Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L
Abstract
1. Introduction
2. Results
2.1. Characterization of the Synthesized Silver Nanoparticles
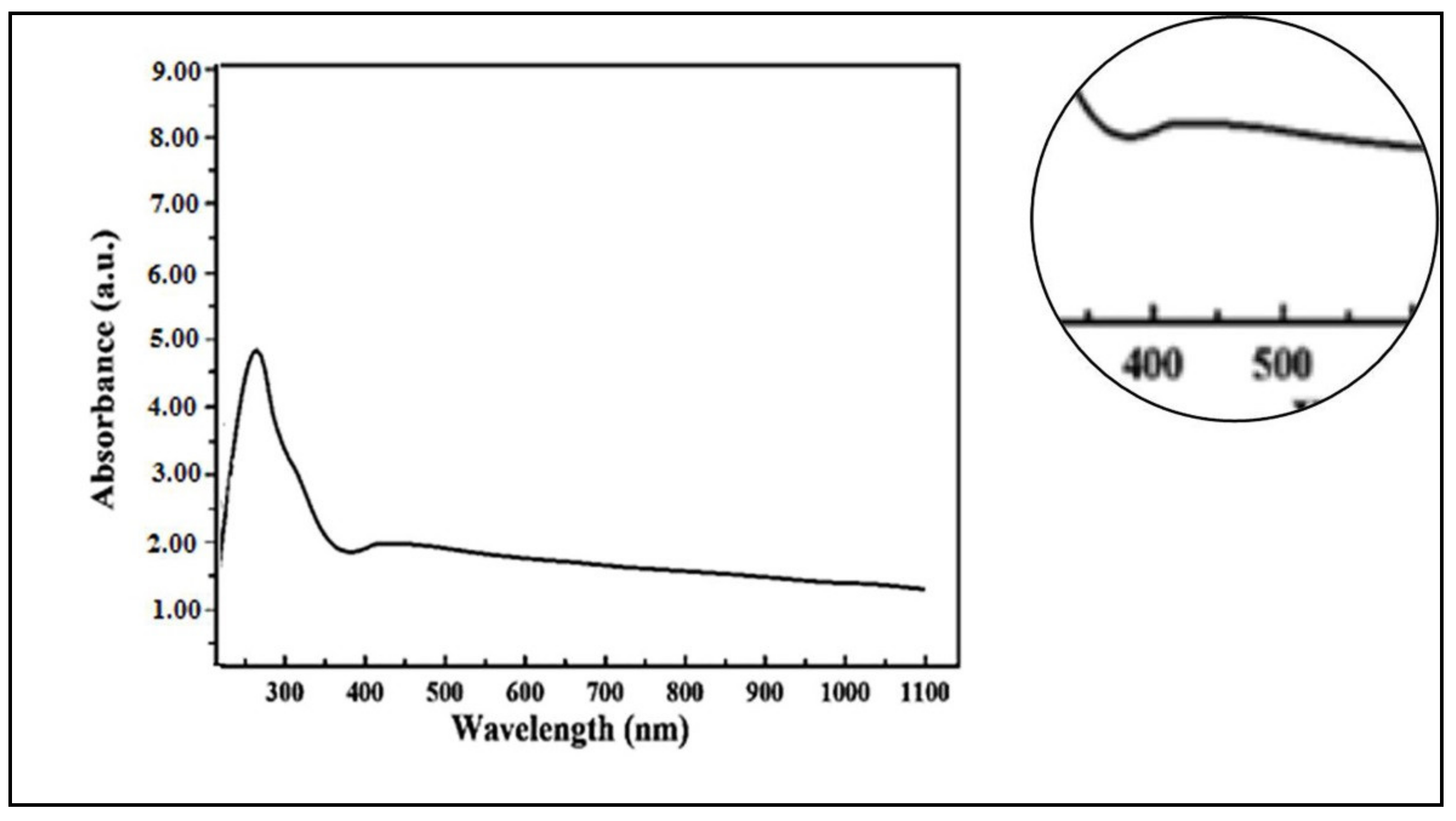
2.1.1. UV/Visible Spectroscopy
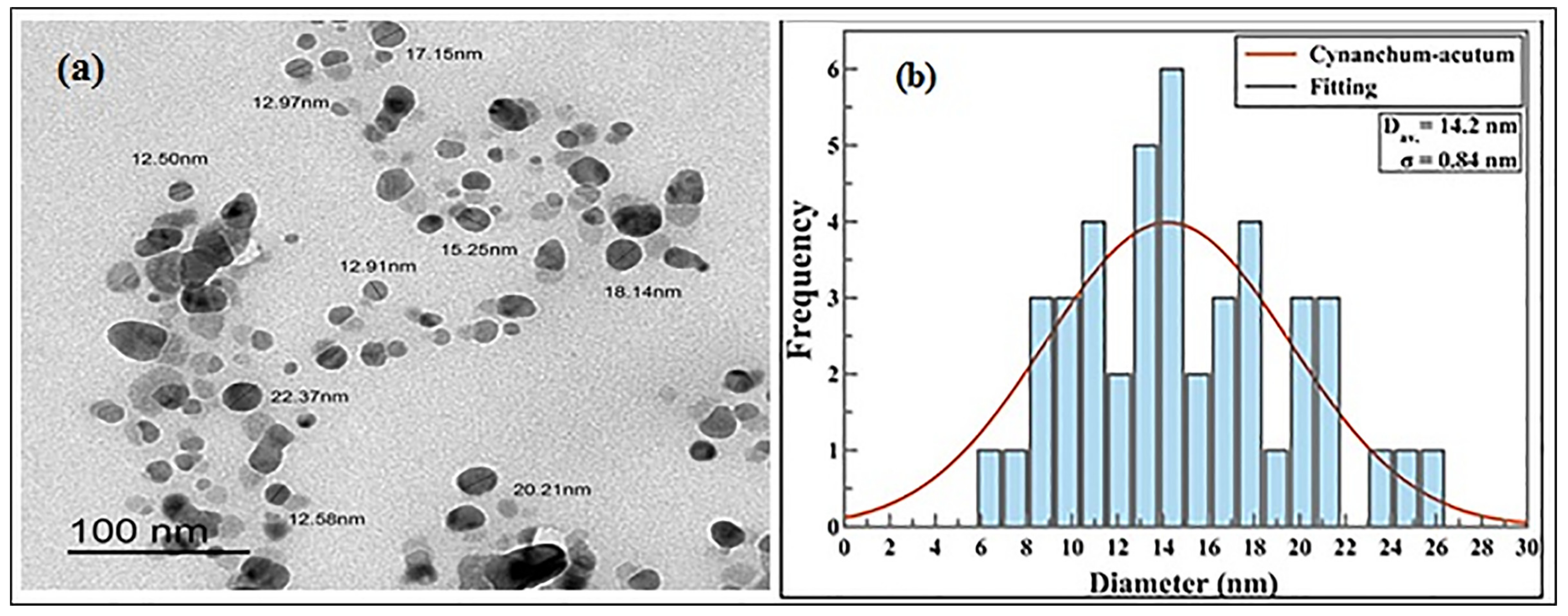
2.1.2. Transmission Electron Microscope Analysis (TEM)
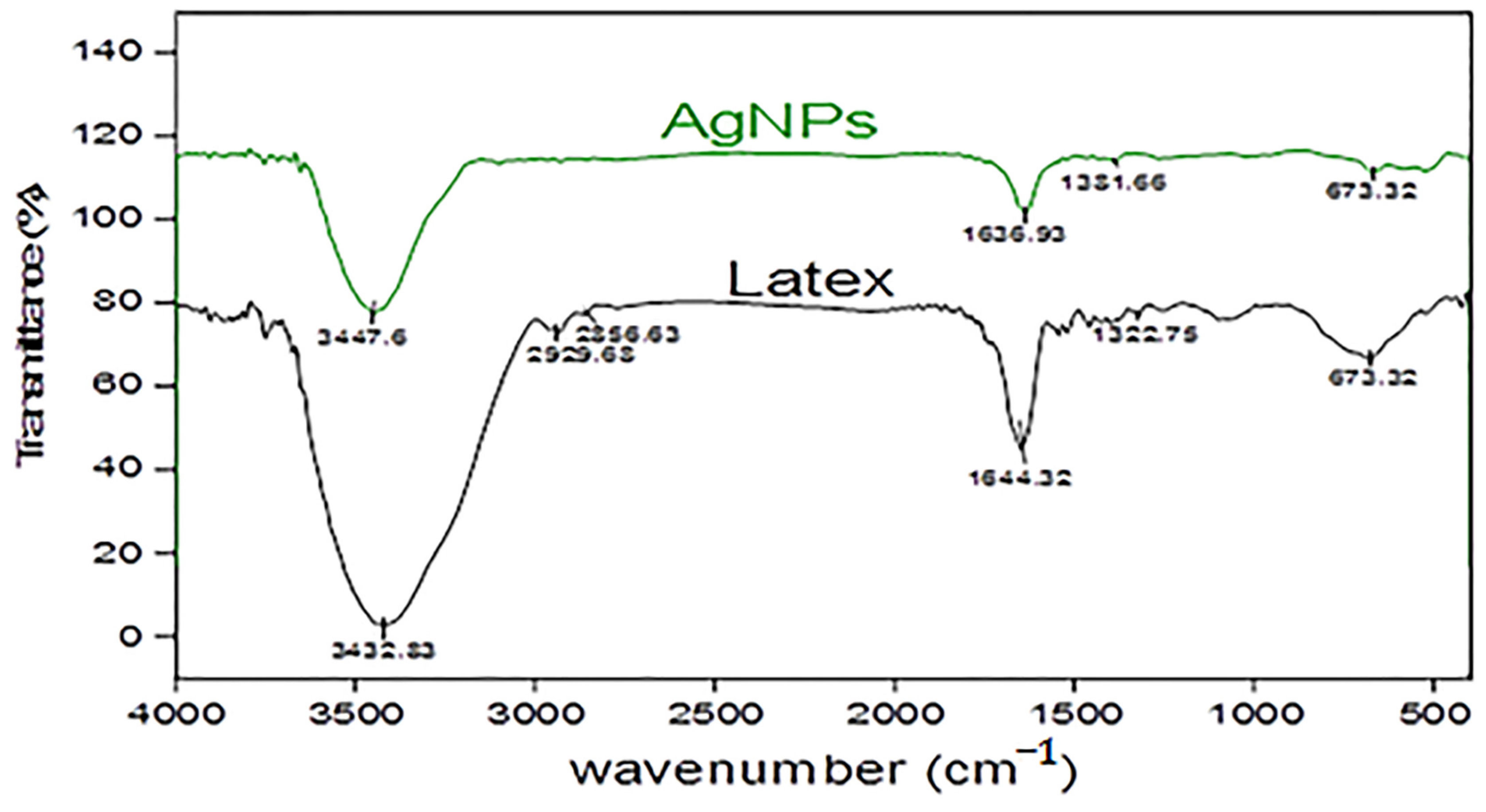
2.1.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2. Phytochemical Analysis
2.3. GC-MS Composition of C. acutum Latex
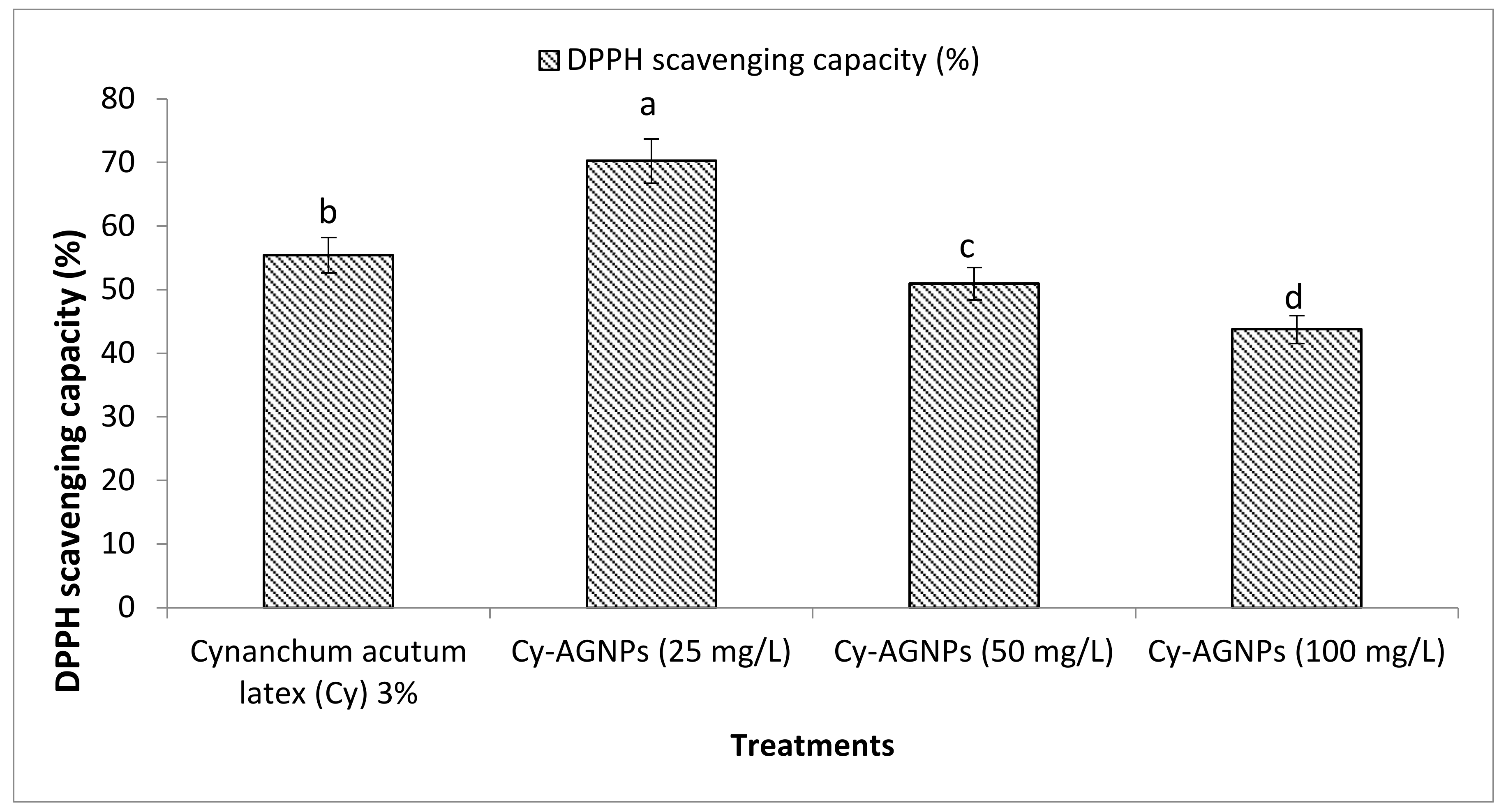
2.4. Antioxidant Activity (DPPH Scavenging Capacity (%))
2.5. Antibacterial Activity
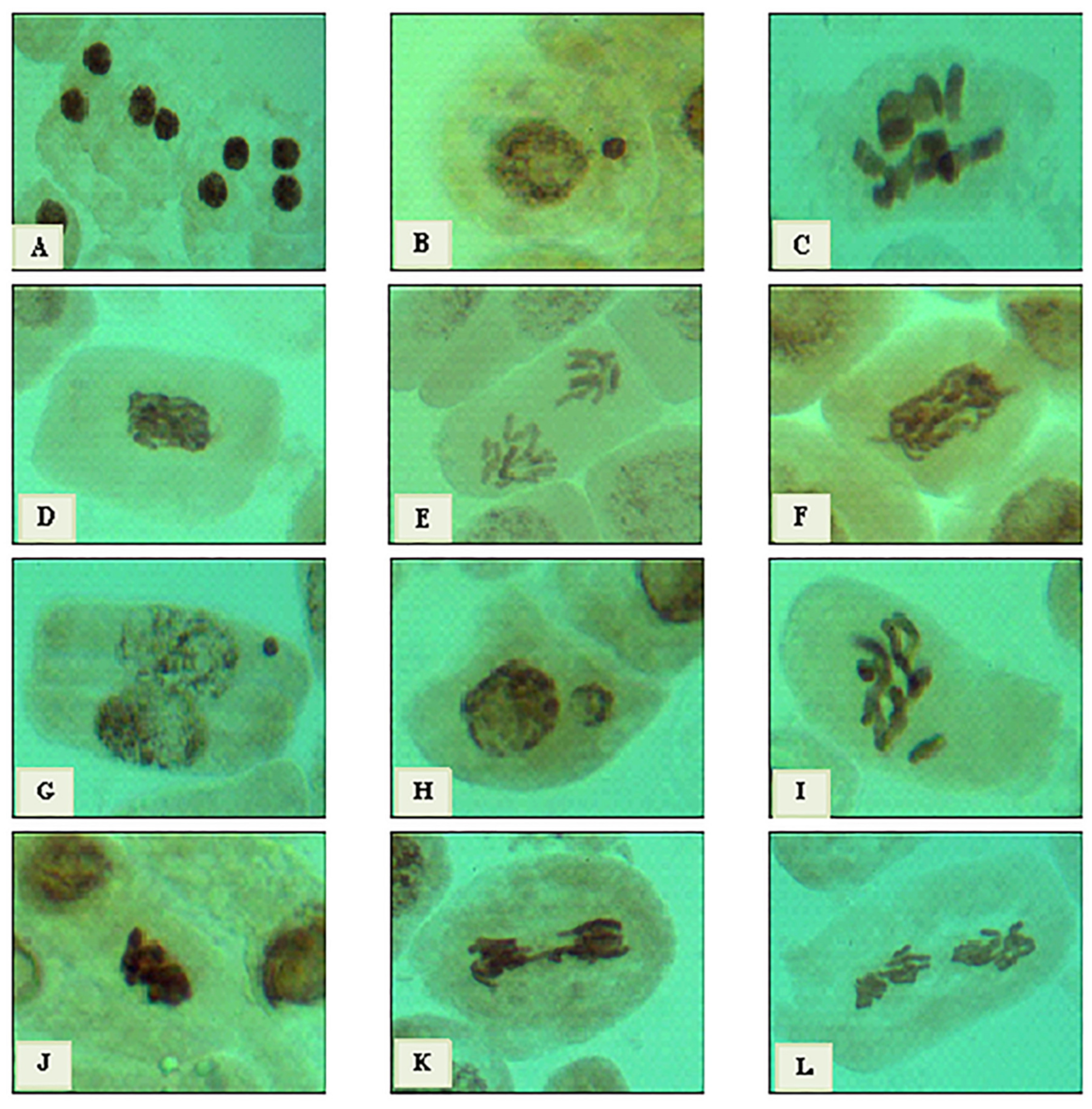
2.6. Cytotoxic Effect
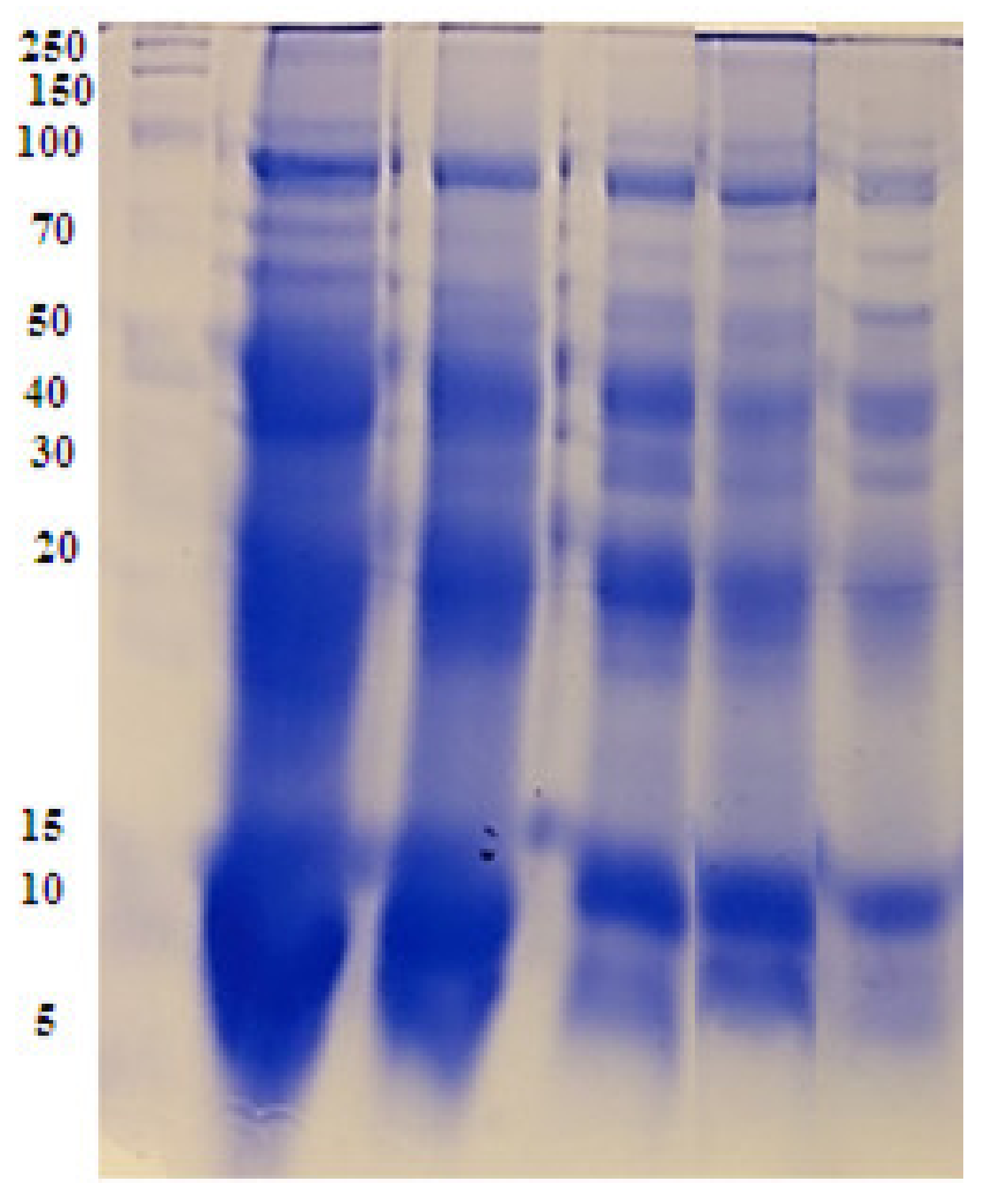
2.7. Biochemical Study Using Seed Protein Profile Electrophoresis of the Treated Vicia Faba Seeds
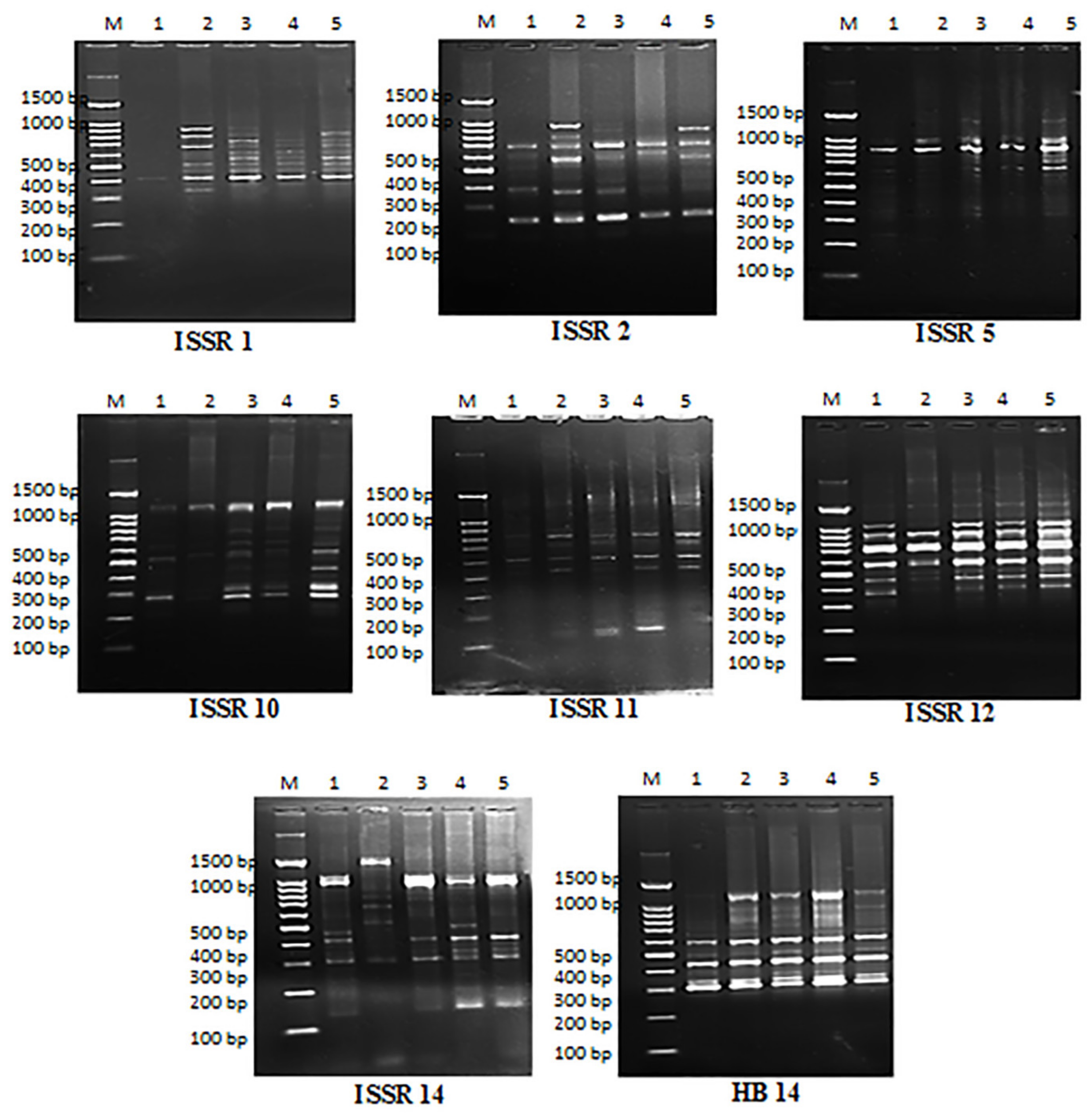
2.8. Molecular Analysis Using ISSR Marker
2.9. Genomic Template Stability
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Green Synthesis of Silver-Latex Nanoparticles
4.3. Characterization of Silver-Latex Nanoparticles
4.4. Phytochemical Analysis
4.4.1. Total phenolic Contents
4.4.2. Total Flavonoid Contents
4.4.3. Total Tannin Contents
4.4.4. Total Alkaloid Contents
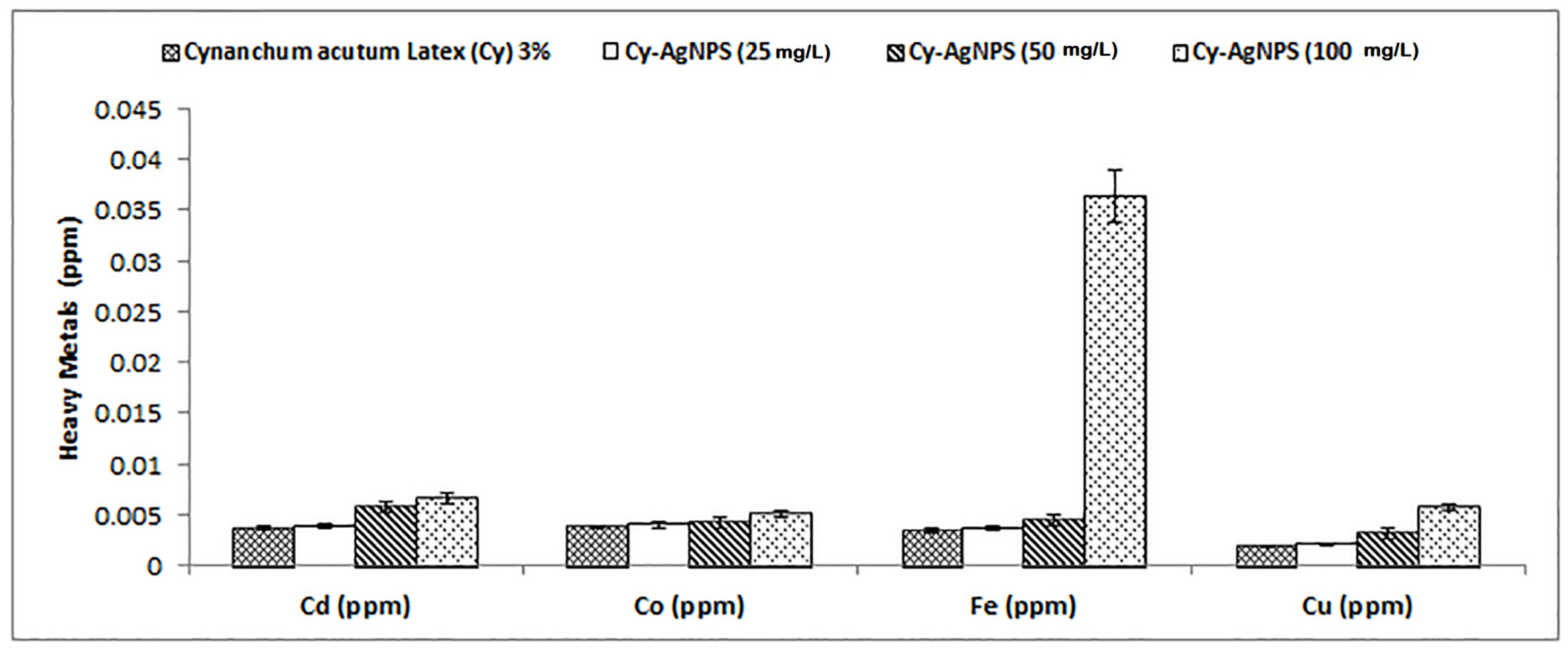
4.4.5. Determination of Heavy Metals in Latex
- a.
- Acid digestion
- b.
- Atomic absorption spectrophotometer analysis
4.5. GC–MS of C. Acutum Latex
4.6. Antioxidant Activity
4.7. Antibacterial Activity of Cy-AgNPs
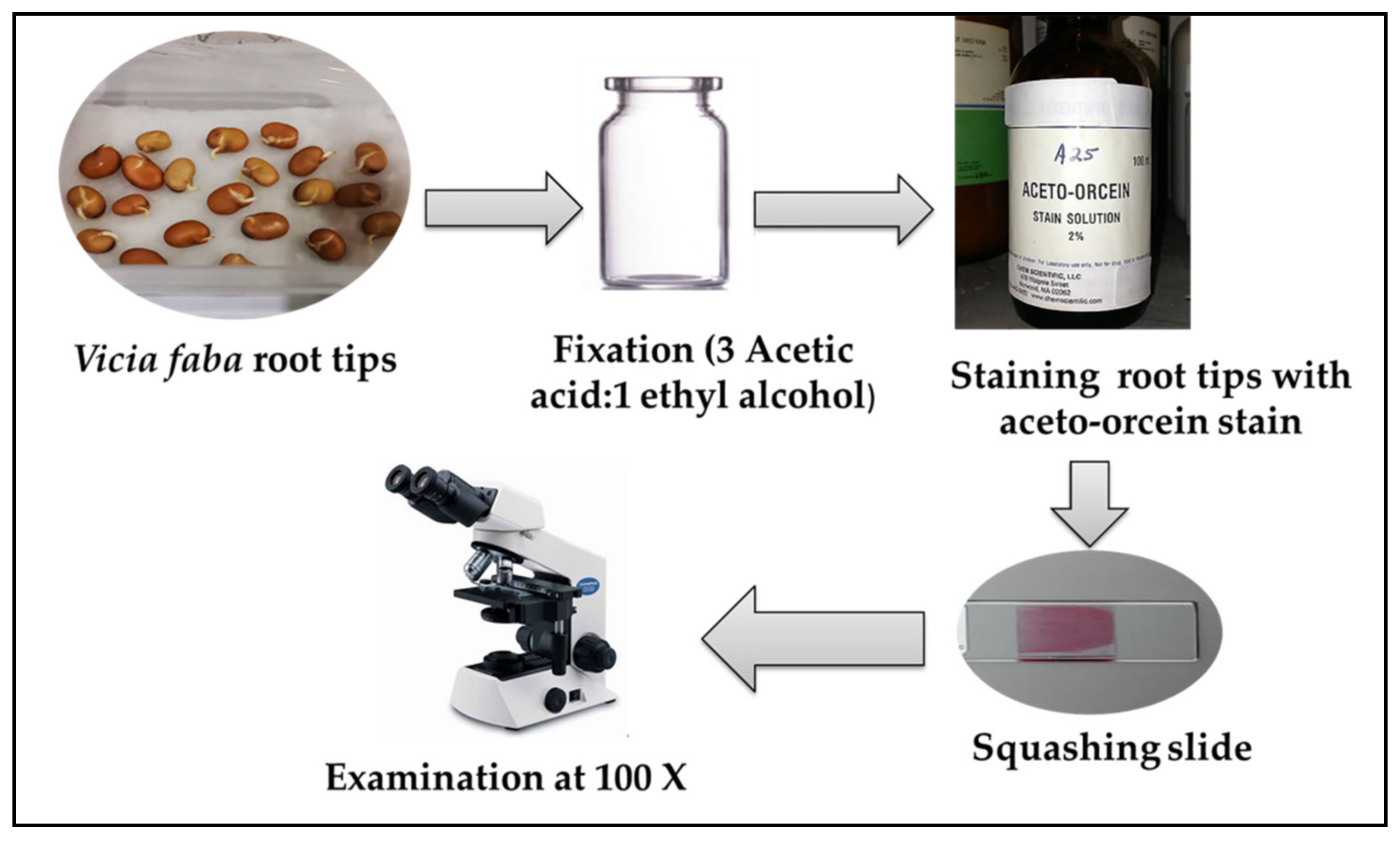
4.8. Determination of Cytotoxicity Using Chromosomal Aberration Assay (Vicia faba Test)
4.8.1. Pre-Treatment
4.8.2. Preparation of Silver-Latex Nanoparticles
4.8.3. Fixation of Roots, Slide Preparation, and Microscopic Examination
4.9. Data Analysis
4.10. Biochemical Study (Protein SDS-PAGE)
4.11. Molecular Study (ISSR Marker)
| Primer | Sequence |
|---|---|
| ISSR-1 | GAGAGAGAGAGAGAGAC |
| ISSR-2 | CACACACACACACACAG |
| ISSR-5 | CACACACACACAGG |
| ISSR-10 | (GA)7GT |
| ISSR-11 | (GACA)4 |
| ISSR-12 | T(GA)9 |
| ISSR-14 | (CTCT)4GTC |
| HB-14 | GAGGAGGAGGC |
4.12. Estimation of Genomic Template Stability (GTS%)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Jaiswal, S. Therapeutic Properties of Ficus Regligiosa. Int. J. Eng. Res. Gen. Sci. 2014, 2, 149–158. [Google Scholar]
- Yilmaz, A.; Yilmaz, M. Bimetallic core-shell nanoparticles of gold and silver via bioinspired polydopamine layer as surface-enhanced Raman spectroscopy (SERS) platform. Nanomaterial 2020, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and application of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang TF, W.; Yang EF, C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Chen, H.; Roco, M.C.; Li, X.; Lin, Y. Trends in nanotechnology patents. Nat. Nanotechnol. 2008, 3, 123–125. [Google Scholar] [CrossRef]
