Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Plant Material, and Growth Conditions
2.2. Evaluation of Plant Growth Performance
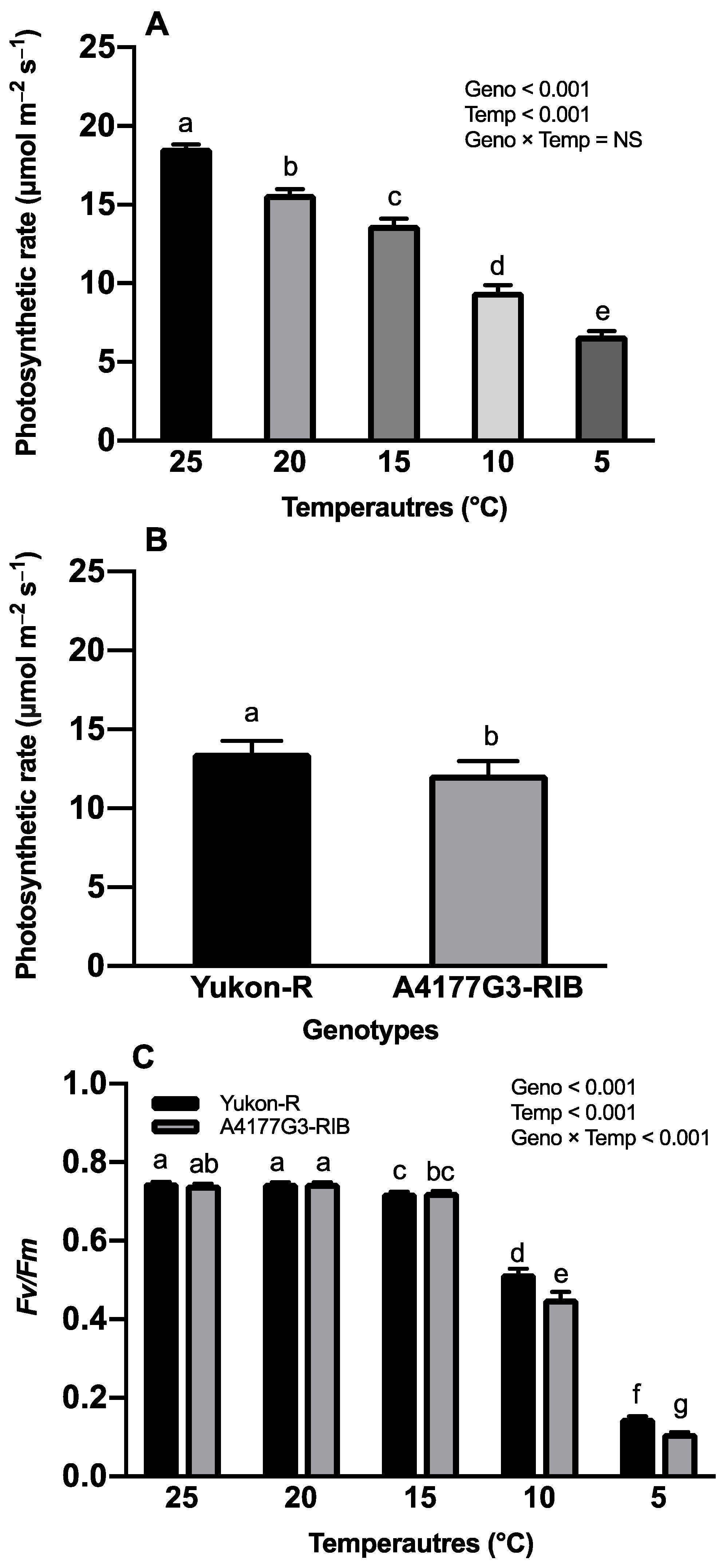
2.2.1. Photosynthesis Rate and Maximum Photochemical Efficiency of Photosystem-II
2.2.2. Plant Growth and Root Characteristics
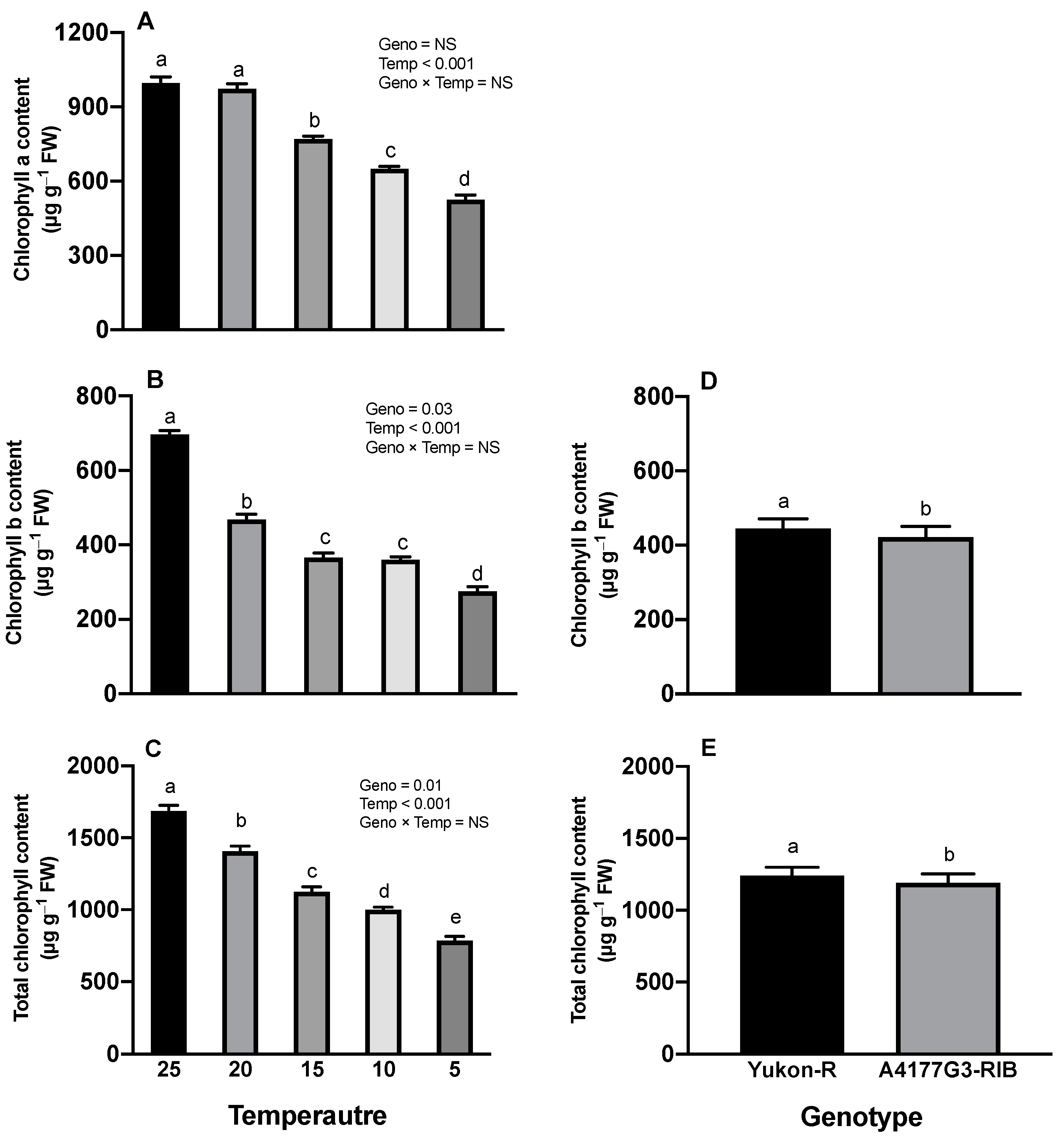
2.2.3. Chlorophyll Contents
2.3. Biochemical Analyses
2.3.1. Proline Contents
2.3.2. Hydrogen Peroxide and Malondialdehyde Contents
2.3.3. Protein Extraction and Antioxidant Enzyme Assays
2.4. Statistical Analyses
3. Results
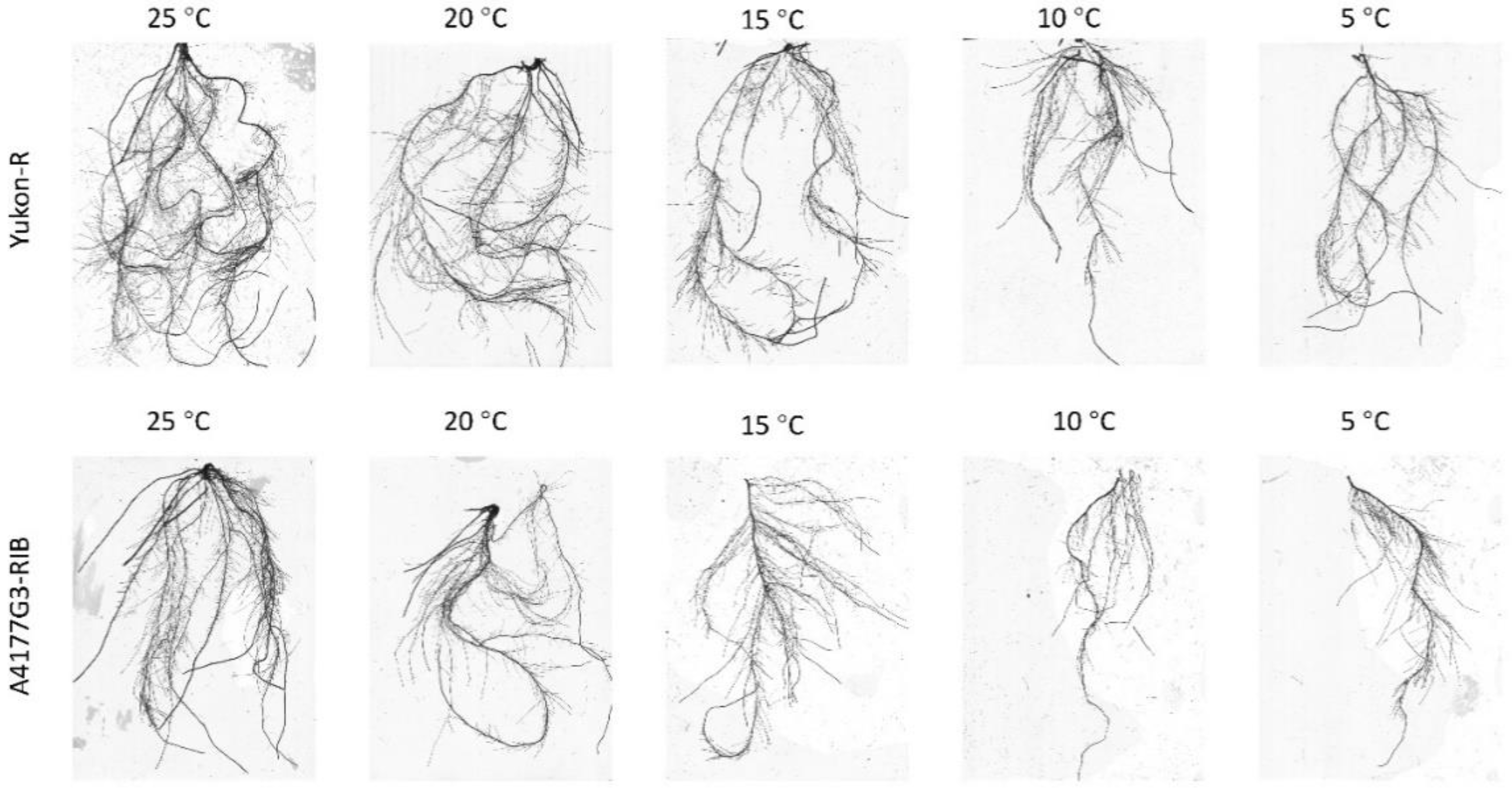
3.1. Seedling Growth and Root Characteristics
3.2. Chlorophyll Content, Photosynthesis Rate, and Maximum Photochemical Efficiency of PS-II
3.3. Hydrogen Peroxide and Malondialdehyde Contents
3.4. Proline Contents and Antioxidant Enzyme Activities
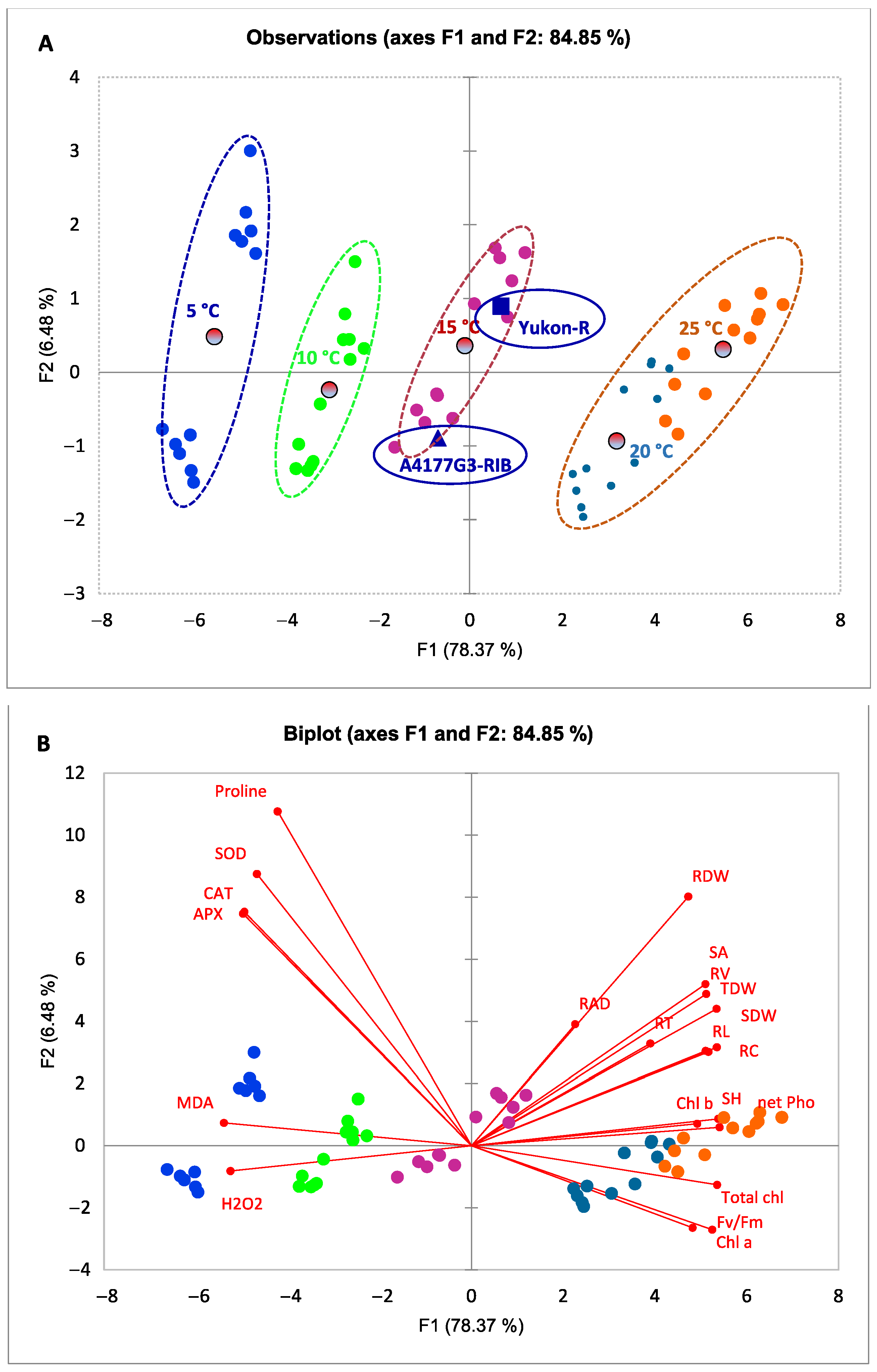
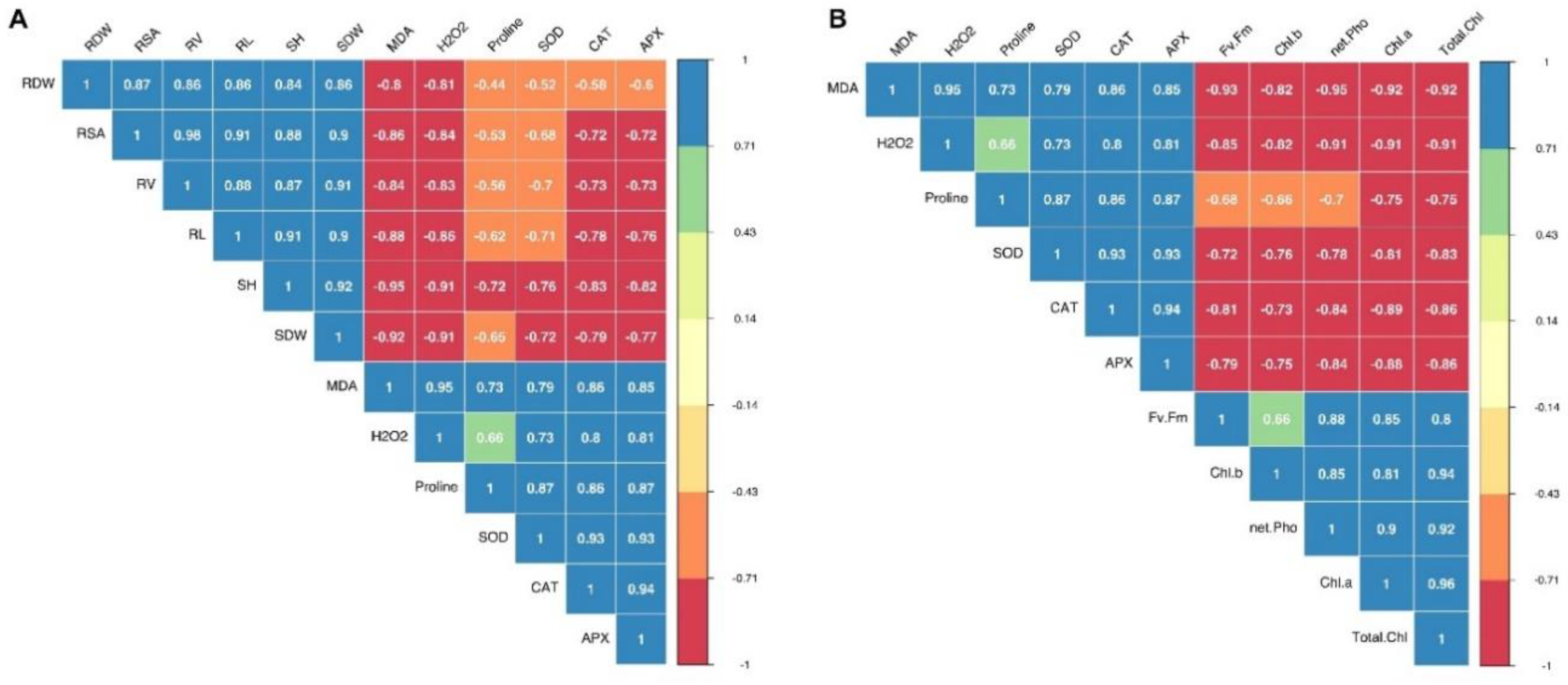
3.5. Relationships between Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes as Influenced by Different Temperature Regiems
4. Discussion
4.1. Changes in Silage Corn Seedling Growth and Photosynthetic Capacity in Response to Different Temperatures
4.2. Chilling Stress-Induced Osmotic Stress, ROS, and Activity of Antioxidant Enzymes in Silage Corn Seedlings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirkland, R.M.; Steen, R.W.J.; Gordon, F.J.; Keady, T.W.J. The influence of grass and maize silage quality on apparent diet digestibility, metabolizable energy concentration and intake of finishing beef cattle. Grass Forage Sci. 2005, 60, 244–253. [Google Scholar] [CrossRef]
- Ericsson, K.; Nilsson, L.J. Assessment of the potential biomass supply in Europe using a resource-focused approach. Biomass Bioenergy 2006, 30, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Phipps, R.H.; Sutton, J.D.; Beever, D.E.; Jones, A.K. The effect of crop maturity on the nutritional value of maize silage for lactating dairy cows. 3. Food intake and milk production. Anim. Sci. 2000, 71, 401–409. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2015, 95, 238–252. [Google Scholar] [CrossRef]
- Guyader, J.; Baron, V.S.; Beauchemin, K.A. Corn Forage Yield and Quality for Silage in Short Growing Season Areas of the Canadian Prairies. Agronomy 2018, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, M.R.C.; Rotz, A.; Kroebel, R.; Beauchemin, K.A.; Hunt, D.; Bittman, S.; Koenig, K.M.; McKenzie, D.B. Prospects of forage production in northern regions under climate and land-use changes: A case-study of a dairy farm in Newfoundland, Canada. Agronomy 2019, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Miedema, P. The Effects of Low Temperature on Zea mays. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1982; Volume 35, pp. 93–128. [Google Scholar]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Nadeem, M.; Pham, T.H.; Thomas, R.; Galagedara, L.; Kavanagh, V.; Zhu, X.; Ali, W.; Cheema, M. Potential role of root membrane phosphatidic acid in superior agronomic performance of silage-corn cultivated in cool climate cropping systems. Physiol. Plant. 2019, 167, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Thomas, R.; Adigun, O.; Manful, C.; Wu, J.; Pham, T.H.; Zhu, X.; Galagedara, L.; Cheema, M. Root membrane lipids as potential biomarkers to discriminate silage-corn genotypes cultivated on podzolic soils in boreal climate. Physiol. Plant. 2020, 170, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, J.; Roychoudhury, A. Cold-Induced Injuries and Signaling Responses in Plants. In Cold Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Herath, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–35. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.-J.; Siddique, K.H.M. Chilling tolerance in maize: Agronomic and physiological approaches. Crop Pasture Sci. 2009, 60, 501–516. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [Green Version]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Long, S.P.; Spence, A.K. Toward cool C4 crops. Annu. Rev. Plant Biol. 2013, 64, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Peeler, T.C.; Naylor, A.W. A comparison of the effects of chilling on leaf gas exchange in pea (Pisum sativum L.) and cucumber (Cucumis sativus L.). Plant Physiol. 1988, 86, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Lianopoulou, V.; Bosabalidis, A.M.; Patakas, A.; Lazari, D.; Panteris, E. Effects of chilling stress on leaf morphology, anatomy, ultrastructure, gas exchange, and essential oils in the seasonally dimorphic plant Teucrium polium (Lamiaceae). Acta Physiol. Plant. 2014, 36, 2271–2281. [Google Scholar] [CrossRef]
- Bilska, A.; Sowiński, P. Closure of plasmodesmata in maize (Zea mays) at low temperature: A new mechanism for inhibition of photosynthesis. Ann. Bot. 2010, 106, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Adam, S.; Murthy, S.D.S. Effect of Cold Stress on Photosynthesis of Plants and Possible Protection Mechanisms. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 219–226. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nature Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Zhang, Y.; Xu, H.; Ke, Y.; Bruno, A.K.; Zhang, L.; Li, J. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [Green Version]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [Green Version]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.-S.; Maali-Amiri, R. Global insights of protein responses to cold stress in plants: Signaling, defence, and degradation. J. Plant Physiol. 2018, 226, 123–135. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Nejadsadeghi, L.; Maali-Amiri, R.; Zeinali, H.; Ramezanpour, S.; Sadeghzade, B. Comparative analysis of physio-biochemical responses to cold stress in tetraploid and hexaploid wheat. Cell Biochem. Biophys. 