Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants
Abstract
1. Introduction
2. Results
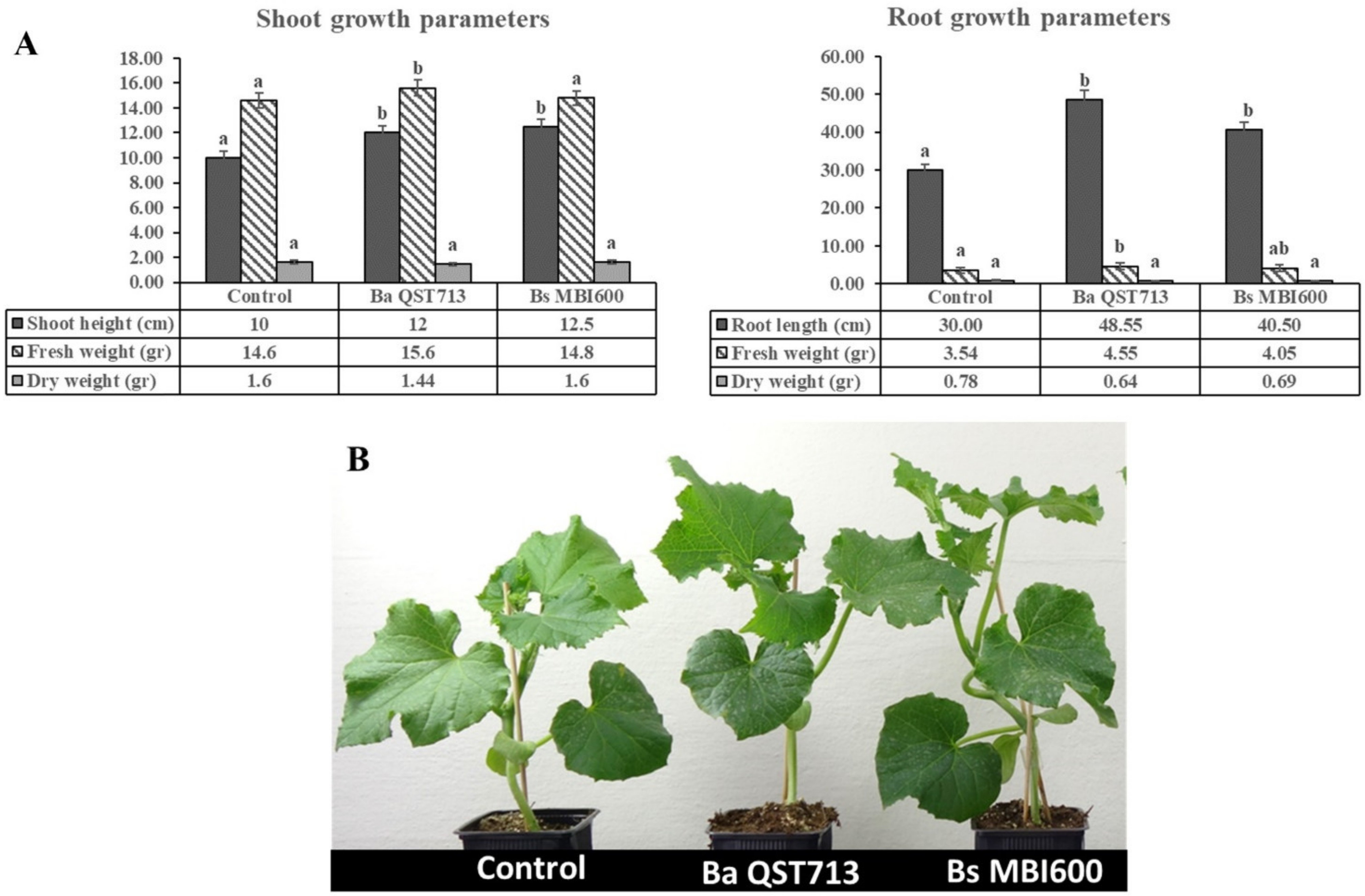
2.1. Treatment with B. subtilis MBI600 Increased Plant Growth
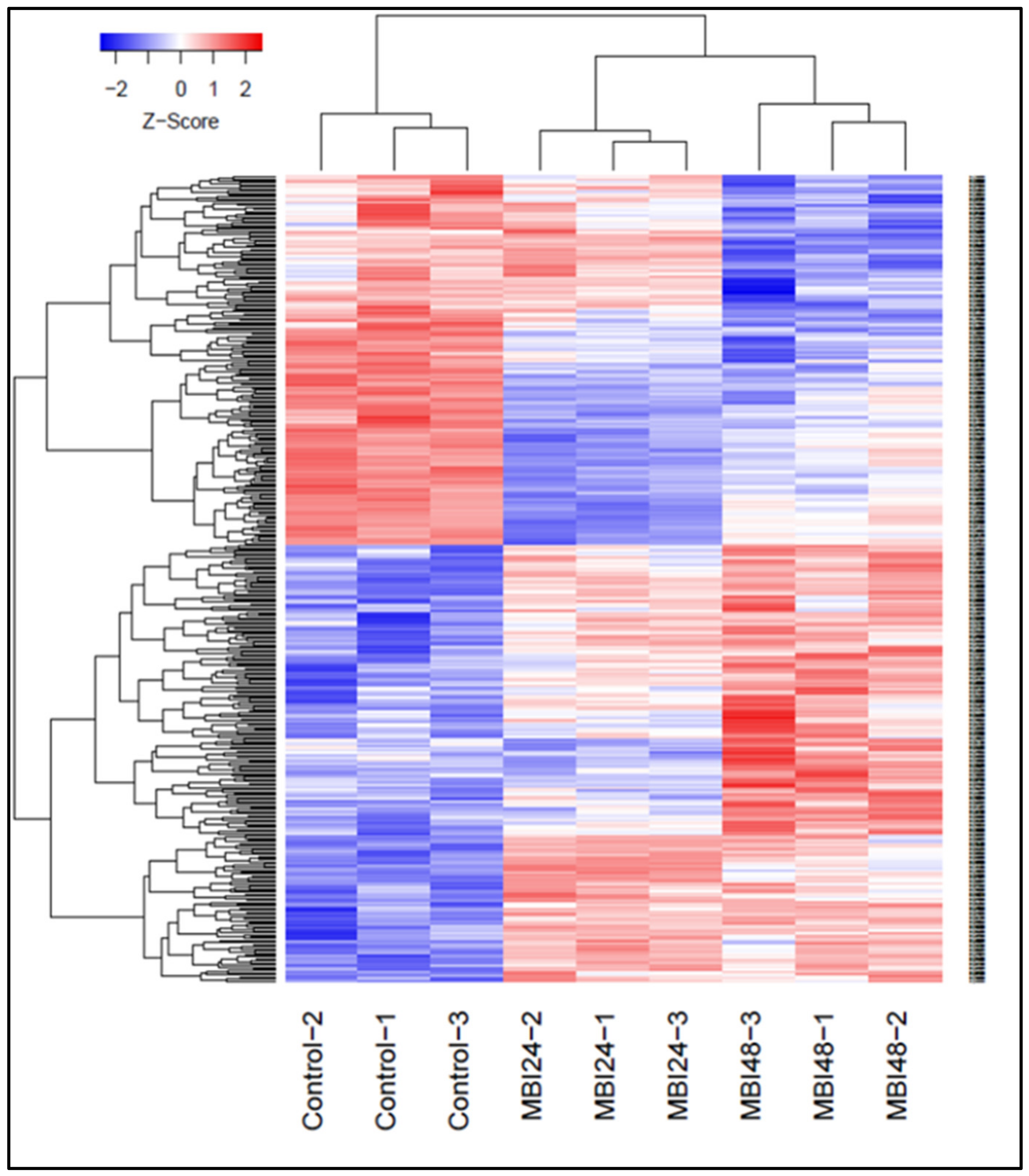
2.2. Differential Gene Expression in Response to Bs MBI600
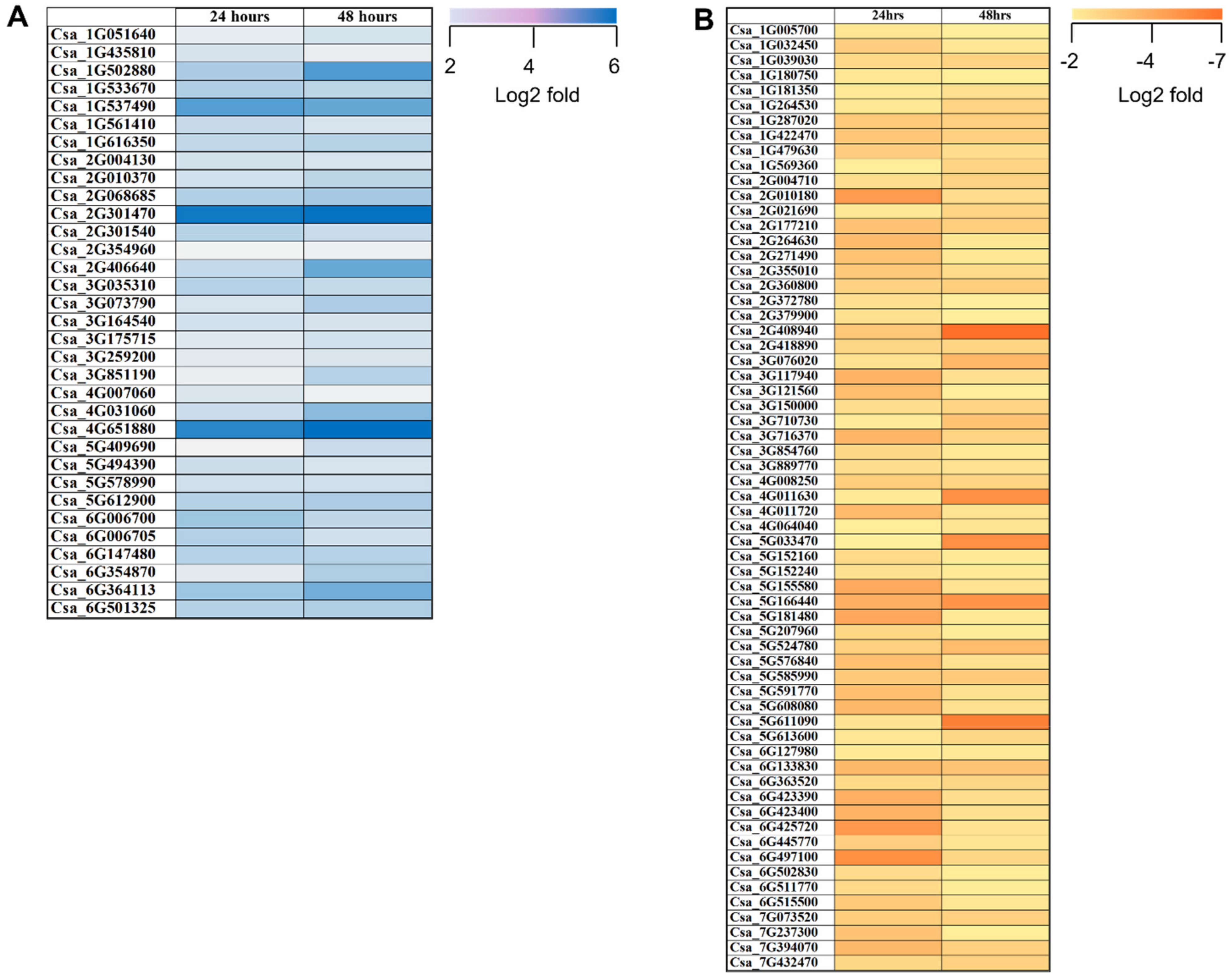
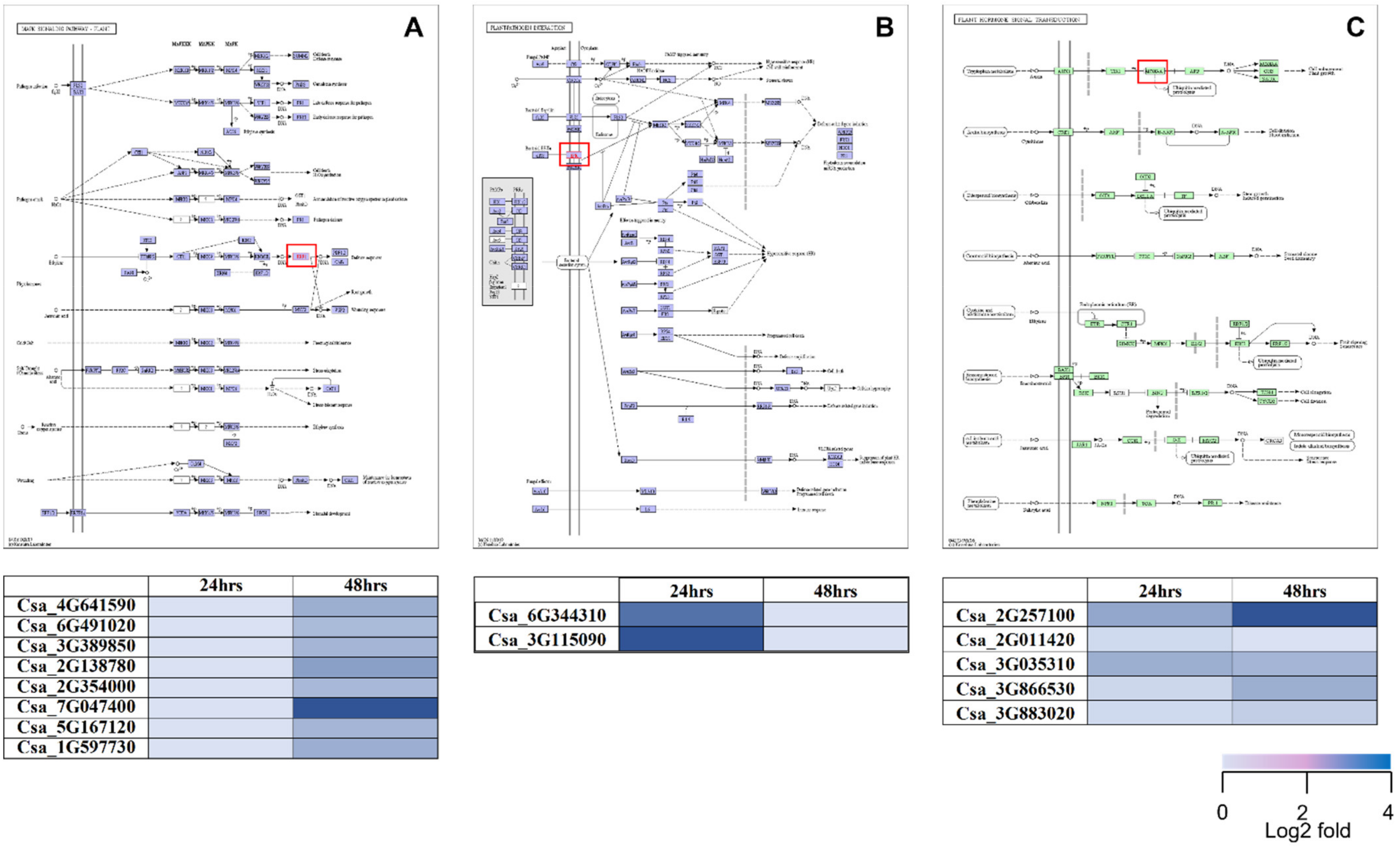
2.3. DEGs Involved in PGPR Biocontrol Mechanisms
2.4. Gene Ontology Enrichment Analysis of DEGs
2.5. KEGG Pathway Analysis
2.6. Gene Validation with qRT-PCR
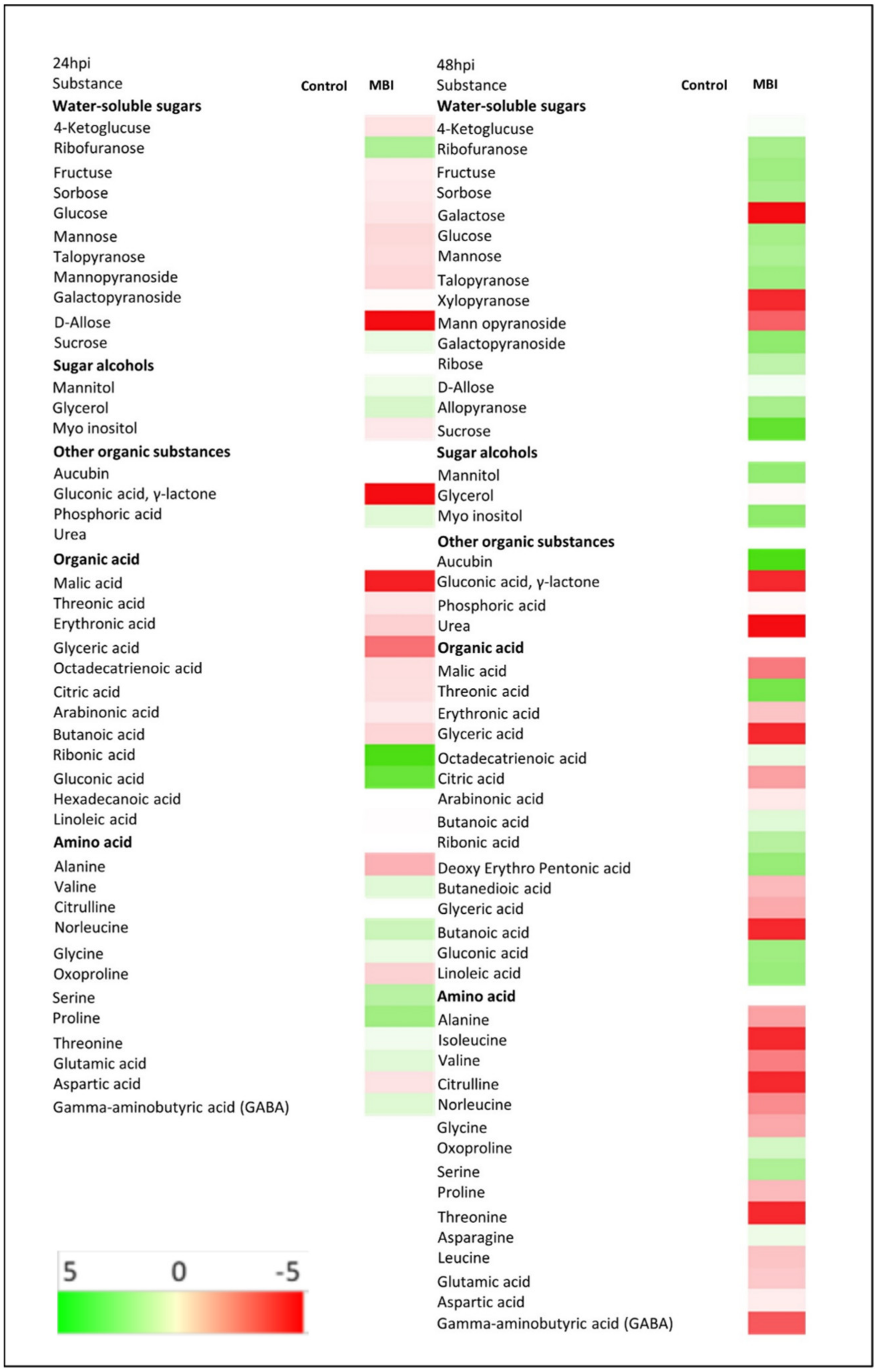
2.7. Metabolite Profile in Cucumber Plants after Application of Bs MBI600
3. Discussion
4. Materials and Methods
4.1. Plant Material and PGPR Application
4.2. Plant Growth and Photosynthesis Parameters
4.3. Metabolite Extraction, Derivatization and Profiling after Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.4. RNA Extraction, Library Construction and RNA Sequencing
4.5. Gene Ontology and KEGG Enrichment Analysis of DEGs
4.6. qRT-PCR Assays
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitrat, M.; Chauvet, C.; Foury, C. Diversity, history and production of cultivated Cucurbits. Acta Hortic. 1999, 492, 21–28. [Google Scholar] [CrossRef]
- Keinath, A.P.; Wintermantel, W.M.; Zitter, T.A. Compendium of Cucurbit Diseases and Pests; APS Press: St. Paul, MN, USA, 2018; pp. 24–159. [Google Scholar]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of plants to adverse chemical soil conditions. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 409–472. