Abstract
Emerging evidence indicates that long non-coding RNAs (lncRNAs) play important roles in diverse biological processes. However, the biological functions of most plant lncRNAs are still unknown. We previously discovered a soybean abiotic-stress-related lncRNA, lncRNA77580, and cloned the entire full-length sequence. Here, in order to fully identify the function of lncRNA77580 in soybean stress response, we created transgenic soybean lines overexpressing lncRNA77580. Compared with the wild type, overexpression of lncRNA77580 enhances the drought tolerance of soybean. However, the transgenic plants exhibit increased sensitivity to high salinity at the seedling stage. We found that lncRNA77580 modulates the transcription of different gene sets during salt and drought stress response. Under water deficit at the reproductive stage, lncRNA77580 overexpression increases the seed yield by increasing the seed number per plant. These results provide insight into the role of lncRNA77580 in soybean stress response.
1. Introduction
Soybean (Glycine max (L.) Merrill.) is a highly nutritious leguminous crop that is widely used as a food source for humans and livestock due to its rich contents of proteins and minerals [1,2]. Furthermore, soybean is also a major oilseed crop, representing around 59% of the overall world oilseed production [3]. However, the growth and yield of soybean, similarly to other crops, are largely affected by abiotic stresses, including drought and salinity stresses. As an example, drought alone can cause up to a 40% yield loss of soybean globally [4]. This also severely limits the space for soybean growth and limits the potential of saline–alkali and arid land to be used as back-up farmland. To solve this problem, it is very important to explore and utilize highly effective stress-resistance genes in soybean.
Long non-coding RNAs (lncRNAs) are RNAs generally longer than 200 nucleotides (nt) that lack protein-coding capability. Emerging evidence indicates that lncRNAs play key roles in gene regulation, development, and environmental responses [5,6,7,8]. Animal lncRNAs have been extensively studied and proven to be functional in different essential biological processes. Large batches of plant lncRNAs have been identified in recent years [9,10,11,12,13], taking advantage of next-generation sequencing technologies. In soybean, 6018 lincRNAs have been identified from transcriptomic data [14]. In our earlier study, we found that more than 75% of discovered lncRNAs in soybean roots were activated or upregulated by continuous salt stress [15]. From two genotypes of soybean grown under different levels of P, 4166 novel lncRNAs, including 525 differentially expressed (DE) lncRNAs, were identified [16].
The functional roles of plant lncRNAs remain largely unknown, and only a few plant lncRNAs are well understood. For example, the rice-specific lncRNA LDMAR has been identified as a key gene in controlling photoperiod-sensitive male sterility [17]. Overexpression of the lncRNA LAIR in rice increases grain yield and regulates the expression of genes in a neighboring cluster, which is known as cis-regulation [18]. Two lncRNAs, COOLAIR and COLDAIR, finely regulate flowering in Arabidopsis [19,20]. In addition, a few lncRNAs were found to play an important role in abiotic stress response through different regulation mechanisms. In Arabidopsis, lncRNA SVALKA governs plant cold acclimation by the SVALKA–asCBF1 cascade mechanism, tightly controlling the level and timing of CBF1 expression, which can be exploited to maximize freezing tolerance with mitigated fitness costs [21]. At the post-transcriptional level, some lncRNAs can interfere with microRNAs (miRNAs), neutralizing their silencing role and thus upregulating the transcript level in response to cold stress [22], heat stress [23], etc. A nucleus-localized Arabidopsis lncRNA, DRIR, enhances drought and salt stress tolerance by modulating the expression of a series of genes involved in the stress response [24]. These studies reveal the key roles of plant lncRNAs in appropriately acclimating to challenging environmental conditions. However, little is known about the functions of lncRNAs in soybean.
In our previous study, we identified a salt-stress-related lncRNA in soybean, lncRNA77580 [15], which was identified as an intergenic lncRNA located on chromosome 20 between the genes Glyma.20G225700 and Glyma.20G225800. We cloned the full length of lncRNA77580 with a 4851 bp DNA fragment and found through overexpression and large DNA fragment deletion in soybean that lncRNA77580 regulates the expression of neighboring genes [25]. In this study, we further investigate the function of lncRNA77580 in transgenic soybean in response to drought and salt stress to support its proposal as a candidate gene for soybean stress tolerance gene mining.