2014, 70, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Nadeem, M.; Ashiq, W.; Zaeem, M.; Gilani, S.S.M.; Rajabi-Khamseh, S.; Pham, T.H.; Kavanagh, V.; Thomas, R.; Cheema, M. The effects of organic and inorganic phosphorus amendments on the biochemical attributes and active microbial population of agriculture podzols following silage corn cultivation in boreal climate. Sci. Rep. 2019, 9, 17297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, W.; Nadeem, M.; Ashiq, W.; Zaeem, M.; Thomas, R.; Kavanagh, V.; Cheema, M. Forage yield and quality indices of silage-corn following organic and inorganic phosphorus amendments in podzol soil under boreal climate. Agronomy 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Stern, D.B. Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol. J. 2020, 18, 1409–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Portland Press Ltd.: London, UK, 1983. [Google Scholar]
- Wehner, G.; Balko, C.; Ordon, F. Experimental design to determine drought stress response and early leaf senescence in barley (Hordeum vulgare L.). Bio-Protocol 2016, 6, e1749. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.B.; Ellouzi, H.; Planchais, S.; De Vos, D.; Faiyue, B.; Carol, P.; Abdelly, C.; Savouré, A. Phospholipases Dζ1 and Dζ2 have distinct roles in growth and antioxidant systems in Arabidopsis thaliana responding to salt stress. Planta 2017, 246, 721–735. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kwabiah, A.B.; MacPherson, M.; McKenzie, D.B. Corn heat unit variability and potential of corn (Zea mays L.) production in a cool climate ecosystem. Can. J. Plant Sci. 2003, 83, 689–698. [Google Scholar] [CrossRef]
- Kwabiah, A.B. Economic evaluation of production methods for sweet corn in a cool climate. J. Veg. Crop Prod. 2004, 10, 73–87. [Google Scholar] [CrossRef]
- Rymen, B.; Fiorani, F.; Kartal, F.; Vandepoele, K.; Inzé, D.; Beemster, G.T.S. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 2007, 143, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercoli, L.; Mariotti, M.; Masoni, A.; Arduini, I. Growth responses of sorghum plants to chilling temperature and duration of exposure. Eur. J. Agron. 2004, 21, 93–103. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Stamp, P. Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. Eur. J. Agron. 2008, 28, 178–185. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Chapter 2 Cold Signalling and Cold Acclimation in Plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 49, pp. 35–150. [Google Scholar]
