Response of Cucumis sativus to Neighbors in a Species-Specific Manner
Abstract
1. Introduction
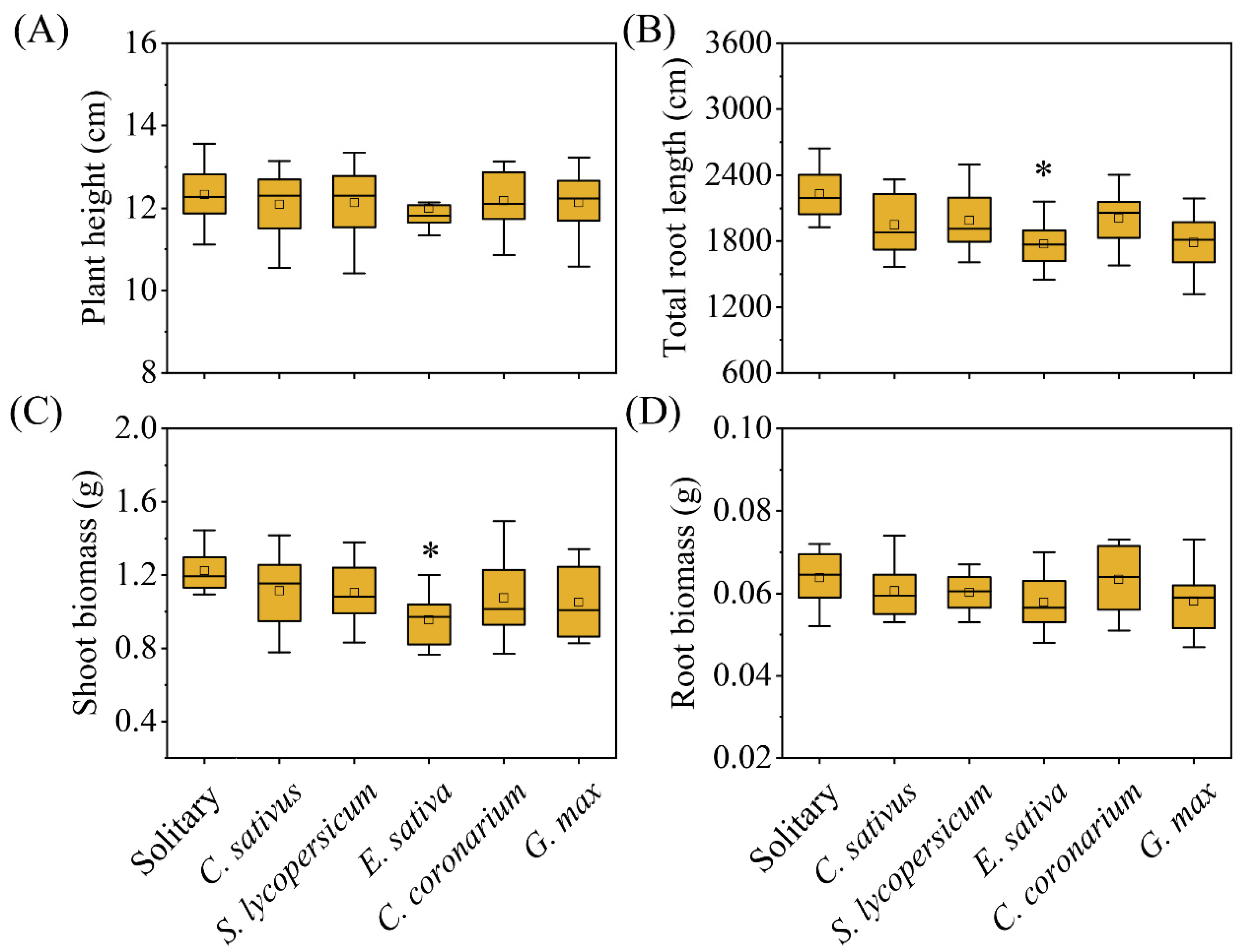
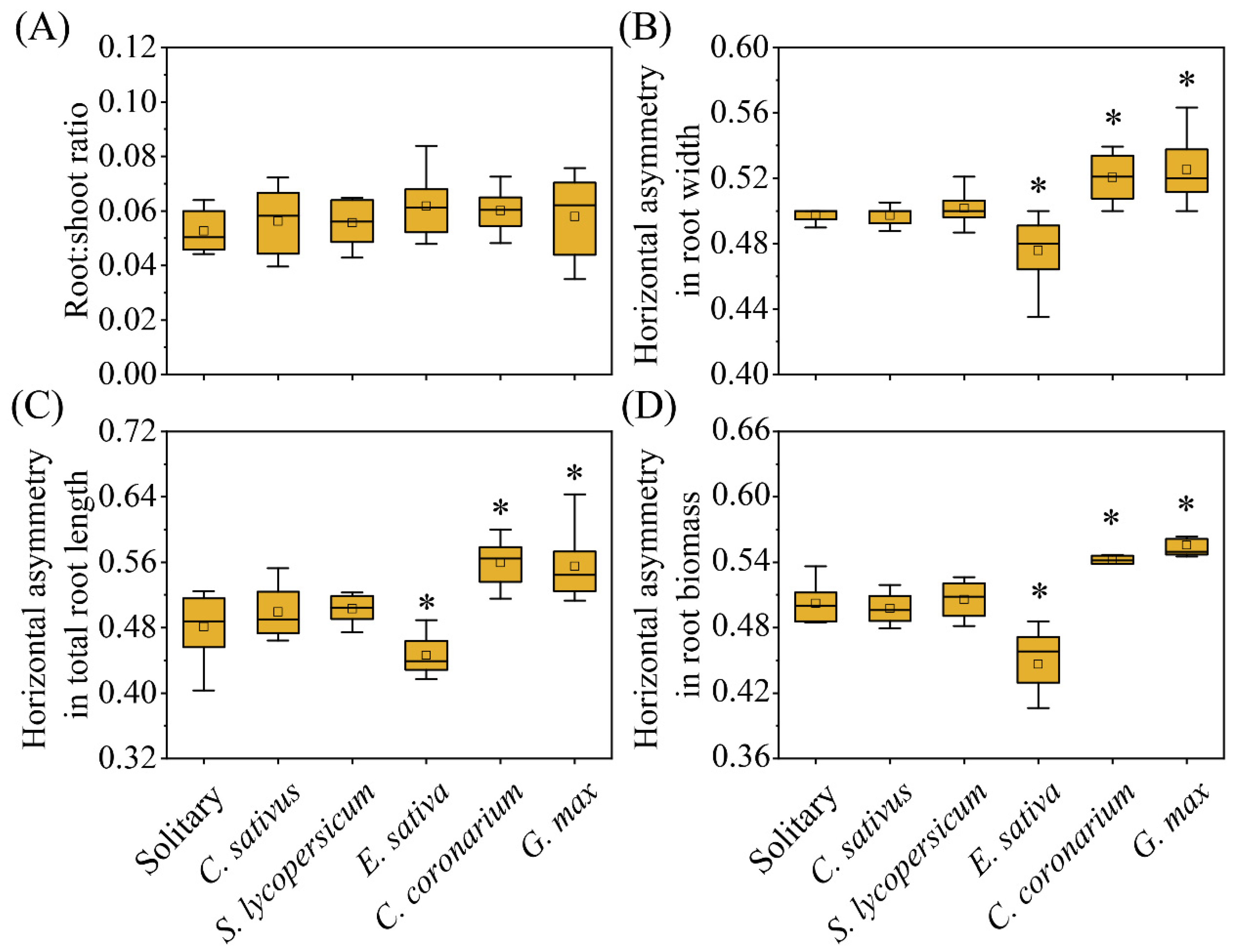
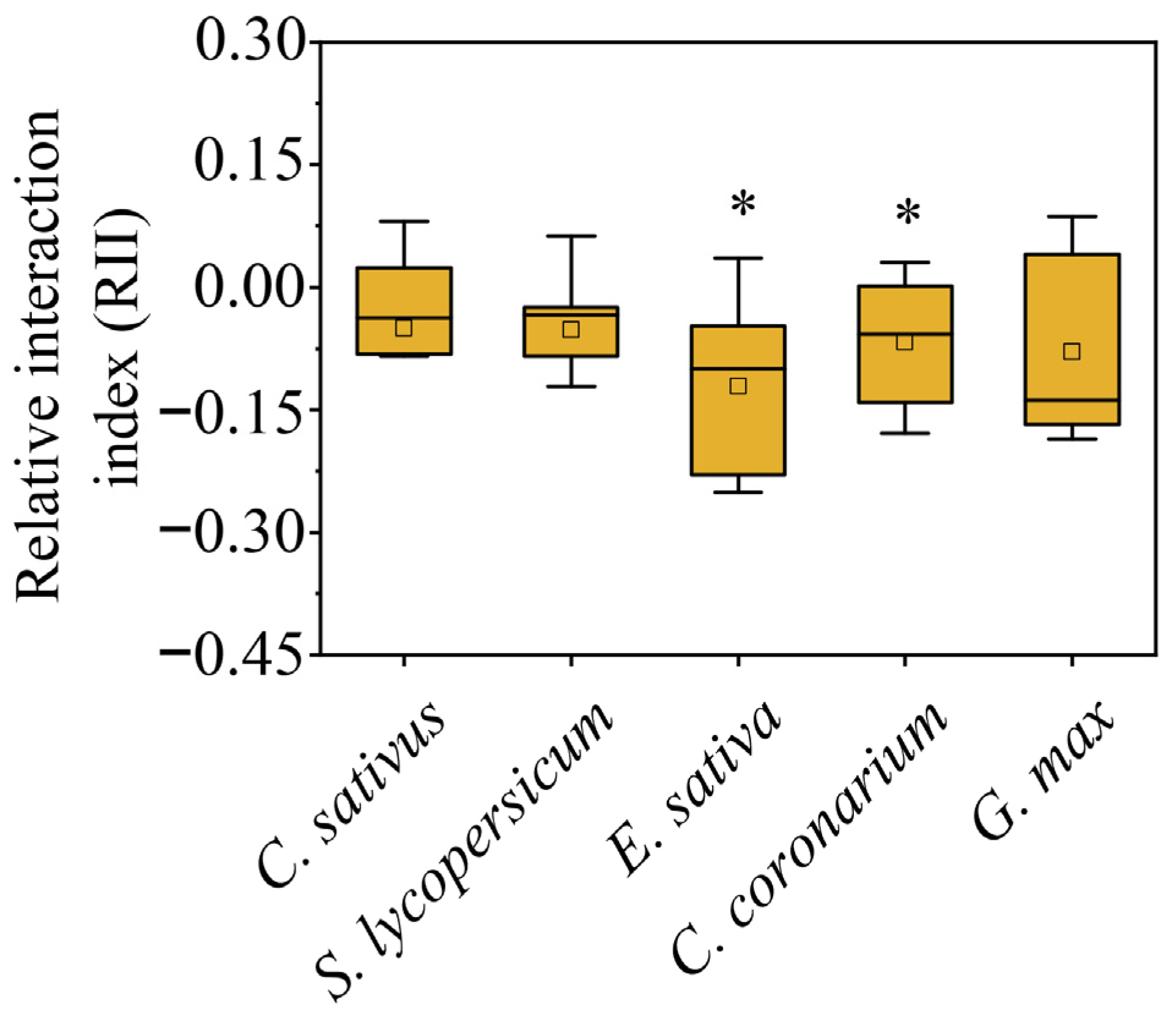
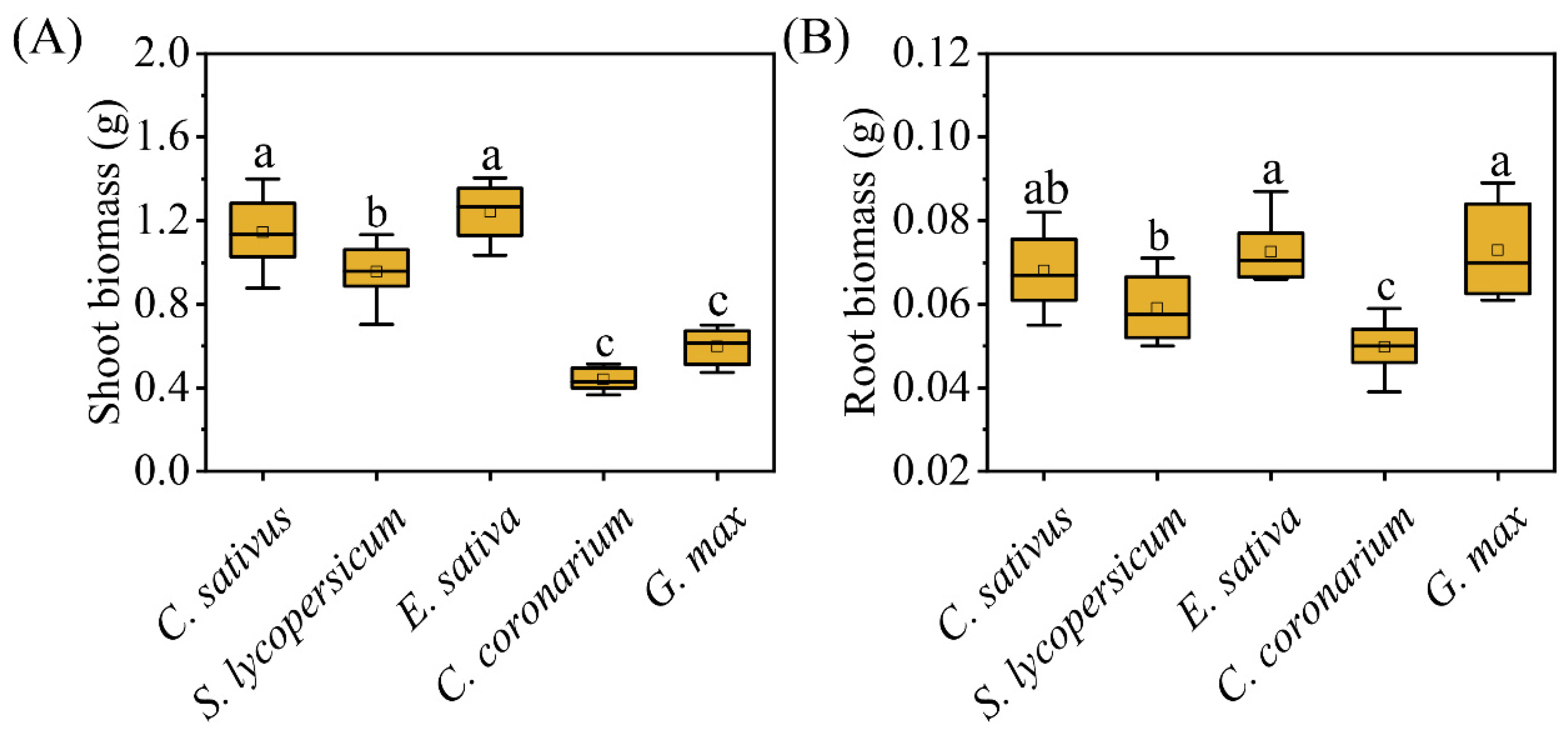
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Rhizoboxes
4.2. Experimental Design
4.2.1. Plasticity Test of C. sativus
4.2.2. Plasticity Test of C. sativus to Different Neighbors
4.3. Growth, Visualization and Harvest
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faget, M.; Nagel, K.A.; Walter, A.; Herrera, J.M.; Jahnke, S.; Schurr, U.; Temperton, V.M. Root-root interactions: Extending our perspective to be more inclusive of the range of theories in ecology and agriculture using in-vivo analyses. Ann. Bot. 2013, 112, 253–266. [Google Scholar] [CrossRef]
- Oduor, A.M. Evolutionary responses of native plant species to invasive plants: A review. New Phytol. 2013, 200, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P. Competition, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.F., Jr.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.F.; McNickle, G.G. The behavioral ecology of nutrient foraging by plants. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 289–311. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. J. Plant Interact. 2022, 17, 685–694. [Google Scholar] [CrossRef]
