Population Genomics Study and Implications for the Conservation of Zabelia tyaihyonii Based on Genotyping-By-Sequencing
Abstract
1. Introduction
2. Results
2.1. SNP Analysis and Genetic Diversity
2.2. Genetic Diversity
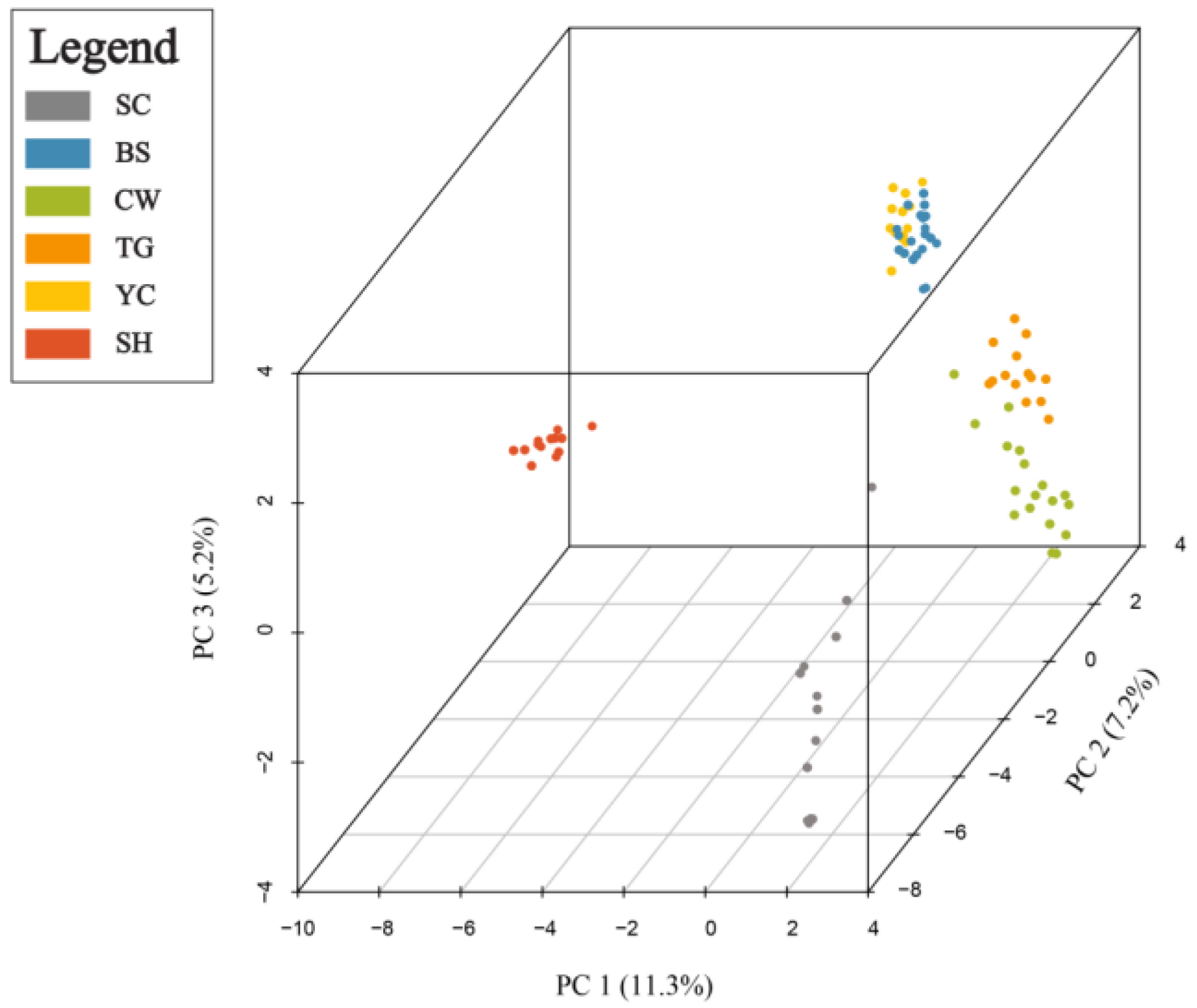
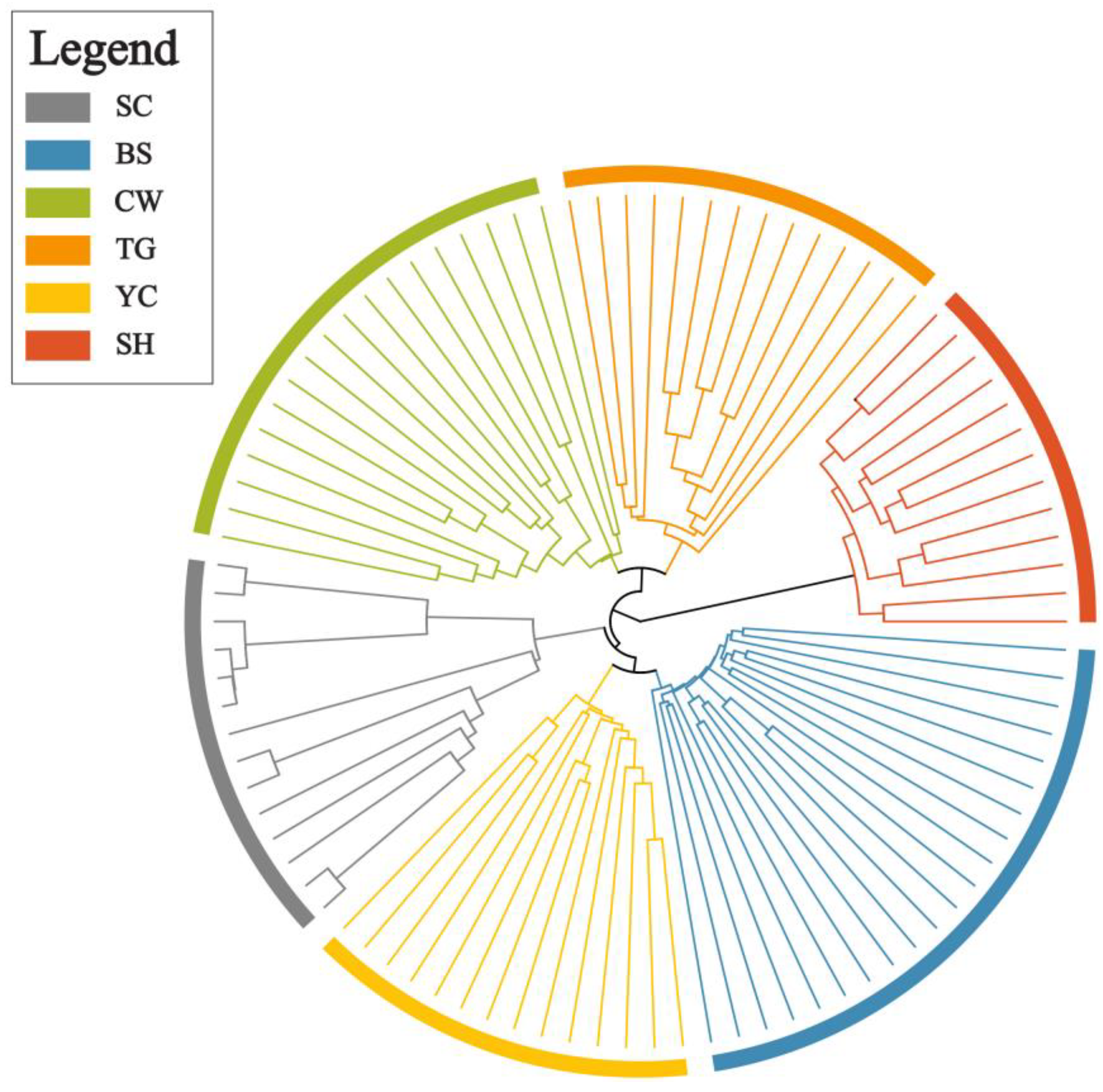
2.3. Genetic Differentiation and Population Structure
3. Discussion
3.1. Genetic Diversity in Z. tyaihyonii Populations
3.2. Genetic Differentiation and Population Structure
3.3. Implications for the Conservation of Z. tyaihyonii
4. Materials and Methods
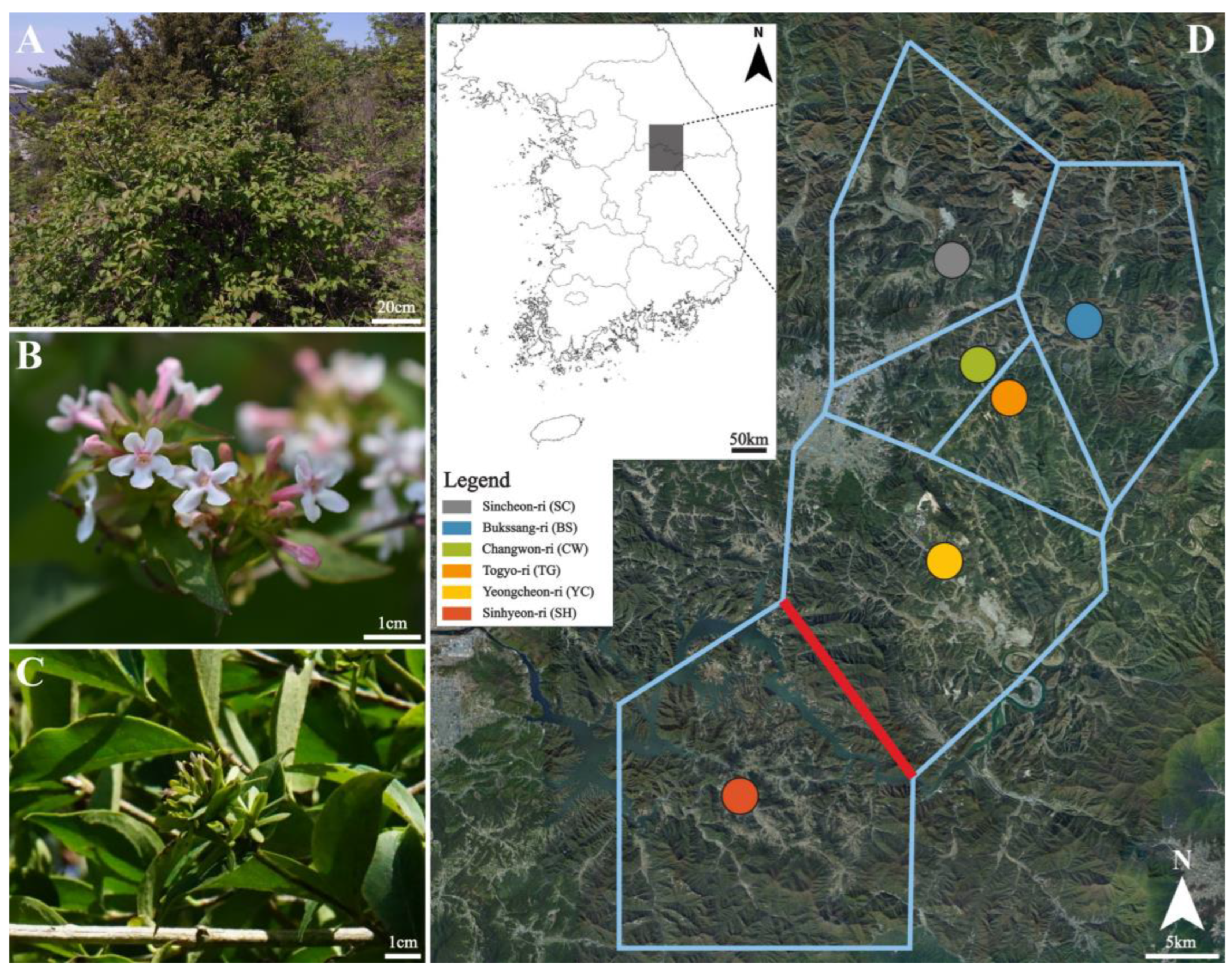
4.1. Plant Materials
4.2. DNA Extraction, Library Preparation, and Sequencing
4.3. Sequence Analyses, Bioinformatics, and SNP Identification
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakai, T. Tentamen systematic carprifoliacearum japonicarum. Journ. Coll. Sci. Univ. 1921, 42, 58. [Google Scholar]
- Hisauchi, K.; Hara, H. On the genus Zabelia Makino. J. Jap. Bot. 1954, 29, 143–144. [Google Scholar]
- Ikuse, M.; Kurosawa, S. Notes on sect. Zabelia Rehder of the genus Abelia. J. Jap. Bot. 1954, 29, 107–111. [Google Scholar]
- Kim, K.A.; Jang, S.K.; Cheon, K.S.; Seo, W.B.; Yoo, K.O. Environmental and ecological characteristics of habitats of Abelia tyahyoni Nakai. Korean J. Plant Taxon. 2010, 40, 135–144. [Google Scholar] [CrossRef]
- Won, H.S.; Kim, T.J. Zabelia. In The Genera of Vascular Plants of Korea; Park, J.W., Ed.; Academy Publishing Co.: Seoul, Republic of Korea, 2007; pp. 1242–1243. [Google Scholar]
- Chung, G.Y.; Chang, K.S.; Chung, J.M.; Choi, H.J.; Paik, W.K.; Hyun, J.O. A checklist of endemic plants on the Korean Peninsula. Korean J. Plant Taxon. 2017, 47, 264–288. [Google Scholar] [CrossRef]
- National Institute of Biological Resources. Red Data Book of Endangered Vascular Plants in Korea; National Institute of Biological Resources: Incheon, Republic of Korea, 2017; p. 128. [Google Scholar]
- Korea National Arboretum. The National Red List of Vascular Plant in Korea; Korea National Arboretum: Pocheon, Republic of Korea, 2021; p. 275. [Google Scholar]
- National Institute of Ecology. Floristic Target Species (FT Species) in Korea; National Institute of Ecology: Seocheon, Republic of Korea, 2018; p. 112. [Google Scholar]