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Pliego, C.; De Weert, S.; Lamers, G.; De Vicente, A.; Bloemberg, G.; Cazorla, F.M.; Ramos, C. Two similar enhanced root-colonizing Pseudomonas strains differ largely in their colonization strategies of avocado roots and Rosellinia necatrix hyphae. Environ. Microbiol. 2008, 10, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Malviya, M.K.; Li, C.N.; Solanki, M.K.; Singh, R.K.; Htun, R.; Singh, P.; Li, Y.R. Comparative analysis of sugarcane root transcriptome in response to the plant growth-promoting Burkholderia anthina MYSP113. PLoS ONE 2020, 15, e0231206. [Google Scholar] [CrossRef]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Chen, H.; Jones, A.D.; Howe, G.A. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar] [CrossRef]
- Doornbos, R.F.; Van Loon, L.C.; Bakker, P.A.H.M. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van Wees, S.C.; Van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; Van Loon, L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Kloepper, J.W.; Tuzun, S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 1991, 81, 1508–1512. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Liang, J.G.; Tao, R.X.; Hao, Z.N.; Wang, L.; Zhang, X. Induction of resistance in cucumber against seedling damping-off by plant growth-promoting rhizobacteria (PGPR) Bacillus megaterium strain L8. Afr. J. Biotechnol. 2011, 10, 6920–6927. [Google Scholar]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.; Karaoglanidis, G.S. Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI600 on cucumber. Front. Microbiol. 2021, 11, 600393. [Google Scholar] [CrossRef]