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Kannivadi Ramakanth, K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell 2017, 170, 102–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, W.; Babcock, G.T. Photosynthetic reaction centers. Plant Physiol. 2001, 125, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S. Melatonin alleviates cold-induced oxidative damage in maize seedlings by up-regulating mineral elements and enhancing antioxidant activity. J. Plant Nutr. Soil Sci. 2015, 178, 433–439. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, U.; Singh, S.K.; Gupta, C.; Singh, M.; Kushwaha, S.R. Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol. Mol. Biol. Plants 2012, 18, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams, W.W. An integrative approach to photoinhibition and photoprotection of photosynthesis. Environ. Exp. Bot. 2018, 154, 1–3. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of Cold Stress on Photosynthetic Traits, Carbohydrates, Morphology, and Anatomy in Nine Cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, T.E.; Langdale, J.A. SCARECROW gene function is required for photosynthetic development in maize. Plant Direct 2020, 4, e00264. [Google Scholar] [CrossRef]
- Slattery, R.A.; VanLoocke, A.; Bernacchi, C.J.; Zhu, X.-G.; Ort, D.R. Photosynthesis, Light Use Efficiency, and Yield of Reduced-Chlorophyll Soybean Mutants in Field Conditions. Front. Plant Sci. 2017, 8, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [Green Version]
- Lidon, F.C.; Loureiro, A.S.; Vieira, D.E.; Bilhó, E.A.; Nobre, P.; Costa, R. Photoinhibition in chilling stressed wheat and maize. Photosynthetica 2001, 39, 161–166. [Google Scholar] [CrossRef]
- Naidu, S.L.; Moose, S.P.; AL-Shoaibi, A.K.; Raines, C.A.; Long, S.P. Cold tolerance of C4 photosynthesis in Miscanthus × giganteus adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiol. 2003, 132, 1688–1697. [Google Scholar] [CrossRef] [Green Version]
- Kubien, D.S.; von Caemmerer, S.; Furbank, R.T.; Sage, R.F. C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiol. 2003, 132, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Ke, D.; Sun, G.; Jiang, Y. Dual role of superoxide radicals in the chilling-induced photoinhibition in maize seedlings. Photosynthetica 2004, 42, 147–152. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Zhang, Z.; Wang, A.; Zhu, J. Cold-regulated gene LeCOR413PM2 confers cold stress tolerance in tomato plants. Gene 2021, 764, 145097. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, M.; Adamczyk, J.; Jończyk, M.; Sobkowiak, A.; Szczepanik, J.; Frankiewicz, K.; Fronk, J.; Sowiński, P. Increased photosensitivity at early growth as a possible mechanism of maize adaptation to cold springs. J. Exp. Bot. 2019, 70, 2887–2904. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracheboud, Y.; Haldimann, P.; Leipner, J.; Stamp, P. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J. Exp. Bot. 1999, 50, 1533–1540. [Google Scholar] [CrossRef]