- Kim, J.D.; Lee, H.J.; Lee, D.H.; Byeon, J.G.; Park, B.J.; Heo, T.I. Characteristics of environmental factors and vegetation community of Zabelia tyaihyonii (Nakai) Hisauti & H.Hara among the target plant species for conservation in Baekdudaegan. J. Korean Soc. For. Sci. 2022, 111, 201–223. [Google Scholar]
- Oh, B.U.; Jo, D.G.; Kim, K.S.; Jang, C.G. Endemic Vascular Plants in the Korean Peninsula; Korea National Arboretum: Pochen, Republic of Korea, 2005. [Google Scholar]
- Coelho, N.; Goncalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Chang, C.S. Genetic analysis and conservation biology of rare plants in Korea. Annu. Rep. Res. Agric. Life Sci. 1998, 2, 200–204. [Google Scholar]
- Hamrick, J.L.; Godt, M.J.W.; Murawski, D.A.; Loveless, M.D. Correlations between species traits allozyme diversity: Implications for conservation biology. In Genetics and Conservation of Rare Plants; Falk, D.A., Holsinger, K.E., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 76–86. [Google Scholar]
- Barrett, S.C.H.; Kohn, J.R. Genetic and evolutionary consequences of small population sizes in plant: Implications for conservation. In Genetics and Conservation of Rare Plants; Falk, D.A., Holsinger, K.E., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 3–30. [Google Scholar]
- Chung, Y.H.; Sun, B.Y. Monographic study on the endemic plants of Korea IV. Taxonomy and interspecific relationships of the genus Abelia. Korean J. Plant Taxon. 1984, 14, 137–152. [Google Scholar] [CrossRef]
- Paik, W.K.; Lee, W.T. A taxonomic study of the genus Abelia in Korea. Korean J. Plant Taxon. 1989, 19, 139–156. [Google Scholar] [CrossRef]
- Kim, T.J.; Sun, B.Y.; Suh, Y. Palynology and cytotaxonomy of the genus Abelia s. l., Caprifoliaceae. Korean J. Plant Taxon. 2021, 31, 91–106. [Google Scholar] [CrossRef]
- Landrein, S.; Prenner, G.; Chase, M.W.; Clarkson, J.J. Abelia and relatives: Phylogenetics of Linnaeeae (Dipsacales-Caprifoliaceae s.l.) and a new interpretation of their inflorescence morphology. Bot. J. Linn. 2012, 169, 692–713. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; Youn, J.S.; Kim, S.C.; Pak, J.H. The complete chloroplast genome sequence of limestone endemic, Zabelia tyahyoni (Caprifoliaceae), in Korea. Mitochondrial DNA B Resour. 2020, 5, 1947–1948. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, H.; Moore, M.J.; Landrein, S.; Liu, B.; Zhu, Z.X.; Wang, H.F. Plastid phylogenomic insights into the evolution of the Caprifoliaceae s.l. (Dipsacales). Mol. Phylogenetics Evol. 2020, 142, 106641. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, K.S.; Lee, C.H.; Kim, Z.S. Genetic diversity and special structure in populations of Abelia tyaihyoni. J. Korean For. Soc. 2007, 96, 667–675. [Google Scholar]