- Samaras, A.; Karaoglanidis, G.S.; Tzelepis, G. Insights into the multitrophic interactions between the biocontrol agent Bacillus subtilis MBI600, the pathogen Botrytis cinerea and their plant host. Microbiol. Res. 2021, 248, 126752. [Google Scholar] [CrossRef]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Liebmann, B.; Kalantidis, K.; Vassilakos, N.; Skandalis, N. Bacillus amyloliquefaciens MBI600 differentially induces tomato defense signaling pathways depending on plant part and dose of application. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G.S. Biocontrol potential and plant-growth-promoting effects of Bacillus amyloliquefaciens MBI600 against Fusarium oxysporum f. sp. radicis-lycopersici on tomato. Acta Hort. 2018, 1207, 139–145. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.; Yao, X.F.; Mi, D.D.; Li, Z.J.; Yang, B.Y.; Zheng, Y.; Guo, J.H. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 against Fusarium wilt on watermelon. Front. Microbiol. 2019, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Gamez, R.M.; Rodríguez, F.; Vidal, N.M.; Ramirez, S.; Alvarez, R.V.; Landsman, D.; Mariño-Ramírez, L. Banana (Musa acuminata) transcriptome profiling in response to rhizobacteria: Bacillus amyloliquefaciens Bs006 and Pseudomonas fluorescens Ps006. BMC Genom. 2019, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Mia, M.B.; Shamsuddin, Z.H. Nitrogen Fixation and Transportation by Rhizobacteria. Int. J. Bot. 2010, 6, 235–242. [Google Scholar] [CrossRef][Green Version]
- Riggs, P.J.; Chelius, M.K.; Iniguez, A.L.; Kaeppler, S.M.; Triplett, E.W. Enhanced maize productivity by inoculation with diazotrophic bacteria. Funct. Plant Biol. 2001, 28, 829–836. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; You, J.; Xiong, L. Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol. Biol. 2009, 70, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Rolland, F.; Moore, B.; Sheen, J. Sugar sensing and signaling in plants. Plant Cell 2002, 14 (Suppl. S1), S185–S205. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Laxmi, A.; St Martin, S.K.; Jang, J.C. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 2004, 16, 2128–2150. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wind, J.J.; Shi, X.; Zhang, H.; Hanson, J.; Smeekens, S.C.; Teng, S. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc. Natl. Acad. Sci. USA 2011, 108, 3436–3441. [Google Scholar] [CrossRef]
- Buchala, A.J. Cotton Fibers: Developmental Biology, Quality Improvement and Textile Processing; Basra, A.S., Ed.; Food Products Press: New York, NY, USA, 1999; pp. 113–136. [Google Scholar]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant–pathogen interactions. Front. Plant Sci. 2014, 5, 228. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A. The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ. Sci. Pollut. Res. 2018, 25, 33370–33380. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological perspectives of microbes in agro-ecosystems. Biotech. Lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef]
- Gassmann, W.; Francisco, R.; Schroeder, J.I. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996, 10, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Pires, R.S.; Szollosi, A.; Morais-Cabral, J.H. The structure of the KtrAB potassium transporter. Nature 2013, 496, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.N.; Shetty, H.S.; Reddy, M.S. Plant growth promoting rhizobacteria: Potential green alternative for plant productivity. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2005; pp. 197–216. [Google Scholar]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Does, D.V.; Van Wees, S.C. Signalling networks involved in induced resistance. In Induced Resistance for Plant Defence: A Sustainable Approach to Crop Protection, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2014; pp. 58–80. [Google Scholar]
- Liu, L.; Kloepper, J.; Tuzun, S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology 1995, 85, 695–698. [Google Scholar] [CrossRef]
- Leeman, M.; Van Pelt, J.A.; Den Ouden, F.M.; Heinsbroek, M.; Bakker, P.; Schippers, B. Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology 1995, 85, 1021–1027. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.; Durairaj, S.; Saravanan, S.; Karikalan, K.; Muralidaran, S.; Islam, V.I.H. Role of ethylene response transcription factor (ERF) and its regulation in response to stress encountered by plants. Plant Mol. Biol. Rep. 2015, 33, 347–357. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yamoto, N.; Suzuki, Y.; Chiba, Y.; Yamazaki, K.I.; Sato, T.; Yamaguchi, J. The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci. 2013, 213, 79–87. [Google Scholar] [CrossRef]
- Higuchi, M.; Pischke, M.S.; Mähönen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Kakimoto, T. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 2004, 101, 8821–8826. [Google Scholar] [CrossRef]
- Borhan, M.H.; Gunn, N.; Cooper, A.; Gulden, S.; Tör, M.; Rimmer, S.R.; Holub, E.B. WRR4 encodes a TIR-NB-LRR protein that confers broad-spectrum white rust resistance in Arabidopsis thaliana to four physiological races of Albugo candida. Mol. Plant Microbe Interact. 