- Yi, Q.; Malvar, R.A.; Álvarez-Iglesias, L.; Ordás, B.; Revilla, P. Dissecting the genetics of cold tolerance in a multiparental maize population. Theor. Appl. Genet. 2020, 133, 503–516. [Google Scholar] [CrossRef]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Bénard, C.; et al. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Song, F.-B.; Xu, H.-W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Périlleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016, 16, 212. [Google Scholar] [CrossRef] [Green Version]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Morsy, M.R.; Jouve, L.; Hausman, J.-F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Weber, H.; Chételat, A.; Reymond, P.; Farmer, E.E. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004, 37, 877–888. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 407–421. [Google Scholar]
- Erdal, S. Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress. Plant Physiol. Biochem. 2012, 57, 1–7. [Google Scholar] [CrossRef]
- Huang, M.; Guo, Z. Responses of antioxidative system to chilling stress in two rice cultivars differing in sensitivity. Biol. Plant. 2005, 49, 81–84. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Herath, V. Redox Regulation of Cold Stress Response. In Cold Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Herath, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 53–64. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Farooqi, M.Q.U.; Zahra, Z.; Lee, J.K. Molecular Genetic Approaches for the Identification of Candidate Cold Stress Tolerance Genes. In Cold Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Herath, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 37–51. [Google Scholar] [CrossRef]
- Duncan, D.R.; Widholm, J.M. Proline accumulation and its implication in cold tolerance of regenerable maize callus. Plant Physiol. 1987, 83, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Samuel, D.; Kumar, T.K.S.; Ganesh, G.; Jayaraman, G.; Yang, P.-W.; Chang, M.-M.; Trivedi, V.D.; Wang, S.-L.; Hwang, K.-C.; Chang, D.-K.; et al. Proline inhibits aggregation during protein refolding. Protein Sci. 2000, 9, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alia, M.J.; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Alia; Pardha Saradhi, P.; Mohanty, P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. B Biol. 1997, 38, 253–257. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Hare, P.D.; Cress, W.A.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406. [Google Scholar] [CrossRef]
- Shafi, A.; Dogra, V.; Gill, T.; Ahuja, P.S.; Sreenivasulu, Y. Simultaneous over-expression of PaSOD and RaAPX in transgenic Arabidopsis thaliana confers cold stress tolerance through increase in vascular lignifications. PLoS ONE 2014, 9, e110302. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Yang, Z.; Yang, Y.; Jiang, L.; Hu, L. CsCAT3, a catalase gene from Cucumis sativus, confers resistance to a variety of stresses to Escherichia coli. Biotechnol. Biotechnol. Equip. 2017, 31, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Kremer, M.L. Mechanism of the Fenton reaction. Evidence for a new intermediate. Phys. Chem. Chem. Phys. 1999, 1, 3595–3605. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reative oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front. Plant Sci. 2018, 9, 1137. [Google Scholar] [CrossRef]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2009, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-G.; Han, S.-Y.; Yang, W.-H.; Wei, H.-L.; Zhang, M.; Qi, L.-W. Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tissue Organ Cult. (PCTOC) 2009, 100, 21. [Google Scholar] [CrossRef] [Green Version]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J. Exp. Bot. 1997, 48, 1105–1113. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme and compound responses to chilling stress and their combining abilities in differentially sensitive maize hybrids. Crop Sci. 1997, 37, 857–863. [Google Scholar] [CrossRef]

| Source of Variance | Seedling Shoot Length (cm Seedling−1) | Shoot Dry Weight (g Seedling−1) | Root Dry Weight (g Seedling−1) | Seedling Ddry Weight (g Seedling−1) |
|---|---|---|---|---|
| Temperature (Temp) | ||||
| 25 °C (control) | 77.41 ± 1.14 a | 0.95 ± 0.02 a | 0.25 ± 0.01 a | 1.20 ± 0.03 a |
| 20 °C | 72.66 ± 1.00 b | 0.79 ± 0.01 b | 0.22 ± 0.01 b | 1.02 ± 0.02 b |
| 15 °C | 63.93 ± 1.84 c | 0.70 ± 0.02 c | 0.19 ± 0.01 c | 0.89 ± 0.03 c |
| 10 °C | 53.79 ± 1.04 d | 0.60 ± 0.02 d | 0.16 ± 0.01 d | 0.75 ± 0.03 d |
| 5 °C | 47.42 ± 0.95 e | 0.49 ± 0.02 e | 0.15 ± 0.01 e | 0.64 ± 0.03 e |
| Genotypes (Geno) | ||||
| Yukon-R | 65.71 ± 2.11 a | 0.75 ± 0.03 a | 0.22 ± 0.01 a | 0.97 ± 0.03 a |
| A4177G3-RIB | 60.37 ± 2.21 b | 0.66 ± 0.03 b | 0.16 ± 0.01 b | 0.83 ± 0.04 b |
| Temp × Geno | ||||
| 25 °C × Yukon-R | 80.02 ± 1.30 a | 0.98 ± 0.03 | 0.27 ± 0.01 | 1.25 ± 0.02 |
| 20 °C ×Yukon-R | 73.25 ± 1.14 b | 0.82 ± 0.03 | 0.25 ± 0.01 | 1.07 ± 0.03 |
| 15 °C × Yukon-R | 68.74 ± 1.73 c | 0.74 ± 0.02 | 0.23 ± 0.01 | 0.97 ± 0.02 |
| 10 °C × Yukon-R | 56.71 ± 1.12 d | 0.65 ± 0.02 | 0.19 ± 0.01 | 0.83 ± 0.02 |
| 5 °C × Yukon-R | 49.87 ± 1.34 e | 0.56 ± 0.02 | 0.18 ± 0.01 | 0.74 ± 0.02 |
| 25 °C × A4177G3-RIB | 74.80 ± 1.96 b | 0.91 ± 0.03 | 0.23 ± 0.01 | 1.14 ± 0.03 |
| 20 °C × A4177G3-RIB | 72.07 ± 0.99 bc | 0.78 ± 0.01 | 0.19 ± 0.01 | 0.96 ± 0.01 |
| 15 °C × A4177G3-RIB | 59.12 ± 0.58 d | 0.67 ± 0.02 | 0.16 ± 0.003 | 0.82 ± 0.02 |
| 10 °C × A4177G3-RIB | 50.87 ± 1.17 e | 0.54 ± 0.01 | 0.13 ± 0.004 | 0.68 ± 0.01 |
| 5 °C × A4177G3-RIB | 44.99 ± 0.52 f | 0.43 ± 0.01 | 0.12 ± 0.003 | 0.54 ± 0.01 |
| Significance | ||||
| Temperatures | *** | *** | *** | *** |
| Genotypes | *** | ** | *** | *** |
| Temp × Gen | * | NS | NS | NS |
| Source of Variance | Total Root Length (RL) (cm Seedling −1) | Root Length 0–0.5 mm (cm Seedling −1) | Root Length >0.5 mm (cm Seedling −1) | Total Root Volume (RV) (cm3 Seedling −1) | Root Volume 0–0.5 mm (cm3 Seedling −1) | Root Volume >0.5 mm (cm3 Seedling −1) | Total Surface Area (SA) (cm2 Seedling −1) | Surface Area 0–0.5 mm (cm2 Seedling −1) | Surface Area >0.5 mm (cm2 Seedling −1) |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (Temp) | |||||||||