2. Results
2.1. Creation of Transgenic Soybean Overexpressing lncRNA77580
To evaluate the contribution of lncRNA77580 in salt and drought stress, we engineered soybean plants to constitutively express lncRNA77580 under a 35S promoter (Figure S1). We selected three independent stable homozygous lines (OE-1, OE-2, and OE-3) for functional analysis. The expression of lncRNA77580 in the three transgenic lines was validated through qPCR (Figure S1). The results of qPCR analysis show that lncRNA77580 transcript levels are significantly higher in lncRNA77580-OE soybean than in the corresponding WT (wild type) lines under normal conditions (Figure S1).
2.2. Transgenic Soybean with lncRNA77580 Overexpression Exhibits Increased Sensitivity to High Salinity
To further verify the biological function of lncRNA77580 in soybean, a lncRNA77580 overexpression vector was transferred into the soybean cultivar Tian Long via Agrobacterium tumefaciens-mediated transformation, and the phenotypes of seedlings under salt stress were evaluated. Under normal conditions, the WT and lncRNA77580-OE soybean seedlings showed similar growth phenotypes. In the experimental group, soybean seedlings were grown in vermiculite and continuously treated with 100 mmol/L NaCl solution starting from seed germination. At 35 days of salt treatment, the leaves of the wild-type and transgenic soybean had turned yellow, and most leaves of the transgenic plants were completely dry, as obvious from their phenotype (Figure 1a). Unlike the OE lines, most of the WT plants had evidently retained chlorophyll in their leaves, indicating continued support of essential life processes.
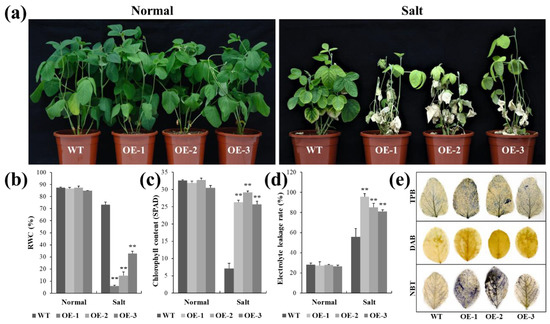
Figure 1.
Phenotypic and physiological analyses of the WT plants and lncRNA77580-OE transgenic soybean under salt stress. (a) Phenotypes of overall plants and (b) relative water content, (c) chlorophyll content, (d) electrolyte leakage rate, and (e) trypan blue, DAB, and NBT staining of the leaves of the WT plants and lncRNA77580-OE transgenic soybean grown under salt treatment or normal control conditions for 35 days. Data are shown as the mean ± standard deviation (n = 3). Significant differences based on ANOVA were set at p < 0.01 (**).
Two physiological indices, i.e., chlorophyll content as an indicator of photosynthetic capacity [26] and relative water content (RWC) as an indicator of plant water status, were measured to quantify the effects of salt and drought on the plant development [27]. The changes in the RWC (Figure 1b) and chlorophyll content (Figure 1c) of plant leaves in response to drought and salt were consistent with the observation of leaf phenotypes. Specifically, the leaves of lncRNA77580 OE lines showed a lower RWC and chlorophyll content than did those of the WT plants.
In order to visually show the degree of damage in soybean WT and OE plant leaves, we used trypan blue, DAB, and NBT staining to measure cell viability in soybean leaves under salt stress. The WT plant leaves were significantly less stained by all three staining methods than the OE plant leaves, suggesting that the leaves of lncRNA77580 OE plants suffered more damage under salt treatments than the WT plants (Figure 1e). The electrolyte leakage rate of the leaves was significantly higher in OE plants than in WT (Figure 1d). These results indicate an evident growth disadvantage for the lncRNA77580 OE lines compared with the WT plants under salt stress.
2.3. lncRNA77580 Positively Regulates Soybean Tolerance to Drought Stress
The lncRNA77580 overexpression soybean was similarly treated with drought stress. At the seedling stage, the WT and lncRNA77580-OE soybean showed similar growth phenotypes under well-watered conditions (Figure 2a). At the fifth day of drought treatment, the three lncRNA77580-OE lines exhibited more robust growth than the WT plants, as indicated by significantly less leaf wilting, curling, and chlorosis (Figure 2a). After rewatering for five days, the control plants were unable to recover and eventually died. By contrast, the majority of lncRNA77580-OE transgenic soybean seedlings appeared to be healthy after rewatering (Figure 2a). Consistent with these results, leaf RWC was significantly higher in the lncRNA77580-OE lines than in WT plants (Figure 2b), and the transgenic plants suffered less damage than the WT plants under drought treatments, as indicated by less intense staining with trypan blue, DAB, and NBT (Figure 2c). Thus, constitutive overexpression of lncRNA77580 positively regulates soybean tolerance to drought stress. Overexpression of lncRNA77580 in soybean has opposite effects on responses when comparing between drought and salt stress.