- Gersani, M.; Brown, J.; O’Brien, E.; Abramsky, M. Tragedy of the commons as a result of root competition. J. Ecol. 2001, 89, 660–669. [Google Scholar] [CrossRef]
- Maina, G.G.; Brown, J.S.; Gersani, M. Intra-plant versus Inter-plant Root Competition in Beans: Avoidance, resource matching or tragedy of the commons. Plant Ecol. 2002, 160, 235–247. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Weiner, J.; Li, F.-M. Root proliferation in response to neighbouring roots in wheat (Triticum aestivum). Basic Appl. Ecol. 2019, 39, 10–14. [Google Scholar] [CrossRef]
- Semchenko, M.; Hutchings, M.J.; John, E.A. Challenging the tragedy of the commons in root competition: Confounding effects of neighbour presence and substrate volume. J. Ecol. 2007, 95, 252–260. [Google Scholar] [CrossRef]
- McNickle, G.G.; Brown, J.S.; Schwinning, S. An ideal free distribution explains the root production of plants that do not engage in a tragedy of the commons game. J. Ecol. 2014, 102, 963–971. [Google Scholar] [CrossRef]
- Mommer, L.; Ruijven, J.V.; Jansen, C.; Steeg, H.M.V.D.; Kroon, H.D. Interactive effects of nutrient heterogeneity and competition: Implications for root fraging theory? Funct. Ecol. 2012, 26, 66–73. [Google Scholar] [CrossRef]
