition of cultivated and wild blueberries and cranberries. Agron. Res. 2018, 16, 454–463. [Google Scholar]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and their Press Cakes. J. Food Sci. 2009, 74, 157–161. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium Macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef]
- Amin, R.; Thalluri, C.; Docea, A.O.; Sharifi-Rad, J.; Calina, D. Therapeutic potential of cranberry for kidney health and diseases. eFood 2022, 3, e33. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive Compounds in Cranberries and their Biological Properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- Kalın, P.; Gülçin, İ.; Gören, A.C. Antioxidant Activity and Polyphenol Content of. Rec. Nat. Prod. 2015, 7, 496–502. [Google Scholar]
- Santana, D.G.; Oliveira, A.S.; Souza, M.T.d.S.; Santos, J.T.d.C.; Hassimotto, N.M.A.; Silva, A.M.d.O.; Grespan, R.; Camargo, E.A. Vaccinium macrocarpon Aiton Extract Ameliorates Inflammation and Hyperalgesia through Oxidative Stress Inhibition in Experimental Acute Pancreatitis. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.R.; Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Mahmoudi, M.; Rezaei, N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Haya, J.; Chedraui, P. Vaccinium macrocarpon: An interesting option for women with recurrent urinary tract infections and other health benefits. J. Obstet. Gynaecol. Res. 2009, 35, 630–639. [Google Scholar] [CrossRef]
- Huang, Y.; Nikolic, D.; Pendland, S.; Doyle, B.J.; Locklear, T.D.; Mahady, G.B. Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, pro-inflammatory cytokine release and the NF-κβ transcriptional response in vitro. Pharm. Biol. 2009, 47, 18–25. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Cranberry Phytochemicals: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2006, 54, 7069–7074. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Jasutienė, I.; Venclovienė, J.; Miliauskienė, I. Evaluation of the Biochemical Components and Chromatic Properties of the Juice of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Plant Foods Hum. Nutr. 2011, 66, 238–244. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L. Development, validation, and application of UPLC-PDA method for anthocyanins profiling in Vaccinium L. berries. J. Berry Res. 2021, 11, 583–599. [Google Scholar] [CrossRef]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and Validation of the UPLC-DAD Methodology for the Detection of Triterpenoids and Phytosterols in Fruit Samples of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Liaudanskas, M.; Janulis, V. Development, Validation, and Application of the UPLC-DAD Methodology for the Evaluation of the Qualitative and Quantitative Composition of Phenolic Compounds in the Fruit of American Cranberry (Vaccinium macrocarpon Aiton). Molecules 2022, 27, 467. [Google Scholar] [CrossRef]
- Çelik, H.; Özgen, M.; Serçe, S.; Kaya, C. Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit. Sci. Hortic. 2008, 117, 345–348. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant Activities and Antitumor Screening of Extracts from Cranberry Fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Heffels, P.; Weber, F.; Schieber, A. Influence of Accelerated Solvent Extraction and Ultrasound-Assisted Extraction on the Anthocyanin Profile of Different Vaccinium Species in the Context of Statistical Models for Authentication. J. Agric. Food Chem. 2015, 63, 7532–7538. [Google Scholar] [CrossRef]
- Lee, J. Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci. Nutr. 2016, 4, 742–752. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Vorsa, N. Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait. Plant Sci. 2004, 167, 1043–1054. [Google Scholar] [CrossRef]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in American cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, B.R. Red light stimulates flowering and anthocyanin biosynthesis in American cranberry. Plant Growth Regul. 2002, 38, 165–171. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and Its Phytochemicals: A Review of In Vitro Anticancer Studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [CrossRef]
- Singh, A.P.; Wilson, T.; Kalk, A.J.; Cheong, J.; Vorsa, N. Isolation of specific cranberry flavonoids for biological activity assessment. Food Chem. 2009, 116, 963–968. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Šedbarė, R.; Siliņa, D.; Janulis, V. Evaluation of the Phytochemical Composition of Phenolic and Triterpene Compounds in Fruit of Large Cranberries (Vaccinium macrocarpon Aiton) Grown in Latvia. Plants 2022, 11, 2725. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Comparison of bioactive potential of cranberry fruit and fruit-based products versus leaves. J. Funct. Foods 2016, 22, 232–242. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, J.; Guo, Y.; Liu, W.; Li, M.; Zhang, L.; Zhang, Y.; Zhou, T.; Zhang, J.; Gao, H.; et al. Suppression of Helicobacter pylori infection by daily cranberry intake: A double-blind, randomized, placebo-controlled trial. J. Gastroenterol. Hepatol. 2021, 36, 927–935. [Google Scholar] [CrossRef]
- Suvanto, J.; Karppinen, K.; Riihinen, K.; Jaakola, L.; Salminen, J.P. Changes in the Proanthocyanidin Composition and Related Gene Expression in Bilberry (Vaccinium myrtillus L.) Tissues. J. Agric. Food Chem. 2020, 68, 7378–7386. [Google Scholar] [CrossRef]