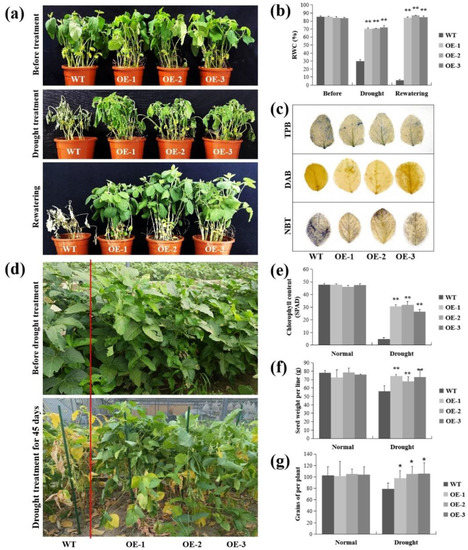
Figure 2.
Phenotypic and physiological analyses of WT plants and lncRNA77580-OE transgenic soybean under drought stress in growth chambers (a–c) vs. the field (d–g). (a) Phenotypes of overall plants and (b) relative water content and (c) trypan blue, DAB, and NBT staining of the leaves of the WT plants and lncRNA77580-OE transgenic soybean grown under drought treatment or well-watered conditions. (d) Phenotypes of overall plants and (e) chlorophyll content, (f) seed weight per line, and (g) seed number per plant of the WT plants and lncRNA77580-OE transgenic soybean grown under drought treatment or in a well-watered field. Data are shown as the mean ± standard deviation (n = 3). Significant differences based on ANOVA were set at p < 0.05 (*) and p < 0.01 (**).
In addition, we planted WT and lncRNA77580-OE soybeans in a field with an automatic control canopy. The control group was well-watered, and the WT and OE soybeans in the experimental group were not watered after flowering. At 45 days of drought treatment, most leaves of the WT soybean had turned yellow or completely dried, while most of the leaves in the three lncRNA77580-OE lines remained green (Figure 2d,e). After the soybeans were ripe and harvested, we investigated some agronomic traits. The results show a significantly lower reduction due to drought stress in the seed yield and seed number per plant (Figure 2f,g), demonstrating once again that transgenic soybean lines have higher drought resistance ability than the WT.
2.4. lncRNA77580 Modulates the Expression of a Series of Genes Involved in the Stress Response
The lncRNA77580-OE soybean showed a salt-sensitive and drought-tolerant phenotype compared with WT. It was hypothesized that this may result from differences in how the expression of some genes associated with stress responses may be changed. Therefore, RNA-seq was conducted to identify which genes are differentially expressed (referred to as DEGs) between the lncRNA77580-OE and WT plants under normal (WT-N, OE-N), salt (WT-S, OE-S), and drought (WT-D, OE-D) stress.
Overexpression of lncRNA77580 in soybean alters the transcript profile under normal and stress conditions (Figure 3). There were 524 upregulated DEGs and 322 downregulated DEGs in OE-N compared with WT-N, 1279 upregulated DEGs and 684 downregulated DEGs in OE-S compared with WT-S, and 483 upregulated DEGs and 509 downregulated DEGs in OE-D compared with WT-D (Figure 3). These data suggest that lncRNA77580 modulates the transcription of genes during salt and drought stress responses. These genes also include many transcription factors. Members from all of the major transcription factor (TF) families (such as ERF/AP2, ZFP, MYB, bHLH, NAC, bZIP, and WRKY) were found to be involved in the regulation of lncRNA77580 under salt and drought stress based on their altered pattern of expression (Figure S3).
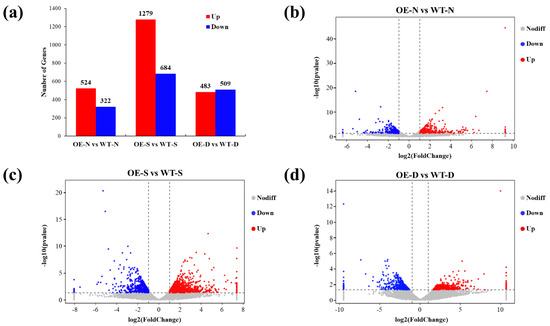
Figure 3.