2008, 21, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Vibhuti, M.; Kumar, A.; Sheoran, N.; Nadakkakath, A.V.; Eapen, S.J. Molecular basis of endophytic Bacillus megaterium-induced growth promotion in Arabidopsis thaliana: Revelation by microarray-based gene expression analysis. J. Plant Growth Regul. 2017, 36, 118–130. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Du, R.; Wang, J.; Li, H.; Xie, D.; Yan, J. Isoleucine Enhances Plant Resistance Against Botrytis cinerea via Jasmonate Signaling Pathway. Front. Plant Sci. 2021, 12, 628328. [Google Scholar] [CrossRef]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 2019, 10, 2351. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Wang, J.; Wang, L.; Yao, W.; Yang, Y.; Wang, Y. The Chinese wild grapevine (Vitis pseudoreticulata) E3 ubiquitin ligase Erysiphe necator-induced RING finger protein 1 (EIRP1) activates plant defense responses by inducing proteolysis of the VpWRKY11 transcription factor. New Phytologist. 2013, 200, 834–846. [Google Scholar] [CrossRef]
- Liu, L.; Jin, L.; Huang, X.; Geng, Y.; Li, F.; Qin, Q.; Li, Y. OsRFPH2-10, a ring-H2 finger E3 ubiquitin ligase, is involved in rice antiviral defense in the early stages of rice dwarf virus infection. Mol. Plant 2014, 7, 1057–1060. [Google Scholar] [CrossRef]
- Bigeard, J.; Hirt, H. Nuclear signaling of plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Máthé, C.; Garda, T.; Freytag, C. The role of serine-threonine protein phosphatase pp2a in plant oxidative stress signaling—facts and hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [Google Scholar] [CrossRef]
- Reddy, P.S.; Leisen, T.C.; Reddy, M.N.; Sarada, C.; Reddanna, P. Differential formation of octadecadienoic acid and octadecatrienoic acid products in control and injured/infected potato tubers. Biochim. Biophys. Acta 2000, 1483, 294–300. [Google Scholar] [CrossRef]