- Nadaroğlu, H.; Güngör, A.A.; İnce, S. Synthesis of Nanoparticles by Green Synthesis Method. Int. J. Innov. Res. Rev. 2017, 1, 6–9. [Google Scholar]
- Jain, N.; Bhargava, A.; Majumdar, S.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism prospective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanotechnol. Biol. Med. Nanomed. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. 2020, 2020, 3189043. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, C.; Chen, J.; Lv, L.; Chen, Z.; Chen, C.; Zheng, L. Cynanchum paniculatum and its major active constituents for inflammatory-related diseases: A review of traditional use, multiple pathway modulations, and clinical applications. Evid. Based Complement. Alternat. Med. 2020, 2020, 7259686. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ren, J.X.; Nan, H.M.; Liu, B.F. Identification of antibacterial and antioxidant constituents of the essential oils of Cynanchum chinense and Ligustrum compactum. Nat. Prod. Res. 2015, 29, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Bamola, N.; Verma, P.; Negi, C. A review on some traditional medicinal plants. Int. J. Life-Sci. Sci. Res. 2018, 4, 1550–1556. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications with Supplement: Search System for Diseases; Medpharm Scientific: Stuttgart, Germany, 2000. [Google Scholar]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Abarca LF, S.; Klinkhamer PG, L.; Choi, H.Y. Plant latex, from ecological interests to bioactive chemical resources. Planta Med. 2019, 85, 85–868. [Google Scholar]
- Salunkhe, P.; Bhoyar, P.; Gode, A.; Shewale, S.P. Application of nanotechnology to the extraction of herbal components for medicinal uses. Curr. Nanomater. 2020, 5, 4–11. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Kathiravan, G.; Srinivasan, K. Antioxidant activity and synthesis of silver nanoparticles using the leaf extract of Limona acidissima. Int. J. Pharm. Biol. Sci. 2016, 7, 201–205. [Google Scholar]
- Farrell, B.D.; Dussourd, D.E.; Mitter, C. Escalation of plant defense: Do latex and resin canals spur plant diversification? Am. Nat. 1991, 138, 881–900. [Google Scholar] [CrossRef]
- Hagel, J.M.; Yeung, E.C.; Facchini, P.J. Got milk? The secret life of laticifers. Trends Plant Sci. 2008, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Bhagyashri, C.A.; Jogendra, H.C.; Avinash, P.V. Plant latex: An inherent spring of pharmaceuticals. World J. Pharm. Pharm. Sci. 2015, 4, 1781–1796. [Google Scholar]
- Mesquita, M.L.; Desrivot, J.; Bories, C.; Fournet, A.; Paula, J.E.; Grellier, P.; Espindola, L.S. Antileishmanial and trypanocidal activity of Brazilian Cerrado plants. Mem. Inst. Oswaldo Cruz. 2005, 100, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Domsalla, A.; Melzig, M.F. Occurrence and properties of proteases in plant latices. Planta Med. 2008, 74, 699–711. [Google Scholar] [CrossRef]