DEGs of soybean seedlings overexpressing lncRNA77580 under normal, salt, and drought conditions. (a) Numbers of up- and downregulated DEGs in lncRNA77580-overexpressing soybean (OE) under normal (N), salt (S), and drought (D) conditions. (b–d) Volcano diagrams of DEGs in lncRNA77580-overexpression soybean under normal, salt, and drought conditions compared with wild-type (WT) soybean. Blue spots represent DEGs whose expression was less than half that of the level in WT. Red spots represent DEGs whose expression was more than double that of the level in WT.
Gene Ontology (GO) analysis of the DEGs revealed potential functions of lncRNA77580 according to three terms: biological process, molecular function, and cellular component (Figure 4). Under the three different conditions, normal, salt, and drought, few of the represented terms are the same. For example, cell wall (GO: 0005618) for cellular component and oxidoreductase activity (GO: 0016491) for molecular function are common and represent the exception. Under salt stress, lncRNA77580 overexpression had a greater impact on the expression of genes related to photosynthesis, and most are associated with terms for locations in the thylakoid, photosystem, and membrane (GO: 0009579, GO: 0009521, and GO: 0016020). Meanwhile, under drought stress, for cellular component, the major category is cell wall (GO: 0005618), and the genes most significantly enriched among all terms are associated with terpenoid catabolic process (GO: 0016107). These data suggest that lncRNA77580 modulates the transcription of different gene sets during salt and drought stress responses.
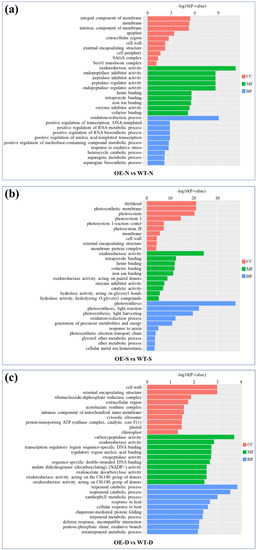
Figure 4.
Gene Ontology analysis of the differentially expressed genes and their associated terms in molecular functions (MF), cellular components (CC), and biological processes (BP). (a–c) GO enrichment analysis of DEGs in lncRNA77580-OE soybean compared with WT under normal (N), salt (S), and drought (D) conditions.
3. Discussion
Although not as well studied as in animals, it is known that lncRNAs, in plants, can regulate gene silencing, flowering time, reproduction, stress responses, organogenesis in roots, and photomorphogenesis in seedlings [11,28,29,30,31,32]. However, the overwhelming majority of plant lncRNAs with clear functions were studied in Arabidopsis, and our understanding of the lncRNAs in crop species remains limited [18]. In this study, we discovered that overexpression of a soybean lncRNA, lncRNA77580, can result in an obvious increase in soybean drought tolerance. However, the transgenic plants exhibited increased sensitivity to high salinity. Similar results have been obtained using transgenic plants overexpressing known positive regulators of abiotic stress tolerance, such as ABF3, ABF4, AtXERICO, AtSDIR1, OsSDIR1, and GmNFYA3 [33,34,35,36,37]. The simplest explanation for this is that transgenic plants produce some compatible solutes that confer tolerance to osmotic stress but not to sodium ion toxicity.
Under salt stress, there was significant inhibition of the root growth of transgenic soybean overexpressing lncRNA77580. The number of lateral roots was significantly lower in transgenic soybean seedlings than in wild type (Figure S4). There was no significant difference in root development between lncRNA77580-OE and WT under drought stress. In saline environments, the roots of plants are the primary point of contact regarding ionic toxicity and osmotic stress. In this study, continuous salt stress from seed germination was imposed on the soybean, while for the drought stress treatment, drought stress was applied at the seedling stage, after 10 days of normal growth. Salt stress may have a more severe effect on young roots, and compared with wild type, lncRNA77580-OE had more DEGs under salt stress than under drought stress.