- Chae, H.H. Conservation Ecology on the Rare Plant Thalictrum petaloideum L. and the Endemic Plant Zabelia tyaihyoni (Nakai) Hisauti & Hara in Korea. Ph.D. Thesis, Gangneung-Wonju National University, Gangneung, Republic of Korea, 2021. [Google Scholar]
- Schuster, S.C. Next-generation sequencing transforms today’s biology. Nat. Methods 2008, 5, 16–18. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, Y.; Wang, H.; Yu, D.; Liu, Y.; Zhang, A.; Gowda, M.; Nair, S.K.; Hao, Z.; Lu, Y.; et al. Applications of genotyping-by-sequencing (GBS) in maize genetics and breeding. Sci. Rep. 2020, 10, 16308. [Google Scholar] [CrossRef] [PubMed]
- Scheben, A.; Batley, J.; Edwards, D. Genotyping-by-sequencing approaches to characterize crop genomes: Choosing the right tool for the right application. Plant. Biotechnol. J. 2017, 15, 149–161. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Gouesnard, B.; Negro, S.; Laffray, A.; Glaubitz, J.; Melchinger, A.; Revilla, P.; Moreno-Gonzalez, J.; Madur, D.; Combes, V.; Tollon-Gordet, C.; et al. Genotyping-by-sequencing highlights original diversity patterns within a European collection of 1191 maize flint lines, as compared to the maize USDA genebank. Theor. Appl. Genet. 2017, 130, 2165–2189. [Google Scholar] [CrossRef]
- Hamon, P.; Grover, C.; Davis, A.; Rakotomalala, J.J.; Albert, V.; Sreenath, H.; Stoffelen, P.; Mitchell, S.; Couturon, E.; Hamon, S.; et al. Genotyping-by-sequencing provides the first well-resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species GBS coffee phylogeny and the evolution of caffeine content. Mol. Phylogenet. Evol. 2017, 109, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Eltaher, S.; Sallam, A.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Poland, J.; Baenziger, P.S. Genetic diversity and population structure of F3:6 Nebraska winter wheat genotypes using genotyping-by-sequencing. Front. Genet. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Hong, U.V.T.; Tamiru-Oli, M.; Hurgobin, B.; Okey, C.R.; Abreu, A.R.; Lewsey, M.G. Insights into opium poppy (Papaver spp.) genetic diversity from genotyping-by-sequencing analysis. Sci. Rep. 2022, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Jara-Arancio, P.; Carvalho, C.S.; Carmona-Ortiz, M.R.; Bustamante, R.O.; Schmidt Villela, P.M.; Andrade, S.C.S.; Peña-Gómez, F.T.; González, L.A.; Fleury, M. Genetic diversity and population structure of Jubaea chilensis, an endemic and monotype gender from Chile, based on SNP markers. Plants 2022, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Rakshit, S.; Kumar, S.; Singh, B.K.; Lahkar, C.; Jha, A.K.; Kumar, K.; Kumar, P.; Choudhary, M.; Singh, S.B.; et al. Genetic diversity, population structure and linkage disequilibrium analyses in tropical maize using genotyping by sequencing. Plants 2022, 11, 799. [Google Scholar] [CrossRef]
- Lee, J.H.; Ong, H.G.; Kim, B.Y.; Kim, Y.I.; Jung, E.K.; Chung, M.G.; Kim, Y.D. Population genomics study for the conservation management of the endangered shrub Abeliophyllum distichum. Conserv. Genet. 2022, 23, 683–697. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.H.; Wang, Y.H.; Shen, S.K. Genetic diversity and population structure of Rhododendron rex subsp. Rex inferred from microsatellite markers and chloroplast DNA sequences. Plants 2020, 9, 338. [Google Scholar]
- Feng, X.Y.; Wang, Y.; Gong, X. Genetic diversity, genetic structure and demographic history of Cycas simplicipinna (Cycadaceae) assessed by DNA sequences and SSR markers. BMC Plant Biol. 2014, 14, 187. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]
- Yun, S.A.; Kim, S.C. Genetic diversity and structure of Saussurea polylepis (Asteraceae) on continental islands of Korea: Implications for conservation strategies and management. PLoS ONE 2021, 16, e0249752. [Google Scholar] [CrossRef]
- Jin, C.; Huixia, K.; Shubin, D. Population genetic structure and gene flow of rare and endangered Tetraena mongolica Maxim. Revealed by reduced representation sequencing. BMC Plant Biol. 2020, 20, 391. [Google Scholar]
- Liu, F.; Hong, Z.; Xu, D.; Jia, H.; Zhang, N.; Liu, X.; Yang, Z.; Lu, M. Genetic diversity of the endangered Dalbergia odorifera revealed by SSR markers. Forests 2019, 10, 225. [Google Scholar] [CrossRef]
- Hedrick, P.W. Genetics of Populations, 2nd ed.; Jones and Bartlett Publishers: Sudbury, MA, USA, 2000; p. 675. [Google Scholar]