- Kitajima, S.; Kamei, K.; Taketani, S.; Yamaguchi, M.; Kawai, F.; Komatsu, A.; Inukai, Y. Two chitinase-like proteins abundantly accumulated in latex of mulberry show insecticidal activity. BMC Biochem. 2010, 11, 6–11. [Google Scholar] [CrossRef]
- Fernandez-Arche, A.; Saenz, M.T.; Arroyo, M.; de la Puerta, R.; Garcia, M.D. Topical anti-inflammatory effect of tirucallol a triperpene isolated form Euphorbia lacteal latex. Phytomedicine 2010, 17, 146–148. [Google Scholar] [CrossRef]
- De Marino, S.; Gala, F.; Zollo, F.; Vitalini, S.; Fico, G.; Visioli, F.; Iorizzi, M. Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity. Molecules 2008, 13, 219–229. [Google Scholar] [CrossRef]
- Mendonça, R.J.; Maurício, V.B.; Teixeira, L.d.B.; Lachat, J.J.; Coutinho-Netto, J. Increased vascular permeability angiogenesis and wound healing induced by the serum of natural latex of the rubber tree Hevea brasiliensis. Phytother. Res. 2010, 24, 764–768. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Yang, M.; Zhou, L.; Xinxin Deng, X.; Wei, S.; Wang, W.; Wang, Z.; Qiao, X.; Bai, C. Ethnobotany, phytochemistry and pharmacological effects of plants in genus Cynanchum Linn. (Asclepiadaceae). Molecules 2018, 23, 1194. [Google Scholar] [CrossRef]
- Boomibalagan, P.; Eswaran, S.; Rathinavel, S. Traditional uses of medicinal plants of Asclepiadaceae by rural people in Madurai District, Tamil Nadu, India. Int. J. Bot. 2013, 9, 133–139. [Google Scholar] [CrossRef]
- Estakhr, J.; Javadian, F.; Ganjali, Z.; Dehghani, M.; Heidari, A. Study on the anti-Inflammatory effects of ethanolic extract of Cynanchum acutum. Curr. Res. J. Biol. Sci. 2012, 4, 630–632. [Google Scholar]
- Dehghani, M.; Ganjali, Z.; Javadian, F.; Estakhr, J.; Heidari, A. Anti-microbial activity of ethanolic and aqueous extract of Cynanchum acutum. Br. J. Pharmacol. Toxicol. 2012, 3, 177–180. [Google Scholar]
- Youssef, A.M.M.; El-Swaify, Z.A.S.; Al-Saraireh, Y.M.; Al-Dalain, S.M. Anticancer effect of different extracts of Cynanchum acutum L. seeds on cancer cell lines. Phcog. Mag. 2019, 15, 261–266. [Google Scholar] [CrossRef]
- Retchkiman-Schabes, P.S.; Canizal, G.; Becerra-Herrera, R.; Zorrilla, C.; Liu, H.B.; Ascencio, J.A. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater. 2006, 29, 95–99. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Supriya; Kaspate, R.; Pal, C.K.; Sengupta, S.; Basu, J.K. Microwave-mediated synthesis of silver nanoparticles on various metal-alginate composites: Evaluation of catalytic activity and thermal stability of the composites in solvent-free acylation reaction of amine and alcohols. SN Appl. Sci. 2020, 2, 282. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef]