- Chen, B.J.W.; Huang, L.; During, H.J.; Wang, X.; Wei, J.; Anten, N.P.R. No neighbour-induced increase in root growth of soybean and sunflower in mesh-divider experiments after controlling for nutrient concentration and soil volume. AoB Plants 2021, 13, plab020. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 2018, 186, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Crepy, M.A.; Casal, J.J. Photoreceptor-mediated kin recognition in plants. New Phytol. 2015, 205, 329–338. [Google Scholar] [CrossRef]
- Cabal, C.; Martínez-García, R.; de Castro Aguilar, A.; Valladares, F.; Pacala, S.W. The exploitative segregation of plant roots. Science 2020, 370, 1197–1199. [Google Scholar] [CrossRef]
- Schmid, C.; Bauer, S.; Bartelheimer, M. Should I stay or should I go? Roots segregate in response to competition intensity. Plant Soil 2015, 391, 283–291. [Google Scholar] [CrossRef]
- Fang, S.; Gao, X.; Deng, Y.; Chen, X.; Liao, H. Crop root behavior coordinates phosphorus status and neighbors: From field studies to three-dimensional in situ reconstruction of root system architecture. Plant Physiol. 2011, 155, 1277–1285. [Google Scholar] [CrossRef]
- Fang, S.; Clark, R.T.; Zheng, Y.; Iyer-Pascuzzi, A.S.; Weitz, J.S.; Kochian, L.V.; Edelsbrunner, H.; Liao, H.; Benfey, P.N. Genotypic recognition and spatial responses by rice roots. Proc. Natl. Acad. Sci. USA 2013, 110, 2670–2675. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, L.L.; Xu, Y.; Kong, C.H. Kin recognition in rice (Oryza sativa) lines. New Phytol. 2018, 220, 567–578. [Google Scholar] [CrossRef]