- Yu, S.W.; Tang, K.X. MAP kinase cascades responding to environmental stress in plants. Acta Bot. Sin. 2004, 46, 127–136. [Google Scholar]
- Bi, G.; Zhou, J.M. MAP kinase signaling pathways: A hub of plant-microbe interactions. Cell Host Microbe 2017, 21, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Kano, A.; Fukumoto, T.; Ohtani, K.; Yoshihara, A.; Ohara, T.; Tajima, S.; Akimitsu, K. The rare sugar D-allose acts as a triggering molecule of rice defense via ROS generation. J. Exp. Bot. 2013, 64, 4939–4951. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Akimitsu, K. The rare sugar d-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Ainalidou, A.; Tanou, G.; Belghazi, M.; Samiotaki, M.; Diamantidis, G.; Molassiotis, A.; Karamanoli, K. Integrated analysis of metabolites and proteins reveal aspects of the tissue-specific function of synthetic cytokinin in kiwifruit development and ripening. J. Proteom. 2016, 143, 318–333. [Google Scholar] [CrossRef]
- Nardozza, S.; Boldingh, H.L.; Osorio, S.; Höhne, M.; Wohlers, M.; Gleave, A.P.; Clearwater, M.J. Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J. Exp. Bot. 2013, 64, 5049–5063. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]

| Gene ID | Protein | Function | Expression Pattern a |
|---|---|---|---|
| Csa_2G138780 | ethylene-responsive transcription factor ERF062 | Signalling | 24 |
| Csa_3G115090 | L-type lectin-domain containing receptor kinase IX.1 | 24 | |
| Csa_7G452180 | LRR receptor-like serine/threonine-protein kinase IOS1 | 24 | |
| Csa_7G047400 | ethylene-responsive transcription factor ERF054 | 48 | |