- Shweta JH, A.; Ramesh, N.P. Molecular mechanism of plant nanoparticle interactions. In Molecular Mechanism of Plant–Nanoparticle Interactions; Kole, C., Kumar, D.S., Khodakovskaya, M.V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 15–181. [Google Scholar]
- Skalska, J.; Strużyńska, L. Toxic effects of silver nanoparticles in mammals—Does a risk of neurotoxicity exist? Folia Neuropathol. 2015, 53, 281–300. [Google Scholar] [CrossRef]
- Lee, T.Y.; Liu, M.S.; Huang, L.J.; Lue, S.I.; Lin, L.C.; Kwan, A.L.; Yang, R.C. Bioenergetic failure correlates with autophagy and apoptosis in rat liver following silver nanoparticle intraperitoneal administration. Part. Fibre Toxicol. 2013, 10, 40. [Google Scholar] [CrossRef]
- El Mahdy, M.M.; Eldin, T.A.; Aly, H.S.; Mohammed, F.F.; Shaalan, M.I. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015, 67, 21–29. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Van Der Ven, L.T.; Sleijffers, A.; Park, M.V.; Jansen, E.H.; Van Loveren, H.; Vandebriel, R.J. Systemic and immune toxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, O.M.; Hussein, R.M. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int. J. Nanomed. 2014, 9, 1505–1517. [Google Scholar]
- Feng, H.; Pyykko, I.; Zou, J. Hyaluronan up-regulation is linked to renal dysfunction and hearing loss induced by silver nanoparticles. Eur. Arch. Otorhinolaryngol. 2015, 272, 2629–2642. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-κB Pathway/miR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef]
- AbouZeid, A.H.S.; Ibrahim, N.A.; Sammour, E.A. Phytochemical, insecticidal and molluscicidal investigations of the aerial parts of Cynanchumacutum L. growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 2001, 39, 235–245. [Google Scholar]
- Rahman, A.; Seth, D.; Mukhopadhyaya, S.K.; Brahmachary, R.L.; Ulrichs, C.; Goswami, A. Surface functionalized amorphous nanosilica and microsilica with nanopores as promising tools in biomedicine. Naturwissenschaften 2009, 96, 31–38. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. Green synthesis of silver nanoparticles from plant latex and their antibacterial and photocatalytic studies. Environ. Technol. 2022, 43, 3064–3074. [Google Scholar] [CrossRef]
- Asare, G.A.; Bugyei, K.; Sittie, A.; Yahaya, E.S.; Gyan, B.; Adjei, S.; Addo, P.; Wiredu, E.K.; Adje, D.N.; Nyarko, A. Genotoxicity, cytotoxicity and toxicological evaluation of whole plant extracts of the medicinal plant Phyllanthus niruri (Phyllathaceae). Genet. Mol. Res. 2012, 11, 100–111. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Role of green silver nanoparticles synthesized from Symphytum officinale leaf extract in protection against UVB-induced photoaging. J. Nanostruct. Chem. 2018, 8, 359–368. [Google Scholar] [CrossRef]