- Campbell, B.D.; Grime, J.P.; Mackey, J.M. A trade-off between scale and precision in resource foraging. Oecologia 1991, 87, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Bartelheimer, M.; Steinlein, T.; Beyschlag, W. Aggregative root placement: A feature during interspecific competition in inland sand-dune habitats. Plant Soil 2006, 280, 101–114. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H.; Xia, Z.C.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cheng, H.F.; Kong, C.H.; Meiners, S.J. Intra-specific kin recognition contributes to inter-specific allelopathy: A case study of allelopathic rice interference with paddy weeds. Plant Cell Environ. 2021, 44, 3479–3491. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, L.L.; Meiners, S.J.; Kong, C.H. Root placement patterns in allelopathic plant-plant interactions. New Phytol. 2022, 237, 563–575. [Google Scholar] [CrossRef]
- Li, L.; Li, S.M.; Sun, J.H.; Zhou, L.L.; Bao, X.G.; Zhang, H.G.; Zhang, F.S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef]
- Sattler, J.; Bartelheimer, M. Root responses to legume plants integrate information on nitrogen availability and neighbour identity. Basic Appl. Ecol. 2018, 27, 51–60. [Google Scholar] [CrossRef]
- Zhang, D.; Lyu, Y.; Li, H.; Tang, X.; Hu, R.; Rengel, Z.; Zhang, F.; Whalley, W.R.; Davies, W.J.; Cahill, J.F., Jr.; et al. Neighbouring plants modify maize root foraging for phosphorus: Coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol. 2020, 226, 244–253. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Vermeulen, P.J. Tragedies and crops: Understanding natural selection to improve cropping systems. Trends Ecol. Evol. 2016, 31, 429–439. [Google Scholar] [CrossRef]
- Gao, H.; Li, S.; Wu, F. Impact of Intercropping on the Diazotrophic Community in the Soils of Continuous Cucumber Cropping Systems. Front. Microbiol. 2021, 12, 630302. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, F.; Zhou, X. Different toxic effects of ferulic and p-hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fertil. Soils 2020, 56, 125–136. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Belter, P.R.; Cahill, J.F., Jr. Disentangling root system responses to neighbours: Identification of novel root behavioural strategies. AoB Plants 2015, 7, plv059. [Google Scholar] [CrossRef]
- Xia, H.-Y.; Zhao, J.-H.; Sun, J.-H.; Bao, X.-G.; Christie, P.; Zhang, F.-S.; Li, L. Dynamics of root length and distribution and shoot biomass of maize as affected by intercropping with different companion crops and phosphorus application rates. Field Crop. Res. 2013, 150, 52–62. [Google Scholar] [CrossRef]
- Gong, X.; Dang, K.; Lv, S.; Zhao, G.; Tian, L.; Luo, Y.; Feng, B. Interspecific root interactions and water-use efficiency of intercropped proso millet and mung bean. Eur. J. Agron. 2020, 115, 126034. [Google Scholar] [CrossRef]