- Aaby, K.; Remberg, S.V. Strawberry phenolics and impact of ripening. In Processing and Impact an Active Components in Food; Preedy, V., Ed.; Elsevier: London, UK, 2015; pp. 157–164. [Google Scholar]
- Dixon, R.A.; Sarnala, S. Proanthocyanidin Biosynthesis—A Matter of Protection. Plant Physiol. 2020, 184, 579–591. [Google Scholar] [CrossRef]
- Occhipinti, A.; Germano, A.; Maffei, M.E. Prevention of Urinary Tract Infection with Oximacro®, A Cranberry Extract with a High Content of A-Type Proanthocyanidins: A Pre-Clinical Double-Blind Controlled Study. Urol. J. 2016, 13, 2640–2649. [Google Scholar]
- Vostalova, J.; Vidlar, A.; Simanek, V.; Galandakova, A.; Kosina, P.; Vacek, J.; Vrbkova, J.; Zimmermann, B.F.; Ulrichova, J.; Student, V. Are High Proanthocyanidins Key to Cranberry Efficacy in the Prevention of Recurrent Urinary Tract Infection?: Are High PAC Key to Cranberry Efficacy in the Prevention rUTI? Phytother. Res. 2015, 29, 1559–1567. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Cheng, M.; Wei, G.; Du, Y.; Fan, Y.; Li, J.; Li, H.; Deng, Z. Serum Cholesterol-Lowering Activity of β-Sitosterol Laurate Is Attributed to the Reduction of Both Cholesterol Absorption and Bile Acids Reabsorption in Hamsters. J. Agric. Food Chem. 2020, 68, 10003–10014. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-Sitosterol: A Promising but Orphan Nutraceutical to Fight Against Cancer. Nutr. Cancer 2015, 67, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Bharanishankar, S.; Vishn, V.; Selvaraj, J. Effects of beta-sitosterol on inflammatory cytokines in high-fat diet-fed type-2 diabetic rats. Drug Invent. Today 2019, 12, 7. [Google Scholar]
- Klavins, L.; Klavins, M. Cuticular Wax Composition of Wild and Cultivated Northern Berries. Foods 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xue, L.; Tata, A.; Song, M.; Neto, C.C.; Xiao, H. Bioactive Components of Polyphenol-Rich and Non-Polyphenol-Rich Cranberry Fruit Extracts and Their Chemopreventive Effects on Colitis-Associated Colon Cancer. J. Agric. Food Chem. 2020, 68, 6845–6853. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018, 244, 705–719. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Becker, R.; Pączkowski, C.; Szakiel, A. Various Patterns of Composition and Accumulation of Steroids and Triterpenoids in Cuticular Waxes from Screened Ericaceae and Caprifoliaceae Berries during Fruit Development. Molecules 2019, 24, 3826. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Ndhlala, A.; Moyo, M.; Van Staden, J. Natural Antioxidants: Fascinating or Mythical Biomolecules? Molecules 2010, 15, 6905–6930. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.I.; Almajano, M. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Liu, T.H.; Hsu, T.H.; Lin, F.Y.; Chen, H.Y. Antioxidant and antiglycation properties of different solvent extracts from Chinese olive (Canarium album L.) fruit. Asian Pac. J. Trop. Med. 2015, 8, 1013–1021. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of different extraction techniques on quantification of oleanolic and ursolic acid in Lamii albi flos. Ind. Crops Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; Silva, B.C.E.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Klavins, M. Comparison of methods of extraction of phenolic compounds from American cranberry (Vaccinium macrocarpon L.) press residues. Agron. Res. 2017, 15, 1316–1329. [Google Scholar]
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; Silva, V.J.G.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, F.; Li, B.; Wei, T.; Guan, H.; Ren, S. Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst. Molecules 2018, 23, 2445. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Janulis, V. Phytogenotypic Anthocyanin Profiles and Antioxidant Activity Variation in Fruit Samples of the American Cranberry (Vaccinium macrocarpon Aiton). Antioxidants 2022, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Vattem, D.A.; Ghaedian, R.; Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005, 14, 120–130. [Google Scholar] [PubMed]
- The Meteorological Data in 2021, Lithuanian Hydrometeorological Service, Monthly Overview of Hydrometeorological Conditions. Available online: http://www.meteo.lt/lt/2021-gruodis (accessed on 17 November 2022).
- Gudžinskaitė, I.; Stackevičienė, E.; Liaudanskas, M.; Zymonė, K.; Žvikas, V.; Viškelis, J.; Urbštaitė, R.; Janulis, V. Variability in the Qualitative and Quantitative Composition and Content of Phenolic Compounds in the Fruit of Introduced American Cranberry (Vaccinium macrocarpon Aiton). Plants 2020, 9, 1379. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; p. 51. [Google Scholar]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of ‘condensed tannins’ as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šedbarė, R.; Pašakinskienė, I.; Janulis, V. Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection. Plants 2023, 12, 202. https://doi.org/10.3390/plants12010202
Šedbarė R, Pašakinskienė I, Janulis V. Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection. Plants. 2023; 12(1):202. https://doi.org/10.3390/plants12010202
Chicago/Turabian StyleŠedbarė, Rima, Izolda Pašakinskienė, and Valdimaras Janulis. 2023. "Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection" Plants 12, no. 1: 202. https://doi.org/10.3390/plants12010202
APA StyleŠedbarė, R., Pašakinskienė, I., & Janulis, V. (2023). Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection. Plants, 12(1), 202. https://doi.org/10.3390/plants12010202