- Bhuyan, B.; Paul, A.; Paul, B.; Dhar, S.S.; Dutta, P. Paederia foetida Linn. promoted biogenic gold and silver nanoparticles: Synthesis, characterization, photocatalytic and in vitro efficacy against clinically isolated pathogens. J. Photochem. Photobiol. 2017, 173, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2017, 45, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Grant, A. Effects of genotoxicity and its consequences at the population level in sexual and asexual Artemia assessed by analysis of inter-simple sequence repeats (ISSR). Mutat. Res. 2013, 757, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Archna, H.R. A review on green synthesis of silver nanoparticle, characterization and optimization parameters. Int. J. Res. Eng. Technol. 2016, 5, 49–53. [Google Scholar]
- Salem, W.M.; Haridyb, M.; Sayeda, W.F.; Hassan, N.H. Antibacterial activity of silver nanoparticles synthesized from latex and leaf extract of Ficus sycomorus. Ind. Crops Prod. 2014, 62, 228–234. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Mustafa, D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Nazeruddin, G.; Prasad, N.; Prasad, S.; Shaikh, Y.; Waghmare, S.; Adhyapak, P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crops Prod. 2014, 60, 212–216. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A.; Jafri, A.; Arshad, M.; Siddiqui, M.A. Green synthesis of silver nanocomposites of Nigella sativa seeds extract for hepatocellular carcinoma. Curr. Nanomater. 2019, 4, 191–200. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.P.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart. Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Raman, R.P.; Parthiban, S.; Srinithya, B.; Kumar, V.V.; Anthony, S.P.; Sivasubramanian, A.; Muthuraman, M.S. Biogenic silver nanoparticles synthesis using the extract of the medicinal plant Clerodendron serratum and its in-vitro antiproliferative activity. Mat. Lett. 2015, 160, 400–403. [Google Scholar] [CrossRef]
- Ayad, Z.M.; Ibrahim, O.M.S.; Omar, L.W. Biosynthesis and characterization of silver nanoparticles by Silybum marianum (silymarin) fruit extract. Adv. Anim. Vet. Sci. 2019, 7, 122–130. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Binupriya, A.R.; Sathishkumar, M.; Soon-Il, Y. Myco-crystallization of silver ions to nanosized particles by live and dead cell filtrates of Aspergillus oryzae var. viridis and its bactericidal activity toward Staphylococcus aureus KCCM 12256. Ind. Eng. Chem. Res. 2010, 49, 852–858. [Google Scholar] [CrossRef]
- Babu, S.A.; Prabu, G.H. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater. Lett. 2011, 65, 1675–1677. [Google Scholar] [CrossRef]