- Chen, B.J.; During, H.J.; Anten, N.P. Detect thy neighbor: Identity recognition at the root level in plants. Plant Sci. 2012, 195, 157–167. [Google Scholar] [CrossRef]
- Dudley, S.A.; File, A.L. Kin recognition in an annual plant. Biol. Lett. 2007, 3, 435–438. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Delory, B.M.; Schempp, H.; Spachmann, S.M.; Störzer, L.; van Dam, N.M.; Temperton, V.M.; Weinhold, A. Soil chemical legacies trigger species-specific and context-dependent root responses in later arriving plants. Plant Cell Environ. 2021, 44, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lv, J.; Xie, J.; Feng, Z.; Ma, N.; Li, J.; Yu, J.; Calderón-Urrea, A. Transcriptome analysis reveals the different response to toxic stress in rootstock grafted and non-grafted cucumber seedlings. Int. J. Mol. Sci. 2020, 21, 774. [Google Scholar] [CrossRef]
- Lyu, J.; Jin, N.; Meng, X.; Jin, L.; Wang, S.; Xiao, X.; Liu, Z.; Tang, Z.; Yu, J. Exogenous silicon alleviates the adverse effects of cinnamic acid-induced autotoxicity stress on cucumber seedling growth. Front. Plant. Sci. 2022, 13, 968514. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Ruijven, J.; de Caluwe, H.; Smit, A.; Wagemaker, C.; Ouborg, N.; Bögemann, G.; van der Weerden, G.M.; Berendse, F.; Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Craine, J.M.; Fargione, J.; Sugita, S. Supply pre-emption, not concentration reduction, is the mechanism of competition for nutrients. New Phytol. 2005, 166, 933–940. [Google Scholar] [CrossRef]