In addition to the roots, the leaves of transgenic soybean were also more severely damaged by salt stress than those of the wild type. Specifically, there was significant leaf degreening and drying in lncRNA77580-OE soybean under salt stress (Figure 1). Moreover, plants can generate hydrogen peroxide (H2O2) and superoxide (O−2) under salt stress. H2O2 and O−2 can be visually detected using DAB and NBT. Programmed cell death was analyzed using trypan blue. Trypan blue can color dead cells blue. However, living cells are not stained [38]. Thus, the staining results of DAB, NBT, and trypan blue indicated that the antioxidant capacity was lower in transgenic soybean than in WT. Transcriptomic profiling also indicates a greater impact of lncRNA77580 overexpression on the expression of genes related to photosynthesis and oxidation–reduction process under salt stress. It may be that high salt stress caused more serious damage to the chloroplasts of the transgenic soybeans. High salinity has multiple effects on chloroplasts, including reduced photosynthetic efficiency, thylakoid membrane damage, oxidative stress, etc. [39]. In addition to functioning in photosynthesis, chloroplasts also contribute to the biosynthesis of amino acids, vitamins, isoprenoids, fatty acids, and lipids [40]. The dysfunction of chloroplasts caused by salt stress can have harmful effects on the physiological, biochemical, and metabolic properties of plant cells. However, many genes related to photosynthesis were upregulated in lncRNA77580-OE soybean under salt stress. Some genes related to oxidative stress, ion homeostasis, and energy metabolism were downregulated (Figure S5a). lncRNA77580-OE soybean may thus be more hypersensitive to salt stress and try to increase the energy supply under salt stress by enhancing photosynthesis, but prolonged salt stress may cause an imbalance in growth and the stress response, eventually leading to plant death [41].
Overexpression of lncRNA77580 enhanced drought tolerance in transgenic soybean at the seedling stage and the stage from flowering to maturity (Figure 2). At the seedling stage, the leaves of WT were more likely to dry out and die than those of lncRNA77580-OE under drought stress. The DAB, NBT, and trypan blue staining results indicate that the antioxidant capacity was higher in transgenic soybean than in WT. Under both salt and drought stresses, the expression of genes related to oxidative stress was changed by lncRNA77580 overexpression. However, unlike under salt stress, many genes related to oxidative stress were upregulated under drought stress (Figure S5b). Transgenic soybean may have a relatively higher ability than WT to remove excess ROS to alleviate the potential harmful effects of drought stress on soybean metabolism. Several genes differentially expressed in lncRNA77580-OE under salt and drought stresses were randomly selected for RT-PCR verification, and all genes exhibited the same expression tendency as shown in the RNA-seq data (Figure S6).
Furthermore, soybean plants are most sensitive to water deficit during the flowering and seed set stages, when water shortage will most seriously affect yield [42,43,44]. In this study, after 55 days in well-watered conditions, soybeans bloomed and were not watered until they were ripe. The yield (grain weight of per line) of both the WT and lncRNA77580-OE soybean decreased under drought, but that of the lncRNA77580-OE soybean was higher than that of WT under drought conditions. Drought mainly affected yield through the seed number per plant, because we found that the lncRNA77580-OE soybean had lower 100-seed weight and smaller seeds than wild-type soybeans (Figure S7). Water shortage during the flowering and seed set stages might influence pollen or ovule function [43], resulting in lower seed set; we found that the WT soybean had more two-seeded pods and empty pods than did the lncRNA77580-OE soybean under drought stress (Figure S7). Another study also reported that when water stress was imposed at the reproductive stage, there was a severe reduction in seed number, and it had different effects on different soybean varieties [43].
Genes that are genetically adjacent and co-expressed in the expression pattern are likely to be regulated by this lncRNA, which is known as the lncRNA cis mechanism of action. In this study, among the 28 genes whose expression changed due to lncRNA77580 overexpression under different conditions, i.e., normal, salt, and drought conditions, the gene Glyma.20G225700 is most closely positioned to lncRNA77580 (Figure S2) and was upregulated in lncRNA77580-OE soybean, and similar results were found in our previous study [25]. It is predicted to be a target gene of lncRNA77580. The functions of a lncRNA can also be predicted by the functions of their target genes, but unfortunately, the function of Glyma.20G225700 is unknown, and no studies have been conducted on this gene have been reported. Perhaps the molecular mechanism of lncRNA77580 can be determined by studying the function of the gene Glyma.20G225700.
In conclusion, we characterized a soybean long non-coding RNA, lncRNA77580, through its overexpression in soybean. With the phenotypic, physiological, and molecular analyses conducted in this work, we found that overexpression of lncRNA77580 enhances salt sensitivity while increasing the drought tolerance of transgenic plants at the seedling stage. Under water deficit at the reproductive stage, lncRNA77580 overexpression increases the seed yield through an increased seed number per plant. lncRNA77580 may regulate soybean response to stress by regulating a protein-coding gene. However, our comprehension of the myriad roles of lncRNA77580 is still in its infancy. Currently, we are further exploring the functional mechanism of lncRNA77580.