- Leimu, R.; Mutikainen, P.; Koricheva, J.; Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation. J. Ecol. 2006, 94, 942–952. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Ramírez-Herrera, C.; López-Upton, J.; Valdez-Hernández, J.I.; López-Sánchez, H.; López-Mata, L. Diversity and Genetic Structure of Dioon holmgrenii (Cycadales: Zamiaceae) in the Mexican Pacific Coast Biogeographic Province: Implications for Conservation. Plants 2021, 10, 2250. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.M.; Allan, G.J.; Howe, M.; Elisens, W.J.; Junak, S.A.; Rieseberg, L.H. Genetic analysis of the endangered island endemic Malacothamnus fasciculatus (Nutt.) Greene var. nesioticus (Rob.) Kearn. (Malvaceae). Conserv Biol. 1995, 9, 404–415. [Google Scholar]
- Sydes, M.A.; Peakall, R. Extensive clonality in the endangered shrub Haloragodendron lucasii (Haloragaceae) revealed by allozymes and RAPDs. Mol Ecol. 1998, 7, 87–93. [Google Scholar] [CrossRef]
- Sagarin, R.D.; Gaines, S.D. The “abundant centre” distribution: To what extent is it a biogeographical rule? Ecol Lett. 2002, 5, 137–147. [Google Scholar] [CrossRef]
- Petit, R.J.; Aguinagalde, I.; de Beaulieu, J.L.; Bittkau, C.; Brewer, S.; Cheddadi, R.; Ennos, R.; Fineschi, S.; Grivet, D.; Lascoux, M.; et al. Glacial refugia: Hotspots but not melting pots of genetic diversity. Science 2003, 300, 1563–1565. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Mol Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef]
- Grant, V. The evolutionary process: A critical study of evolutionary theory. Stud. Hist. Phil. Sci. 1986, 17, 65–98. [Google Scholar]
- Wang, S.Q. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implication for its conservation. BMC Plant Biol. 2020, 20, 510. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Evolution and the Genetic of Population, Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978; Volume 4, pp. 213–220. [Google Scholar]
- Govindaraju, D.R. Relationship between dispersal ability and levels of gene flow in plants. Oikos 1988, 52, 31–35. [Google Scholar] [CrossRef]
- Manchester, S.R.; Donoghue, M.J. Winged fruits of Linnaeeae (Caprifoliaceae) in the tertiary of western North America: Diplodipelta gen. nov. Int. J. Plant Sci. 1995, 156, 709–722. [Google Scholar] [CrossRef]