- Mahall, B.E.; Callaway, R.M. Root communication among desert shrubs. Proc. Natl. Acad. Sci. USA 1991, 88, 874–876. [Google Scholar] [CrossRef]
- Liao, M.; Palta, J.A.; Fillery, I.R.P. Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 2006, 57, 1097. [Google Scholar] [CrossRef]
- Semchenko, M.; John, E.A.; Hutchings, M.J. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol. 2007, 176, 644–654. [Google Scholar] [CrossRef]
- Nagel, K.A.; Putz, A.; Gilmer, F.; Heinz, K.; Fischbach, A.; Pfeifer, J.; Faget, M.; Blossfeld, S.; Ernst, M.; Dimaki, C.; et al. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct. Plant Biol. 2012, 39, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef]

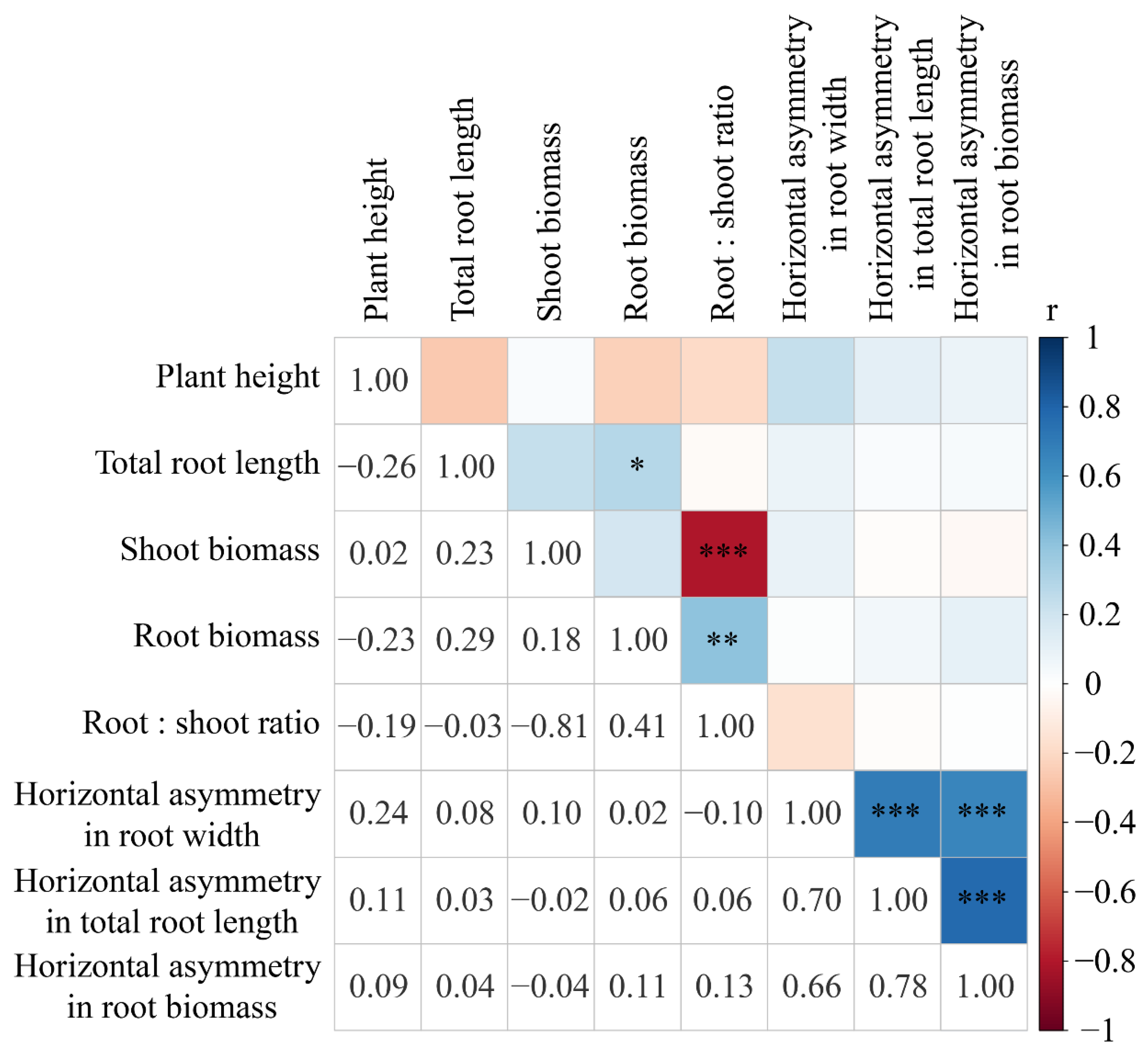
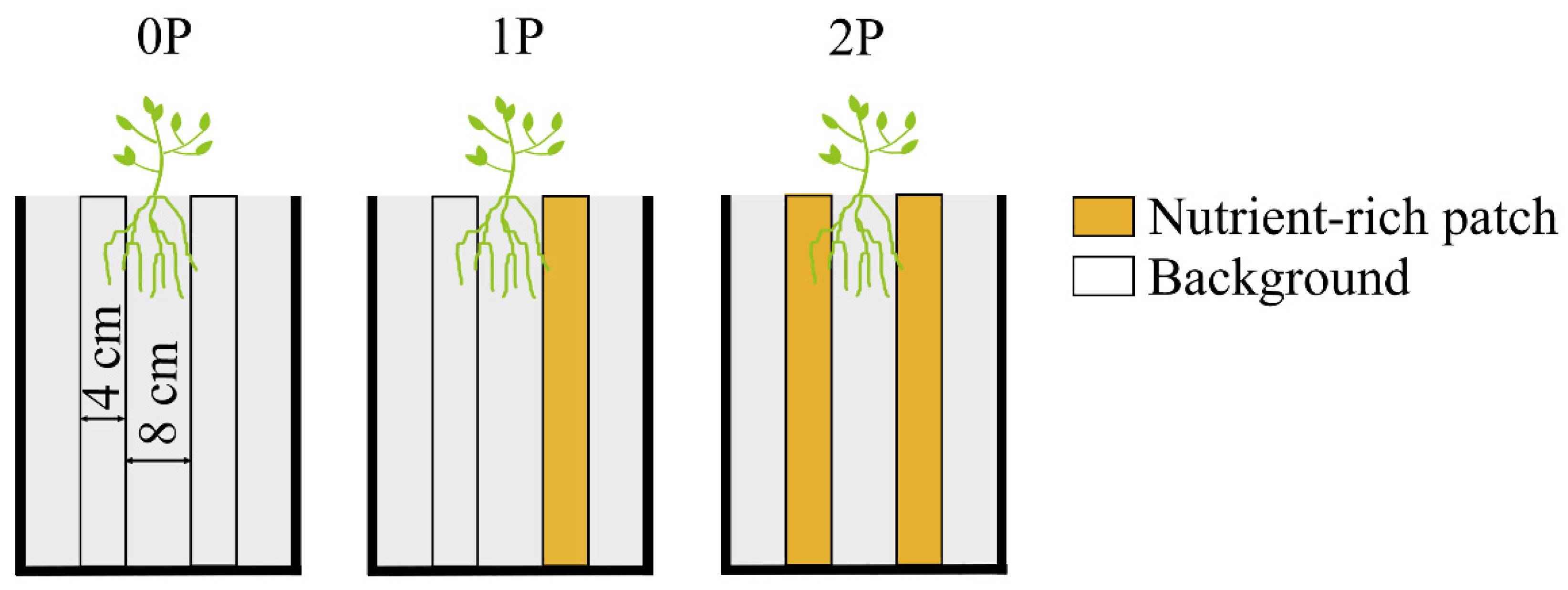
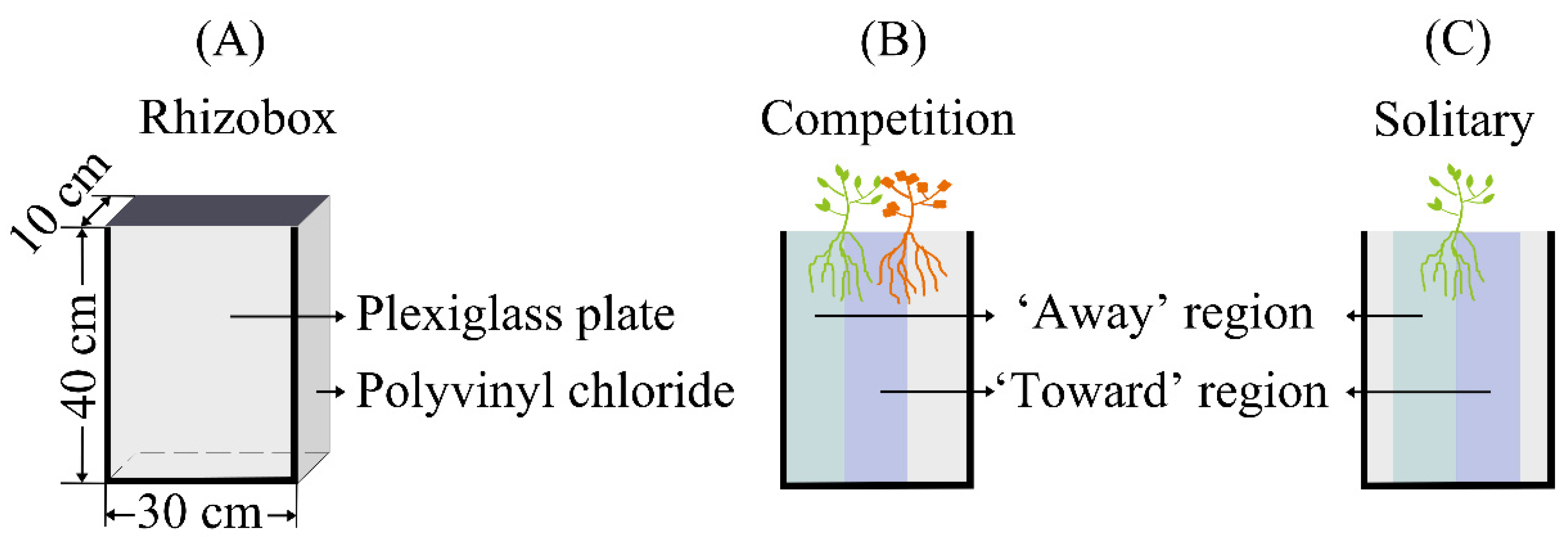
| Treatments | Description |
|---|---|
| Solitary | A C. sativus plant |
| C. sativus | A pair of C. sativus plants |
| S. lycopersicum | A C. sativus plant and a S. lycopersicum neighbor |
| E. sativa | A C. sativus plant and a E. sativa neighbor |
| C. coronarium | A C. sativus plant and a C. coronarium neighbor |
| G. max | A C. sativus plant and a G. max neighbor |
| Response Variables | Description |
|---|---|
| Plant height | The height (cm) of the aboveground part of the plant (cm) |