4. Materials and Methods
4.1. Plant Transformation and Transgenic Plant Selection
For lncRNA77580 overexpression, the full-length sequence was cloned from soybean root cDNA using the primer C3 and subcloned into the pCAMBIA3301 vector under the control of the 35S CaMV promoter.
lncRNA77580-overexpression constructs were electroporated into the Agrobacterium tumefaciens strain EHA105 and then used to transform soybean cultivar Tianlong via the Agrobacterium-mediated cotyledonary node transformation method. Transgenic soybean was examined using the QuickStix Kit for PAT/bar (EnviroLogix, America), Basta spraying, and PCR amplification of the lncRNA77580 fragment. The expression of lncRNA77580 in transgenic soybean was further confirmed by qPCR. The primers used in this study are listed in Table S1.
4.2. Evaluation of Salt and Drought Tolerance of Transgenic Plants
The WT and lncRNA77580-OE soybean seeds were planted in vermiculite with Hoagland nutrient solution or 100 mmol/L NaCl–Hoagland solution. The whole cultivation process was accomplished in a growth chamber with a 16 h/8 h light/dark photoperiod at 28 °C. The whole plants of 15-day-old seedlings were collected and immediately frozen in liquid nitrogen until total RNA isolation. After 35 days of continuous salt stress, the phenotype of salt stress was observed, and histochemical and physiological analyses were performed according to a previously described protocol [39]. The leaves of the WT and lncRNA77580-OE under normal and stress treatments for 2 days were stained with 3,3′-diaminobenzidine (DAB), nitroblue tetrazolium (NBT), and trypan blue solution (TPB) to assay H2O2, O−2, and programmed cell death. The relative water content, chlorophyll content, and electrolyte leakage were measured in the leaves of the WT and OE soybean under normal and stress treatment. Each leaf sample came from three different plants. All experiments were replicated three times.
For drought stress treatment at the seedling stage, 10 days of normal growth was followed by 5 days of drought stress treatment, and rewatering was then conducted. The growing conditions and the histochemical and physiological analyses were the same as those for the salt stress treatment mentioned above. In addition, the WT and lncRNA77580-OE soybean seeds were planted in the field under an auto-rain-shelter. After 55 days of growth under well-watered conditions, the soybeans bloomed and were not watered again until they were ripe. At 44 days of drought stress treatment, we observed the phenotype and measured the chlorophyll content of the WT and OE soybean leaves. After harvest, we characterized the agronomic traits of the soybean, including the grain weight per line, grain numbers per plant, and 100-grain weight.
4.3. RNA-Seq Analysis of Transgenic Soybean
4.3.1. Library Construction and RNA Sequencing
Total RNA was extracted from the whole plant of 15-day-old WT and lncRNA77580-OE seedlings. The total RNA was then submitted to Personalbio in Shanghai (www.personalbio.cn) for library construction and RNA sequencing. The RNA-seq experiment was conducted with three biological replicates.
Messenger RNA was enriched by oligo(dT)-attached magnetic beads for cDNA synthesis. Size-selected and adaptor-ligated cDNA fragments were then purified for library construction. After library preparation, the libraries were sequenced on an Illumina Novaseq 6000 platform, and 150 bp paired-end reads were generated. Both generated raw data and clean data from each library were no less than 6G in sequencing depth.
4.3.2. Differential Expression Analysis
All clean reads were mapped to the soybean reference genome (https://www.soybase.org/SequenceIntro.php) using HISAT2 v2.0.5. We used HTSeq (0.9.1) statistics to compare the read count values for each gene as the original expression of the gene, and FPKM was then used to standardize the expression. The difference in expression of genes was analyzed using DESeq (1.39.0) with screened conditions as follows: expression difference multiple |log2FoldChange| > 1, significant p-value < 0.05. At the same time, we used the R language Pheatmap (1.0.8) software package to perform bi-directional clustering analysis of all different genes from the samples. We obtained a heatmap according to the expression level of the same gene in different samples and the expression patterns of different genes in the same sample using the Euclidean method to calculate the distance and the Complete Linkage method to cluster. Some DEGs were verified by qPCR. The primers used this test were listed in Table S1.
4.3.3. Gene Ontology Enrichment Analysis
All genes were mapped to terms in the Gene Ontology database and calculated the numbers of differentially expressed genes enriched according to each term. Using topGO (2.40.0) to perform GO enrichment analysis on the differentially expressed genes, we calculated the p-value using the hypergeometric distribution method (the standard of significant enrichment is p-value < 0.05), and we found the significantly enriched GO terms associated with differentially expressed genes to determine the main biological functions performed by these DEGs. ClusterProfiler (3.16.1) software was used to carry out the enrichment analysis of the KEGG pathways of the differentially expressed genes, focusing on the significant enrichment pathways with a p-value of <0.05.