- Ndikau, M.; Noah, N.M.; Andala, D.M.; Masika, E. Green Synthesis and Characterization of Silver Nanoparticles Using Citrullus lanatus Fruit Rind Extract. Hindawi 2017, 2017, 8108504. [Google Scholar] [CrossRef]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional cellulose nanocrystal/metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Ranade, A.V.; Acharya, R.N.; Shukla, V.J.; Maji, J. Monitoring of seasonal variation in latex of Calotropis procera AIT. and Calotropis gigantea L.R.BR using FTIR spectroscopy. J. Res. Educ. Indian Med. 2017, 23, 59–74. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nano Med. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, K.; Reddy, P.S. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 121, 164–172. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Chen, J.; Zhu, Y.; Zhang, Z. The Effects of Several Metal Nanoparticles on Seed Germination and Seedling Growth: A Meta-Analysis. Coatings 2022, 12, 183. [Google Scholar] [CrossRef]
- Sre, P.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta Part A 2015, 135, 1137–1144. [Google Scholar]
- Shakirin, F.H.; Azlan, A.; Ismail, A.; Amom, Z.; Cheng, Y. Protective Effect of Pulp Oil Extracted from Canarium odontophyllum Miq. Fruit on Blood Lipids, Lipid Peroxidation, and Antioxidant Status in Healthy Rabbits. Oxidative Med. Cell. Longev. 2012, 2012, 840973. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Gupta, T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants 2017, 23, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.; Demirci, B.; Yilmaz, G.; Can Baser, K.H. Essential oil composition of three species of Scutellaria from Turkey. Nat. Prod. Res. 2011, 25, 1720–1726. [Google Scholar] [CrossRef]
- He, L. The Studies on the Bacteriostatic Effects of Loquat Flower’s Extracts and its Relative Active Compositions. Master’s Thesis, Sichuan Normal University, Chengdu, China, 2009. [Google Scholar]
- Hassan, A.; Akmal, Z.; Khan, N. The phytochemical screening and antioxidants potential of Schoenoplectus triqueter L. Palla. Hindawi 2020, 2020, 3865139. [Google Scholar] [CrossRef]
- Banso, A.; Adeyemo, S.O. Phytochemical and antimicrobial evaluation of ethanolic extract of Dracaena manni. Bark Nig. J. Biotechnol. 2007, 18, 27–32. [Google Scholar]
- Ashokkumar, R.; Ramaswamy, M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian Medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 395–406. [Google Scholar]
- Joshi, H.; Choudhary, P.; Mundra, S. Future prospects of nanotechnology in agriculture. Int. J. Chem. Stud. 2019, 7, 957–963. [Google Scholar]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-3; International Organization for Standardization—ISO. 1992a: ISO 10.993-3: International Standard: Biological Evaluation of Medical Devices—Part 3. Tests for Cytotoxicity: In Vitro Methods. ISO: Geneva, Switzerland, 1992.
- ISO 10993-5; International Organization for Standardization—ISO. 1992b: ISO 10.993-5: International Standard: Biological Evaluation of Medical Devices—Part 5. Tests for Cytotoxicity: In Vitro Methods. ISO: Geneva, Switzerland, 1992.