| Total root length (cm) | Total length of roots analyzed using LA-S (Wseen, Hangzhou, China) |
| Shoot biomass | Dry weight (g) of the aboveground part of the plant (g) |
| Root biomass | Dry weight (g) of the belowground part of the plant (g) |
| Root:shoot ratio | Ratio of root biomass to shoot biomass |
| Horizontal asymmetry in root width | Proportion of the root width for the ‘Toward’ region of focal species |
| Horizontal asymmetry in total root length | Proportion of the total root length for the ‘Toward’ region of focal species |
| Horizontal asymmetry in root biomass | Proportion of the root biomass for the ‘Toward’ region of focal species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yan, J.; Wu, F. Response of Cucumis sativus to Neighbors in a Species-Specific Manner. Plants 2023, 12, 139. https://doi.org/10.3390/plants12010139
Zhang X, Yan J, Wu F. Response of Cucumis sativus to Neighbors in a Species-Specific Manner. Plants. 2023; 12(1):139. https://doi.org/10.3390/plants12010139
Chicago/Turabian StyleZhang, Xiu, Jingfan Yan, and Fengzhi Wu. 2023. "Response of Cucumis sativus to Neighbors in a Species-Specific Manner" Plants 12, no. 1: 139. https://doi.org/10.3390/plants12010139
APA StyleZhang, X., Yan, J., & Wu, F. (2023). Response of Cucumis sativus to Neighbors in a Species-Specific Manner. Plants, 12(1), 139. https://doi.org/10.3390/plants12010139