4.4. Data Analysis
Statistical analyses were performed using Microsoft Excel, and the significance of the differences among control and treatments was analyzed by ANOVA, followed by Tukey–Kramer at a significance level of 1%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12010181/s1, Figure S1: lncRNA77580 was overexpressed in soybean; Figure S2: Common DEGs under normal condition, salt stress, and drought stress; Figure S3: Differential expression of transcription factors (TFs) in soybean seedlings with lncRNA77580 overexpression under normal, salt, and drought conditions; Figure S4: Root number and length of WT and lncRNA77580-OE transgenic soybean seedlings; Figure S5: Heatmap of some DEGs in lncRNA77580-OE soybean; Figure S6: Quantitative RT-PCR verification for randomly selected differentially expressed genes. The data were displayed as fold changes; Figure S7: The pods and seeds of WT and OE transgenic soybean under drought stress; Table S1: Sequences of the primers used in this study.
Author Contributions
Conceptualization, Q.J., Z.H., and H.Z.; methodology, F.N.; validation, X.C., X.J., and F.N.; investigation, Q.J.,X.C., X.J., and F.N.; data analysis, Q.J., X.S., and F.G.; writing—original draft preparation, Q.J., and F.N.; writing-review and editing, Q.J., Z.H., and X.S.; project administration, Q.J., and X.S.; funding acquisition, Q.J., H.Z., and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Program of Hainan Province (ZDYF2022XDNY135), the Agricultural Science and Technology Innovation Program (ASTIP No. CAAS-ZDRW202201), the National Key Research and Development Program of China (2021YFD1201603-2), and the National Natural Science Foundation of China (31601302).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the supplement or upon request.
Acknowledgments
This study was supported by the Key Research and Development Program of Hainan Province (ZDYF2022XDNY135), the Agricultural Science and Technology Innovation Program (ASTIP No. CAAS-ZDRW202201), the National Key Research and Development Program of China (2021YFD1201603-2), and the National Natural Science Foundation of China (31601302).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; He, J.; Wang, Y.; Xing, G.; Zhao, J.; Li, Y.; Yang, S.; Palmer, R.G.; Zhao, T.; Gai, J. Establishment of a 100-seed weight quantitative trait locus-allele matrix of the germplasm population for optimal recombination design in soybean breeding programmes. J. Exp. Bot. 2015, 66, 6311–6325. [Google Scholar] [CrossRef]
- Vanlliyodan, B.; Ye, H.; Song, L.; Murphy, M.; Shannon, J.G.; Nguyen, H.T. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J. Exp. Bot. 2017, 68, 1835–1849. [Google Scholar] [CrossRef]
- El-Hamidi, M.; Zaher, F.A. Production of vegetable oils in the world and in Egypt: An overview. Bull. Natl. Res. Cent. 2018, 42, 19. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Jha, R.; Khurshid, M.; Zhou, M.; Mantri, N.; Siddique, K.H.M. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020, 20, 466. [Google Scholar] [CrossRef]
- Lucero, L.; Ferrero, L.; Fonouni-Farde, C.; Ariel, F. Functional classification of plant long noncoding RNAs: A transcript is known by the company it keeps. New Phytol. 2021, 229, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jung, C.; Xu, J.; Wang, H.; Deng, S.; Bernad, L.; Arenas-Huertero, C.; Chua, N.H. Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 2012, 24, 4333–4345. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Eichten, S.R.; Shimizu, R.; Petsch, K.; Yeh, C.T.; Wu, W.; Chettoor, A.M.; Givan, S.A.; Cole, R.A.; Fowler, J.E.; et al. Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol. 2014, 15, R40. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Liao, J.Y.; Li, Z.Y.; Yu, Y.; Zhang, J.P.; Li, Q.F.; Qu, L.H.; Shu, W.S.; Chen, Y.Q. Genome-wide screening and functional analysis identify a large number of long noncoding RNAs involved in the sexual reproduction of rice. Genome Biol. 2014, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, W.; Hao, J.; Lv, S.; Wang, C.; Tong, W.; Wang, Y.; Wang, Y.; Liu, X.; Ji, W. Genome-wide identification and functional prediction of novel and fungi-responsive lincRNAs in Triticum aestivum. BMC Genom. 2016, 17, 238. [Google Scholar] [CrossRef]
- Qi, X.; Xie, S.; Liu, Y.; Yi, F.; Yu, J. Genome-wide annotation of genes and noncoding RNAs of foxtail millet in response to simulated drought stress by deep sequencing. Plant Mol. Biol. 2013, 83, 459–473. [Google Scholar] [CrossRef]