| Csa_1G642550 | jasmonate-induced protein | 48 | |
| Csa_2G301540 | RING-H2 finger protein ATL78 | 24 + 48 | |
| Csa_3G175715 | pentatricopeptide repeat-containing protein | 24 | |
| Csa_7G007930 | indole-3-acetic acid-induced protein ARG7 | Plant growth-related mechanisms | 24 + 48 |
| Csa_5G409690 | potassium channel SKOR | 24 + 48 | |
| Csa_2G011420 | auxin-responsive protein IAA4 | 24 +48 | |
| Csa_4G007060 | potassium transporter 5 | 24 + 48 | |
| Csa_3G035310 | auxin-responsive protein SAUR32 | 24 + 48 | |
| Csa_3G866530 | auxin-responsive 6B | 24 + 48 | |
| Csa_5G609820 | zinc finger protein GIS4 | 48 | |
| Csa_2G406640 | peroxidase 55 | Defense-related mechanisms | 24 + 48 |
| Csa_3G743950 | thaumatin-like protein 1b | 24 | |
| Csa_7G044780 | peroxidase 28 | 48 | |
| Csa_5G643380 | endo-1,3(4)-beta-glucanase 3 | 48 | |
| Csa_2G010370 | pathogenesis-related protein PR-4 | 24 + 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. https://doi.org/10.3390/plants11091218
Samaras A, Kamou N, Tzelepis G, Karamanoli K, Menkissoglu-Spiroudi U, Karaoglanidis GS. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants. 2022; 11(9):1218. https://doi.org/10.3390/plants11091218
Chicago/Turabian StyleSamaras, Anastasios, Nathalie Kamou, Georgios Tzelepis, Katerina Karamanoli, Urania Menkissoglu-Spiroudi, and George S. Karaoglanidis. 2022. "Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants" Plants 11, no. 9: 1218. https://doi.org/10.3390/plants11091218
APA StyleSamaras, A., Kamou, N., Tzelepis, G., Karamanoli, K., Menkissoglu-Spiroudi, U., & Karaoglanidis, G. S. (2022). Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants, 11(9), 1218. https://doi.org/10.3390/plants11091218