- Nam, J.I.; Kim, M.S.; Song, J.H.; Seo, J.M.; Choi, G.E.; Kim, Y.K. Analysis of morphological characteristics and variation in five populations of Zabelia tyaihyonii in South Korea. J. People Plants Environ. 2021, 24, 619–628. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.N.; Mangel, M. Modeling investments in seeds, clonal offspring, and translocation in a clonal plant. Ecology 1999, 80, 1202–1220. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhang, D.Y. Asexual and sexual reproductive strategies in clonal plant. Acta Phytoecol. Sin. 2006, 30, 174–183. [Google Scholar] [CrossRef]
- Allison, J.R.; Stevens, T.E. Vascular flora of Ketona dolomite outcrops in Bibb Country, Alabama. Castanea 2001, 66, 154–205. [Google Scholar]
- Jones, H.T. Magnesium as a plant nutrient. Chem. Ind. 1951, 15, 1108–1110. [Google Scholar]
- Mota, J.F.; Medina-Cazorla, J.M.; Mavarro, F.B.; Pérez-Gracía, F.J.; Pérez-Latorre, A.; Sánchez-Gómez, P.; Torres, J.A.; Benavente, A.; Blanca, G.; Gil, C.; et al. Dolomite flora of the Baetic Ranges glades (South Spain). Flora 2008, 203, 359–375. [Google Scholar] [CrossRef]
- Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Salmerón-Sánchez, E.S. Plant on rich-magnesium dolomite barrens: A global phenomenon. Biology 2021, 10, 38. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Z.; Dai, J.; Wu, X.; Peng, J.; Wang, H.; Meersmans, J.; Green, S.M.; Quine, T.A. Rock crevices determine woody and herbaceous plant cover in the karst critical zone. Sci. China Earth Sci. 2019, 62, 1756–1763. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Z.C.; Jin, G.Q.; Fan, H.H.; Hu, H.B. Genetic diversity analysis and provenance zone allocation of Schima superba in China using RAPD markers. Sci. Silvae Sin. 2006, 42, 38–42. [Google Scholar]
- Zong, X.X.; Guan, J.P.; Gu, J.; Wang, H.; Ma, Y. Differentiation on population structure and genetic diversity of pea core collections separately constituted from Chinese land races and international genetic resources. J. Plant Genet. Resour. 2009, 10, 347–353. [Google Scholar]
- Tan, X.J.; Wu, Z.K.; Cheng, W.D. Association analysis and its application in plant genetic research. Chin. Bull. Bot. 2011, 46, 108–118. [Google Scholar]
- Zhang, J.S.; Yang, C.Y.; Wu, C.; Hu, Z.Y.; Wang, R.G.; Guo, Y.S.; Ren, X.L. Study on genetic diversity, population structure and specificity of subpopulations of fluecured tobacco germplasm. Acta Tab. Sin. 2012, 18, 21–49. [Google Scholar]