| 25 °C (control) | 1416 ± 59 a | 1145 ± 59 a | 271 ± 8 a | 2.24 ± 0.05 a | 0.42 ± 0.02 a | 1.82 ± 0.03 a | 158.19 ± 3.88 a | 64.21 ± 3.19 a | 77.23 ± 2.54 a |
| 20 °C | 1291 ± 49 a | 1082 ± 47 a | 209 ± 7 a | 1.50 ± 0.07 b | 0.36 ± 0.02 b | 1.14 ± 0.08 b | 121.31 ± 6.82 b | 59.89 ± 3.81 a | 47.79 ± 3.49 b |
| 15 °C | 966 ± 58 b | 775 ± 50 b | 189 ± 8 c | 1.30 ± 0.05 b | 0.33 ± 0.03 b | 0.97 ± 0.05 c | 109.33 ± 5.64 c | 51.07 ± 3.88 b | 46.28 ± 2.05 b |
| 10 °C | 612 ± 48 c | 484 ± 45 c | 127 ± 5 d | 0.80 ± 0.03 c | 0.19 ± 0.01 c | 0.61 ± 0.03 d | 66.68 ± 3.06 d | 29.19 ± 2.09 c | 30.13 ± 1.38 c |
| 5 °C | 589 ± 48 c | 472 ± 35 c | 116 ± 13 d | 0.75 ± 0.04 c | 0.17 ± 0.01 c | 0.58 ± 0.06 d | 61.45 ± 4.52 d | 27.25 ± 1.68 c | 27.85 ± 3.11 c |
| Genotypes (Geno) | |||||||||
| Yukon-R | 1108 ± 69 a | 906 ± 60 a | 201 ± 9 a | 1.48 ± 0.06 a | 0.34 ± 0.02 a | 1.14 ± 0.08 a | 104.55 ± 6.19 a | 54.22 ± 3.56 a | 50.33 ± 2.95 a |
| A4177G3-RIB | 842 ± 64 b | 677 ± 52 b | 164 ± 12 b | 1.15 ± 0.06 b | 0.24 ± 0.02 b | 0.91 ± 0.09 b | 79.80 ± 6.41 b | 38.42 ± 2.61 b | 41.38 ± 4.08 b |
| Temp × Geno | |||||||||
| 25 °C × Yukon-R | 1594 ± 41 | 1302 ± 41 | 272 ± 15 a | 2.33 ± 0.08 | 0.47 ± 0.02 a | 1.86 ± 0.03 a | 148.04 ± 3.54 | 72.56 ± 3.65 a | 75.47 ± 2.43 a |
| 20 °C ×Yukon-R | 1513 ± 59 | 1182 ± 67 | 223 ± 9 b | 1.73 ± 0.08 | 0.43 ± 0.02 ab | 1.31 ± 0.11 b | 125.04 ± 5.56 | 70.52 ± 2.99 a | 54.52 ± 3.66 b |
| 15 °C × Yukon-R | 1047 ± 66 | 904 ± 54 | 213 ± 7 bc | 1.45 ± 0.06 | 0.41 ± 0.02 b | 1.09 ± 0.05 c | 113.62 ± 4.95 | 62.07 ± 3.03 b | 51.56 ± 2.09 b |
| 10 °C × Yukon-R | 848 ± 33 | 581 ± 68 | 140 ± 9 e | 0.88 ± 0.08 | 0.22 ± 0.02 ef | 0.66 ± 0.02 fg | 67.33 ± 3.19 | 34.56 ± 2.56 de | 32.77 ± 1.26 de |
| 5 °C × Yukon-R | 608 ± 60 | 562 ± 47 | 158 ± 8 de | 0.95 ± 0.04 | 0.19 ± 0.01 fg | 0.77 ± 0.03 ef | 68.73 ± 3.54 | 31.38 ± 2.24 e | 37.35 ± 1.50 cd |
| 25 °C × A4177G3-RIB | 1284 ± 42 | 988 ± 65 | 269 ± 6 a | 2.41 ± 0.03 | 0.36 ± 0.02 c | 1.79 ± 0.06 a | 134.85 ± 6.02 | 55.86 ± 1.89 bc | 78.99 ± 4.60 a |
| 20 °C × A4177G3-RIB | 1186 ± 69 | 981 ± 38 | 195 ± 6 c | 1.27 ± 0.04 | 0.30 ± 0.02 d | 0.98 ± 0.09 cd | 90.306 ± 7.24 | 49.25 ± 3.11 c | 41.05 ± 4.71 c |
| 15 °C × A4177G3-RIB | 765 ± 25 | 647 ± 42 | 166 ± 7 d | 1.11 ± 0.03 | 0.26 ± 0.02 de | 0.85 ± 0.04 de | 81.09 ± 3.16 | 40.07 ± 2.95 d | 41.01 ± 1.72 c |
| 10 °C × A4177G3-RIB | 476 ± 54 | 387 ± 22 | 115 ± 6 f | 0.71 ± 0.02 | 0.15 ± 0.01 g | 0.55 ± 0.04 g | 51.29 ± 2.31 | 23.81 ± 1.06 f | 27.48 ± 2.00 e |
| 5 °C × A4177G3-RIB | 429 ± 18 | 343 ± 14 | 74 ± 7 g | 0.54 ± 0.02 | 0.14 ± 0.0 g | 0.39 ± 0.05 h | 41.47 ± 1.81 | 23.11 ± 0.79 f | 18.36 ± 2.09 f |
| Significance | |||||||||
| Temperatures | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Genotypes | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Temp × Gen | NS | NS | *** | NS | *** | * | NS | * | *** |