- Ma, T.H. Vicia cytogenetic tests for environmental mutagens. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1982, 99, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Grant, V. Plant Speciation, 2nd ed.; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Ghosh, M.; Ghosh, I.; Godderis, L.; Hoet, P.; Mukherjee, A. Genotoxicity of engineered nanoparticles in higher plants. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Sousa, T.R.; Arruda, A.S.; Peixoto, N.; Gonçalves, P.J.; Almeid, L.M. Evaluation of cytotoxicity and genotoxicity of Hancornia speciosa latex in Allium cepa root model. Braz. J. Biol. 2016, 76, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tan, L.; Zhang, S.H.; Zuo, Y.T.; Han, X.; Liu, N.; Lu, W.Q.; Liu, A.L. Detection of genotoxic effects of drinking water disinfection byproducts using Vicia faba bioassay. Environ. Sci. Pollut. Res. 2017, 24, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Sudhakar, R.; Gowda, N.; Venu, G. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia 2001, 66, 235–239. [Google Scholar] [CrossRef]
- Liman, R.; Hakki, I.; Cigerci, I.H.; Ozturuk, N.S. Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome abbreviation, and comet assay. Pestic. Biochem. Physiol. 2015, 118, 38–42. [Google Scholar] [CrossRef]
- Sobieh, S.S.; Kheiralla, Z.M.H.; Rushdy, A.A.; Yakob, N.A.N. In vitro and in vivo genotoxicity and molecular response of silver nanoparticles on different biological model systems. Caryologia 2016, 69, 2165–5391. [Google Scholar] [CrossRef][Green Version]
- Mahfouz, H.M.; Barakat, H.M.; Halem, A.S.; El- Hahdy, M.M. Effects of Jasmonic and Salicylic Acids on Cell Division and Cell Cycle Progression. Egypt. J. Bot. 2014, 54, 185–201. [Google Scholar]
- Fouad, A.S.; Hafez, R.M. The effects of silver ions and silver nanoparticles on cell division and expression of cdc2 gene in Allium cepa root tips. Biol. Plant. 2018, 62, 166–172. [Google Scholar] [CrossRef]
- Albertini, R.J.; Anderson, D.; Douglas, G.R.; Hagmar, L.; Hemmink, K.; Merlo, F.; Natarajan, A.T.; Norppa, H.; Shuker, D.E.; Tice, R.; et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res. 2000, 463, 111–172. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmadi, M.S. Cytogenetic and molecular assessment of some nanoparticles using Allium sativum assay. Afr. J. Biotechnol. 2019, 18, 783–796. [Google Scholar]
- Babu, K.; Deepa, M.; Shankar, S.; Rai, S. Effect of nano-silver on cell division and mitotic chromosomes: A prefatory siren. Internet J. Nanotechnol. 2008, 2, 1–7. [Google Scholar]
- Hafez, R.M.; Fouad, A.S. Mitigation of genotoxic and cytotoxic effects of silver nanoparticles on Onion root tips using some antioxidant scavengers. Egypt. J. Bot. 2020, 60, 133–145. [Google Scholar] [CrossRef]
- Srecec, S. Phenotypic and genotypic analysis of spike abnormality in bread wheat (Triticum aestivum L. em Thell) cv. Pitoma. Cereal Res. Commun. 1995, 32, 63–69. [Google Scholar]
- Panda, K.K.; Achary, M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitr. 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- Pesnya, D.S. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia Int. J. Cytol. Cytosyst. Cytogen. 2013, 66, 275–281. [Google Scholar]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S.; El-Mehasseb, I.M. Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanz. 2020, 72, 113–127. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Shehab, A.S.; Tawab, S.A.; Morsi, M.M. Stimulation of mitotic process and gene expression of Vicia faba L. by Azadirachta indica A. juss. Egypt. J. Biotech. 2004, 17, 499–514. [Google Scholar]
- Telma, E.S.; Zanor, M.L.; Valle, E.M. Investigating the role of plant heat shock proteins during oxidative stress. Plant Signal Behav. 2008, 3, 856–857. [Google Scholar]
- Vannini, C.; Domingoa, G.; Onelli, E.; Mattiac, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.P.; Gottschelk, M.A. Quantitative and qualitative situation of Pisum sativum. In Nuclear Techniques for Seed Protein Improvement. IAEA Vienna 1973, 45, 235–253. [Google Scholar]
- Atienzar, F.A.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Use of the random amplified polymorphic DNA (RAPD) for the detection of DNA damage and mutations: Possible implication of confounding factors. Biomarkers 2002, 7, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Cenkci, S.; Yildiz, M.; Ciğerci, I.H.; Konuk, M.; Bozdağ, A. Toxic chemicals induced genotoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere 2009, 76, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, K. Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. Int. J. Indust. Entomol. 2005, 10, 79–86. [Google Scholar]
- Karaca, M.; Izbirak, A. Comparative analysis of genetic diversity in Turkish durum wheat cultivars using RAPD and ISSR markers. J. Food Agric. Environ. 2008, 6, 219–225. [Google Scholar]
- Abdelmigid, H.M. Qualitative assessment of cadmium stress using genome template stability in Hordeum vulgare. Egypt J. Genet. Cytol. 2010, 39, 291–303. [Google Scholar] [CrossRef]
- Neeratanaphan, L.; Sudmoon, R.; Chaveerach, A. Assessment of Genotoxicity through ISSR Marker in Pistia stratiotes induced by Lead. EnvironmentAsia 2014, 7, 99–107. [Google Scholar]
- Savva, D. Use of DNA fingerprinting to detect genotoxic effects. Ecotoxicol. Environ. Saf. 1998, 41, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Galal, O.A.; Thabet, A.F. Cytological and molecular effects of silver nanoparticles (AgNPs) on Vicia faba M1 Plants. J. Agric. Chem. Biotechnol. 2018, 9, 269–275. [Google Scholar] [CrossRef]
- Mahfouz, H. Molecular and cytogenetic assessment of zinc oxide nanoparticles on Vicia faba plant cells. Egypt. J. Exp. Biol. 2019, 15, 39–49. [Google Scholar]
- Savva, D. The use of arbitrarily primed PCR (AP-PCR) fingerprinting detects exposure to genotoxic chemicals. Ecotoxicology 2000, 9, 341–353. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.Y.; Mclellan, J.; Siekkenen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Kalaichelvan, P.T. Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract, and coated on cotton cloth for effective antibacterial activity. Int. J. Nanomed. 2015, 10 (Suppl. S1), 87–97. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Issa, N.K.; Abdul Jabar, R.S.; Hammo, Y.H.; Kamal, I.M. Antioxidant activity of apple peels bioactive molecules extractives. Sci. Technol. 2016, 10, 76–88. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. Research on antioxidant activity of flavonoids from natural materials. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Burlingame, B. Wild nutrition. J. Food Compost. Anal. 2000, 13, 99–100. [Google Scholar] [CrossRef]
- Aberoumand, A. Nutritional evaluation of edible Portulaca oleracia as plant food. Food Anal. Methods 2009, 2, 204–207. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of plant analysis. In Phytochemical Methods; Chapman and Hall: London, UK, 1973. [Google Scholar]
- Jones, J.B.; Case, V.W. Sampling, handling and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Book Series No. 3; Soil Science Society of America: Madison, WI, USA, 1990. [Google Scholar]