- Lai, G.; Lai, Z.; Liu, W.; Ye, W.; Lin, Y.; Liu, S.; Chen, Y.; Zhang, Z.; Wu, G. ISSR analysis of 3 natural populations of the wild banana distributed in the middle of Fujian province based on NTSYS and STRUCTURE. Chin. J. Trop. Crops 2014, 35, 223–231. [Google Scholar]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.M.; Van Groenendael, J.M.; Vosman, B. Genetic diversity and the survival or populations. Plant Biol. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Milligan, B.G.; Leebens-Mack, J.; Strand, A.E. Conservation genetics: Beyond the maintenance of marker diversity. Mol Ecol. 1994, 3, 423–435. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Carson, H.L.; Templeton, A.R. Genetic revolution in relation to speciation phenomena: The founding of new populations. Ann. Rev. Ecol. Syst. 1984, 15, 97–131. [Google Scholar] [CrossRef]
- Levin, D.A. Metapopulations: An arena for local speciation. J. Evol. Biol. 1995, 8, 635–644. [Google Scholar] [CrossRef]
- Heywood, V.H.; Iriondo, J.M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- Perea, C.; De La Hoz, J.F.; Cruz, D.F.; Lobaton, J.D.; Izquierdo, P.; Quintero, J.C.; Raatz, B.; Duitama, J. Bioinformatic analysis of genotype by sequencing (GBS) data with NGSEP. BMC Genom. 2016, 17, 498. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. 1000 Genomes Project Analysis Group. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Lischer, H.E.L.; Excoffier, L. PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 2012, 28, 298–299. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Ligges, U.; Mächler, M. Scatterplot3d-an R package for visualizing multivariate data. J. Stat. Sofw. 2003, 8, 1–20. [Google Scholar]
- Meirmans, P.G.; Van Tienderen, P.H. GENOTYPE and GENODIVE: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 2004, 4, 792–794. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple metods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Puechmaille, S.J. The program structure does not reliably recover the correct population structure when sampling is uneven: Subsampling and new estinators alleviate the problem. Mol. Ecol. Resour. 2016, 16, 608–627. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Manni, F.; Guerard, E.; Heyer, E. Geographic patterns of (genetic, morphologic, linguistic) variation: How barriers can be detected by using Monmonier’s algorithm. Hum. Biol. 2004, 76, 173–190. [Google Scholar] [CrossRef]

| Pop. | N | Na (±SE) | Ne (±SE) | I (±SE) | Ho (±SE) | He (±SE) | GIS |