- Golicz, A.A.; Singh, M.B.; Bhalla, P.L. The long intergenic noncoding RNA (LincRNA) landscape of the soybean genome. Plant Physiol. 2017, 176, 2133–2147. [Google Scholar] [CrossRef]
- Chen, R.; Li, M.; Zhang, H.; Duan, L.; Sun, X.; Jiang, Q.; Zhang, H.; Hu, Z. Continuous salt stress-induced long non-coding RNAs and DNA methylation patterns in soybean roots. BMC Genom. 2019, 20, 730. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Yang, Y.; Zhang, X.; Huang, Z.; Zhang, D. Genome-wide analysis of long non-coding RNAs (lncRNAs) in two contrasting soybean genotypes subjected to phosphate starvation. BMC Genom. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lu, Q.; Ouyang, Y.; Mao, H.; Zhang, P.; Yao, J.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc. Natl. Acad. Sci. USA 2012, 109, 2654–2659. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Sung, S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef]
- Marquardt, S.; Raitskin, O.; Wu, Z.; Liu, F.; Sun, Q.; Dean, C. Functional consequences of splicing of the antisense transcript COOLAIR on FLC transcription. Mol. Cell 2014, 54, 156–165. [Google Scholar] [CrossRef]
- Kindgren, P.; Ard, R.; Ivanov, M.; Marquardt, S. Transcriptional read-through of the long non-coding RNA SVALKA governs plant cold acclimation. Nat. Commun. 2018, 9, 4561. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Lei, N.; Cheng, Z.; Zhao, P.; He, Y.; Wang, W.; Peng, M. Genome-wide identification and functional prediction of cold and/or drought-responsive lncRNAs in cassava. Sci. Rep. 2017, 7, 45981. [Google Scholar] [CrossRef]
- Bhatia, G.; Singh, A.; Verma, D.; Sharma, S.; Singh, K. Genomewide investigation of regulatory roles of lncRNAs in response to heat and drought stress in Brassica juncea (Indian mustard). Environ. Exp. Bot. 2020, 171, 103922. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Jiang, Q.; Sun, X.; Hu, Z.; Wang, L.; Zhang, H. Large DNA fragment deletion in lncRNA77580 regulates neighboring gene expression in soybean (Glycine max). Funct. Plant Biol. 2021, 48, 1139–1147. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.P.; Ashok, R.K.; Manohar, R.D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Berry, S.; Dean, C. Environmental perception and epigenetic memory: Mechanistic insight through FLC. Plant J. 2015, 83, 133–148. [Google Scholar] [CrossRef]
- Li, S.; Yamada, M.; Han, X.; Ohler, U.; Benfey, P.N. High-resolution expression map of the Arabidopsis root reveals alternative splicing and lincRNA regulation. Dev. Cell. 2016, 39, 508–522. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Wang, D.; Qu, Z.; Yang, L.; Zhang, Q.; Liu, Z.H.; Do, T.; Adelson, D.L.; Wang, Z.Y.; Searle, I.; Zhu, J.K. Transposable elements (TEs) contribute to stress-related long intergenic noncoding RNAs in plants. Plant J. 2017, 90, 133–146. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Lin, F.; He, G.; Terzaghi, W.; Zhu, D.; Deng, X.W. Arabidopsis noncoding RNA mediates control of photomorphogenesis by red light. Proc. Natl. Acad. Sci. USA 2014, 111, 10359–10364. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Hu, Z.; Jiang, Q.; Zhang, H. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol. Biol. 2013, 82, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Choi, H.I.; Im, M.Y.; Kim, S.Y. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 2002, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Yang, S.H.; Han, K.H. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006, 47, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Li, Y.; Zheng, N.; Chen, H.; Zhao, Q.; Gao, T.; Guo, H.; Xie, Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007, 19, 1912–1929. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Y.; Zhang, Y.; Liu, L.; Ning, Y.; Wang, D.; Tong, H.; Chen, S.; Chu, C.; Xie, Q. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 2011, 76, 145–156. [Google Scholar] [CrossRef]
- Li, M.; Chen, R.; Jiang, Q.; Sun, X.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity response in chloroplasts: Insights from gene characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological Response of Soybean Plants to Water Deficit. Front. Plant Sci. 2022, 12, 809692. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, L.; Kashiwagi, J.; Gaur, P.M.; Upadhyaya, H.D.; Vadez, V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Res. 2020, 119, 322–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).