- Allen, S.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Material; Blackwell Scientific Publication: Oxford, UK, 1974; p. 521. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- M02-A12; Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. CLSI: Wayne, PA, USA, 2015.
- Chattopadhyay, D.; Sharma, A.K. A new technique for orcein banding with acid treatment. Stain Technol. 1988, 63, 283–287. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.A. Statistical Methods, 6th ed.; The Iowa State University Press: Ames, IA, USA, 1976. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W. Electrophoretic patterns of esterase and acid phosphatase in the grain of registered varieties of barley (Hordum vulgare L.). Genet. Slechteri 1973, 31, 113–122. [Google Scholar]
- Atienzar, F.A.; Conradi, M.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Qualitative assessment of genotoxicity using Random Amplified Polymorphic DNA: Comparison of genomic template stability with key fitness parameters in Daphnia magna exposed to benzo[a] pyrene. Environ. Toxicol. Chem. 1999, 18, 2275–2282. [Google Scholar] [CrossRef]

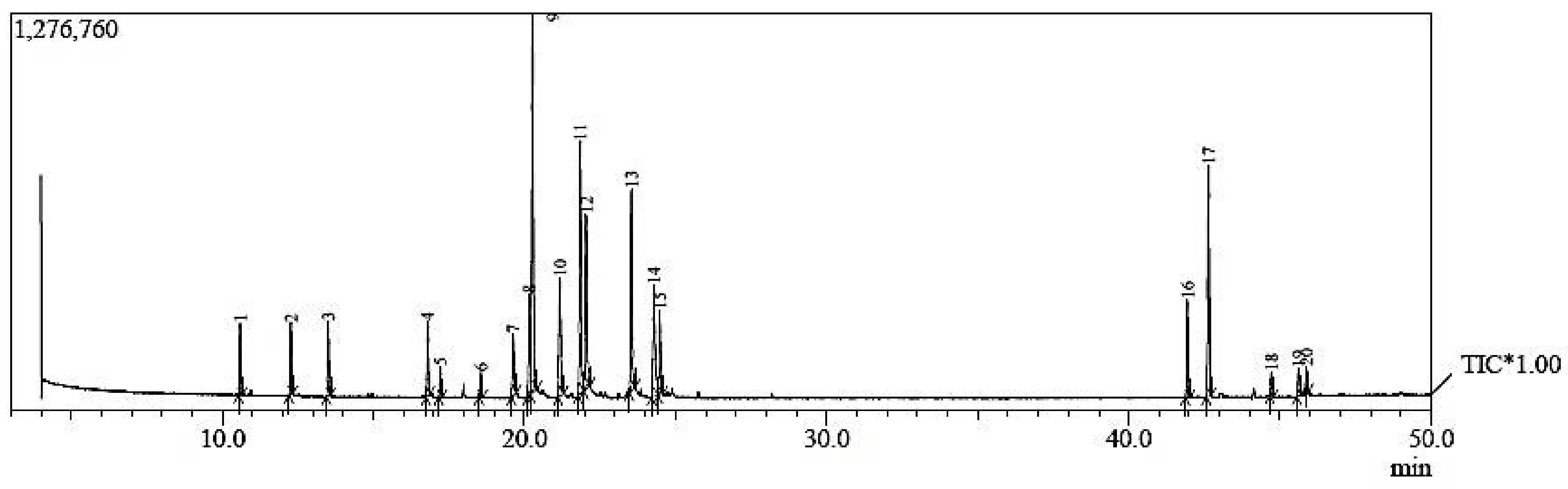



| Quantitative ID | Component Identified | Retention Time (min) | Retention Index (RI) | Area (%) | Identification |
|---|---|---|---|---|---|
| 1 | Octanoic Acid | 10.6 | 1015 | 3.62 | RI, MS * |
| 2 | L-Glucose, 6-deoxy-3-Omethyl- | 12.3 | 236 | 3.15 | RI, MS |
| 3 | Octanal | 13.5 | 874 | 2.99 | RI, MS |
| 4 | Hexanoic acid | 16. 9 | 963 | 2.67 | RI, MS |
| 5 | Thymol | 17.21 | 1290 | 2.11 | RI, MS |
| 6 | Curcumene | 18.44 | 1483 | 1.99 | RI, MS |
| 7 | Elemenone | 19.56 | 1593 | 2.86 | RI, MS |
| 8 | Germacrone | 20.22 | 1693 | 4.45 | RI, MS |
| 9 | Lupeol | 20.56 | 3270 | 15.36 | RI, MS |
| 10 | tetradecanoic acid | 21.12 | 1761 | 6.56 | RI, MS |
| 11 | Hexadecanoic acid (palmitic acid) | 21.73 | 2023 | 10.72 | RI, MS |
| 12 | Phytol | 21.95 | 2111 | 6.51 | RI, MS |
| 13 | Octadecanoic acid | 23. 42 | 2102 | 8.78 | RI, MS |
| 14 | Naphthalene, | 24.38 | 1179 | 5.21 | RI, MS |
| 15 | 7-nonenamide | 24.65 | 2523 | 3.91 | RI, MS |
| 16 | 1-Octadecyne | 41.71 | 1793 | 4.98 | RI, MS |
| 17 | Neophytadiene | 42.56 | 1827 | 9.15 | RI, MS |
| 18 | Cis-Vaccenic acid | 44.65 | 2141 | 1.78 | RI, MS |
| 19 | Glycerol-1-Palmitate | 45.67 | 3734 | 1.66 | RI, MS |
| 20 | Tridecanal | 45.87 | 1510 | 1.54 | RI, MS |
| Treatment | %MI | Phase Index | % Total Abnormal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Prophase | % Metaphase | % Anaphase | % Telophase | Interphase | Mitosis | |||||||
| Samples | Conc. | Mitotic | Abn. | Mitotic | Abn. | Mitotic | Abn. | Mitotic | Abn. | |||
| Untreated sample | 10.70 ± 0.45 | 22.76 | 0.00 | 69.88 | 16.77 | 0.51 | 2.47 | 6.75 | 1.56 | 0.03 ± 0.02 | 20.81 ± 2.09 | |
| Latex of C.acutum L. | 3% Crude Latex | 7.98 ± 0.49 ns | 20.81 | 0.00 | 79.19 | 25.96 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.02 | 25.96 ± 2.87 * |
| Cy-AgNPs 25 mg/L | 9.08 ± 0.59 ns | 15.67 | 0.00 | 49.04 | 15.73 | 9.73 | 2.25 | 25.56 | 5.43 | 0.16 ± 0.11 | 23.41 ± 3.90 * | |
| Cy-AgNPs 50 mg/L | 4.95 ± 0.52 ns | 1.64 | 0.00 | 84.72 | 55.82 | 3.64 | 3.64 | 10.00 | 7.28 | 0.93 ± 0.32 | 66.73 ± 6.82 ns | |
| Cy-AgNPs 100 mg/L | 4.04 ± 0.30 ns | 5.48 | 0.00 | 91.13 | 62.20 | 1.69 | 3.39 | 1.69 | 2.54 | 0.53 ± 0.15 | 68.14 ± 5.40 ns | |
| No. | Treatments | Polymorphism | |||||
|---|---|---|---|---|---|---|---|
| Control | 3% Cynanchum Latex | 25 mg/L Cy-AgNPs | 50mg/L Cy-AgNPs | 100mg/L Cy-AgNPs | |||
| 1 | 100 | 1 | 1 | 1 | 1 | 1 | M. |
| 2 | 90 | 1 | 1 | 1 | 1 | 1 | M. |
| 3 | 70 | 1 | 1 | 1 | 1 | 1 | M. |
| 4 | 60 | 1 | 1 | 1 | 1 | 1 | M. |
| 5 | 50 | 1 | 1 | 1 | 1 | 1 | M. |
| 6 | 40 | 1 | 1 | 1 | 1 | 1 | M. |
| 7 | 30 | 1 | 1 | 1 | 1 | 1 | M. |
| 8 | 28 | 1 | 1 | 1 | 0 | 0 | P. |
| 9 | 23 | 0 | 0 | 1 | 1 | 1 | P. |
| 10 | 20 | 1 | 1 | 1 | 1 | 1 | M. |
| 11 | 19 | 1 | 1 | 1 | 1 | 0 | P. |
| 12 | 18 | 1 | 1 | 1 | 0 | 0 | P. |
| 13 | 17 | 1 | 0 | 0 | 0 | 0 | P. |
| 14 | 15 | 1 | 1 | 1 | 1 | 1 | M. |
| 15 | 10 | 1 | 1 | 0 | 1 | 0 | P. |
| 16 | 5 | 1 | 1 | 1 | 1 | 1 | M. |
| Total bands | 15 | 14 | 14 | 13 | 11 | ||
| Polymorphic bands % | 33 | 29 | 29 | 23 | 9 | 37.5% | |
| GTS% | 100 | 75.33% | 80% | 73.33% | 60% | -------- | |
| Primer | Monomorphic Bands | Polymorphic Bands | Total Bands | Polymorphism % | |
|---|---|---|---|---|---|
| Unique Bands | Non-Unique Bands | ||||
| ISSR 1 | 1 | 1 | 8 | 10 | 90 |
| ISSR 2 | 3 | 3 | 3 | 9 | 66.67 |
| ISSR 5 | 2 | 1 | 6 | 9 | 77.78 |
| ISSR 10 | 4 | 2 | 4 | 10 | 60 |
| ISSR 11 | 3 | 0 | 3 | 6 | 50 |
| ISSR 12 | 7 | 1 | 2 | 10 | 30 |
| ISSR 14 | 1 | 4 | 9 | 14 | 92.86 |
| HB 14 | 5 | 0 | 6 | 11 | 54.5 |
| Total | 23 | 12 | 44 | 79 | 70.89 |
| Primer Name | Control | 3% Latex | 25 ppm AgNPs | 50 ppm AgNPs | 100 ppm AgNPs | ||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | a | b | A | b | a | b | ||
| ISSR 1 | 1 | 5 | 0 | 0 | 0 | 7 | 0 | 7 | 0 |
| ISSR 2 | 5 | 3 | 0 | 1 | 0 | 1 | 2 | 1 | 2 |
| ISSR 5 | 4 | 1 | 1 | 2 | 1 | 2 | 1 | 5 | 1 |
| ISSR 10 | 4 | 0 | 0 | 4 | 0 | 3 | 0 | 6 | 0 |
| ISSR 11 | 3 | 3 | 0 | 3 | 0 | 3 | 0 | 1 | 0 |
| ISSR 12 | 10 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 |
| ISSR 14 | 7 | 4 | 5 | 3 | 1 | 5 | 1 | 3 | 1 |
| HB 14 | 5 | 5 | 0 | 4 | 0 | 6 | 0 | 6 | 0 |
| Total number of bands | 39 | 21 | 8 | 17 | 3 | 27 | 6 | 29 | 6 |
| a + b | ----- | 29 | 20 | 33 | 35 | ||||
| GTS% | 100% | 25.64% | 51.28% | 15.38% | 10.26% | ||||
| Treatments | Code |
|---|---|
| Control | 1 |
| Crude latex of C. acutum L. (3%) | 2 |
| Silver nanoparticles of C. acutum L. (25 Cy-AgNPsmg/L) | 3 |
| Silver nanoparticles of C. acutum L. (50 Cy-AgNPs mg/L) | 4 |
| Silver nanoparticles of acutum L. (100 Cy-AgNPs mg/L) | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, M.I.; Mohammed, N.S.; EL-Sherbeny, G.; Safhi, F.A.; ALshamrani, S.M.; Alyamani, A.A.; Alharthi, B.; Qahl, S.H.; Al Kashgry, N.A.T.; Abd-Ellatif, S.; et al. Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L. Plants 2023, 12, 172. https://doi.org/10.3390/plants12010172
Soliman MI, Mohammed NS, EL-Sherbeny G, Safhi FA, ALshamrani SM, Alyamani AA, Alharthi B, Qahl SH, Al Kashgry NAT, Abd-Ellatif S, et al. Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L. Plants. 2023; 12(1):172. https://doi.org/10.3390/plants12010172
Chicago/Turabian StyleSoliman, Magda I., Nada S. Mohammed, Ghada EL-Sherbeny, Fatmah Ahmed Safhi, Salha Mesfer ALshamrani, Amal A. Alyamani, Badr Alharthi, Safa H. Qahl, Najla Amin T. Al Kashgry, Sawsan Abd-Ellatif, and et al. 2023. "Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L" Plants 12, no. 1: 172. https://doi.org/10.3390/plants12010172
APA StyleSoliman, M. I., Mohammed, N. S., EL-Sherbeny, G., Safhi, F. A., ALshamrani, S. M., Alyamani, A. A., Alharthi, B., Qahl, S. H., Al Kashgry, N. A. T., Abd-Ellatif, S., & Ibrahim, A. A. (2023). Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L. Plants, 12(1), 172. https://doi.org/10.3390/plants12010172