| Source of Variance | Root Average Diameters (mm) | Total Root Tips | Root Tips 0–0.05 mm | Root Tips >0.05 mm | Root Forks | Root Crossings |
|---|---|---|---|---|---|---|
| Temperature (Temp) | ||||||
| 25 °C (control) | 0.366 ± 0.007 a | 3649 ± 358 a | 3592 ± 346 a | 39 ± 4 a | 5341 ± 211 a | 1298 ± 65 a |
| 20 °C | 0.352 ± 0.004 a | 2705 ± 144 b | 2685 ± 144 b | 19 ± 2 b | 4702 ± 290 b | 1233 ± 69 a |
| 15 °C | 0.350 ± 0.005 ab | 2569 ± 222 b | 2566 ± 220 b | 30 ± 2 c | 3908 ± 242 c | 922 ± 65 b |
| 10 °C | 0.350 ± 0.009 ab | 1794 ± 168 c | 1778 ± 167 c | 16 ± 3 c | 2106 ± 166 d | 427 ± 41 c |
| 5 °C | 0.332 ± 0.006 b | 1684 ± 127 c | 1676 ± 126 c | 18 ± 2 c | 1933 ± 124 d | 411 ± 43 c |
| Genotypes (Geno) | ||||||
| Yukon-R | 0.35 ± 0.004 | 2665 ± 227 | 2631 ± 222 | 27 ± 2 | 4090 ± 290 a | 1009 ± 79 a |
| A4177G3-RIB | 0.34 ± 0.004 | 2310 ± 145 | 2288 ± 143 | 22 ± 2 | 3107 ± 258 b | 707 ± 69 b |
| Temp × Geno | ||||||
| 25 °C × Yukon-R | 0.376 ± 0.004 | 4248 ± 495 | 4174 ± 472 | 37 ± 6 | 5711 ± 238 | 1461 ± 58 |
| 20 °C ×Yukon-R | 0.357 ± 0.004 | 2677 ± 276 | 2611 ± 274 | 16 ± 2 | 5351 ± 331 | 1391 ± 86 |
| 15 °C × Yukon-R | 0.347 ± 0.005 | 2915 ± 349 | 2881 ± 345 | 34 ± 4 | 4632 ± 138 | 1119 ± 38 |
| 10 °C × Yukon-R | 0.342 ± 0.016 | 1652 ± 267 | 1630 ± 264 | 21 ± 5 | 2508 ± 211 | 539 ± 41 |
| 5 °C × Yukon-R | 0.343 ± 0.009 | 1833 ± 175 | 1809 ± 173 | 23 ± 2 | 2245 ± 167 | 534 ± 48 |
| 25 °C × A4177G3-RIB | 0.356 ± 0.014 | 3050 ± 419 | 3009 ± 412 | 40 ± 7 | 4971 ± 291 | 1134 ± 68 |
| 20 °C × A4177G3-RIB | 0.343 ± 0.005 | 2732 ± 125 | 2710 ± 125 | 21 ± 3 | 4054 ± 305 | 1075 ± 60 |
| 15 °C × A4177G3-RIB | 0.356 ± 0.008 | 2277 ± 234 | 2251 ± 233 | 26 ± 2 | 3184 ± 174 | 724 ± 41 |
| 10 °C × A4177G3-RIB | 0.357 ± 0.008 | 1937 ± 212 | 1926 ± 211 | 10 ± 1 | 1703 ± 112 | 314 ± 27 |
| 5 °C × A4177G3-RIB | 0.321 ± 0.005 | 1556 ± 181 | 1542 ± 180 | 13 ± 2 | 1621 ± 33 | 288 ± 4 |
| Significance | ||||||
| Temperatures | * | *** | *** | *** | *** | *** |
| Genotypes | NS | NS | NS | NS | *** | *** |
| Temp × Gen | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Nadeem, M.; Galagedara, L.; Thomas, R.; Cheema, M. Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment. Plants 2022, 11, 1217. https://doi.org/10.3390/plants11091217
Wu J, Nadeem M, Galagedara L, Thomas R, Cheema M. Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment. Plants. 2022; 11(9):1217. https://doi.org/10.3390/plants11091217
Chicago/Turabian StyleWu, Jiaxu, Muhammad Nadeem, Lakshman Galagedara, Raymond Thomas, and Mumtaz Cheema. 2022. "Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment" Plants 11, no. 9: 1217. https://doi.org/10.3390/plants11091217
APA StyleWu, J., Nadeem, M., Galagedara, L., Thomas, R., & Cheema, M. (2022). Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment. Plants, 11(9), 1217. https://doi.org/10.3390/plants11091217