|---|---|---|---|---|---|---|---|
| BS | 21 | 1.881 (±0.006) | 1.428 (±0.006) | 0.406 (±0.004) | 0.266 (±0.003) | 0.263 (±0.003) | 0.014 |
| CW | 18 | 1.821 (±0.007) | 1.419 (±0.006) | 0.384 (±0.004) | 0.262 (±0.004) | 0.252 (±0.003) | −0.013 |
| TG | 14 | 1.797 (±0.007) | 1.405 (±0.006) | 0.375 (±0.004) | 0.273 (±0.004) | 0.245 (±0.003) | −0.079 |
| SC | 14 | 1.671 (±0.008) | 1.380 (±0.007) | 0.339 (±0.005) | 0.267 (±0.005) | 0.225 (±0.003) | −0.150 |
| YC | 14 | 1.791 (±0.007) | 1.405 (±0.006) | 0.375 (±0.004) | 0.260 (±0.004) | 0.245 (±0.003) | −0.025 |
| SH | 13 | 1.441 (±0.009) | 1.299 (±0.007) | 0.249 (±0.005) | 0.209 (±0.005) | 0.170 (±0.004) | −0.194 |
| mean | 1.734 (±0.003) | 1.389 (±0.003) | 0.355 (±0.002) | 0.256 (±0.002) | 0.233 (±0.001) | −0.057 |
| Source | Df | SS | MS | Est. Var. | % | F-Statistics | p Value | Nm |
|---|---|---|---|---|---|---|---|---|
| Among populations | 5 | 14,276.121 | 2855.224 | 79.490 | 17.00 | FST = 0.170 | p < 0.001 | 1.221 |
| Among individuals | 88 | 33,547.943 | 381.227 | −6.993 | −1.5 | FIS = −0.018 | p > 0.05 | |
| Within individuals | 94 | 37,150.0 | 395.213 | 395.213 | 84.50 | FIT = 0.155 | p < 0.001 | |
| Total | 187 | 84,974.064 | 467.71 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; An, S.-M.; Park, Y.-J.; Lee, Y.-B.; Lee, J.-H.; Cheon, K.-S.; Kim, K.-A. Population Genomics Study and Implications for the Conservation of Zabelia tyaihyonii Based on Genotyping-By-Sequencing. Plants 2023, 12, 171. https://doi.org/10.3390/plants12010171
Kang H, An S-M, Park Y-J, Lee Y-B, Lee J-H, Cheon K-S, Kim K-A. Population Genomics Study and Implications for the Conservation of Zabelia tyaihyonii Based on Genotyping-By-Sequencing. Plants. 2023; 12(1):171. https://doi.org/10.3390/plants12010171
Chicago/Turabian StyleKang, Halam, Sung-Mo An, Yoo-Jung Park, Yoo-Bin Lee, Jung-Hyun Lee, Kyeong-Sik Cheon, and Kyung-Ah Kim. 2023. "Population Genomics Study and Implications for the Conservation of Zabelia tyaihyonii Based on Genotyping-By-Sequencing" Plants 12, no. 1: 171. https://doi.org/10.3390/plants12010171
APA StyleKang, H., An, S.-M., Park, Y.-J., Lee, Y.-B., Lee, J.-H., Cheon, K.-S., & Kim, K.-A. (2023). Population Genomics Study and Implications for the Conservation of Zabelia tyaihyonii Based on Genotyping-By-Sequencing. Plants, 12(1), 171. https://doi.org/10.3390/plants12010171







