Photosynthetic Parameters and Oxidative Stress during Acclimation of Crepe-Myrtle (Lagerstroemia speciosa (L.) Pers.) in a meta-Topolin-Based Micropropagation System and Genetic Fidelity of Regenerated Plants
Abstract
:1. Introduction
2. Results
2.1. In Vitro Establishment and Optimizing Shoot Proliferation and Rooting of Shoots
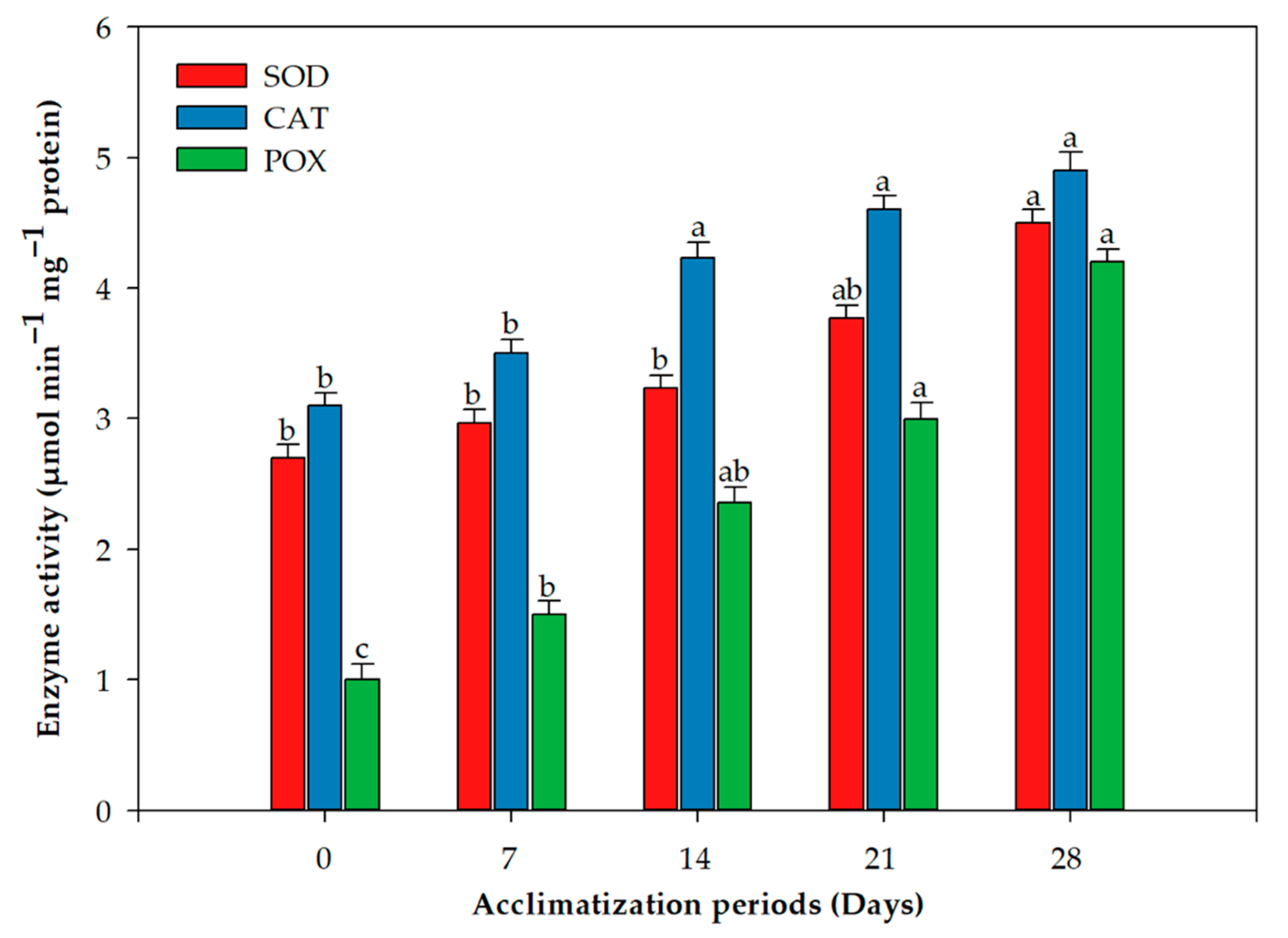
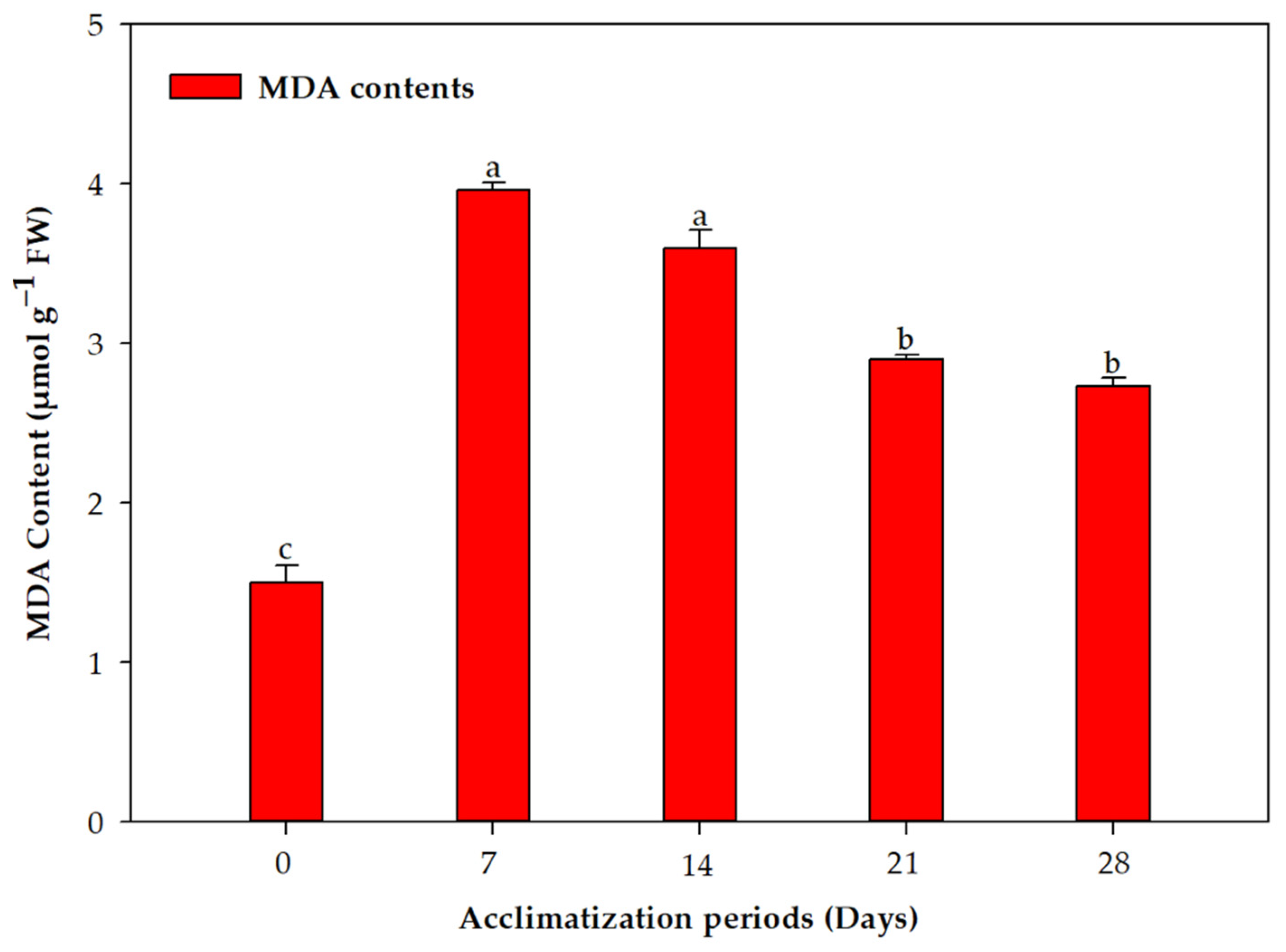
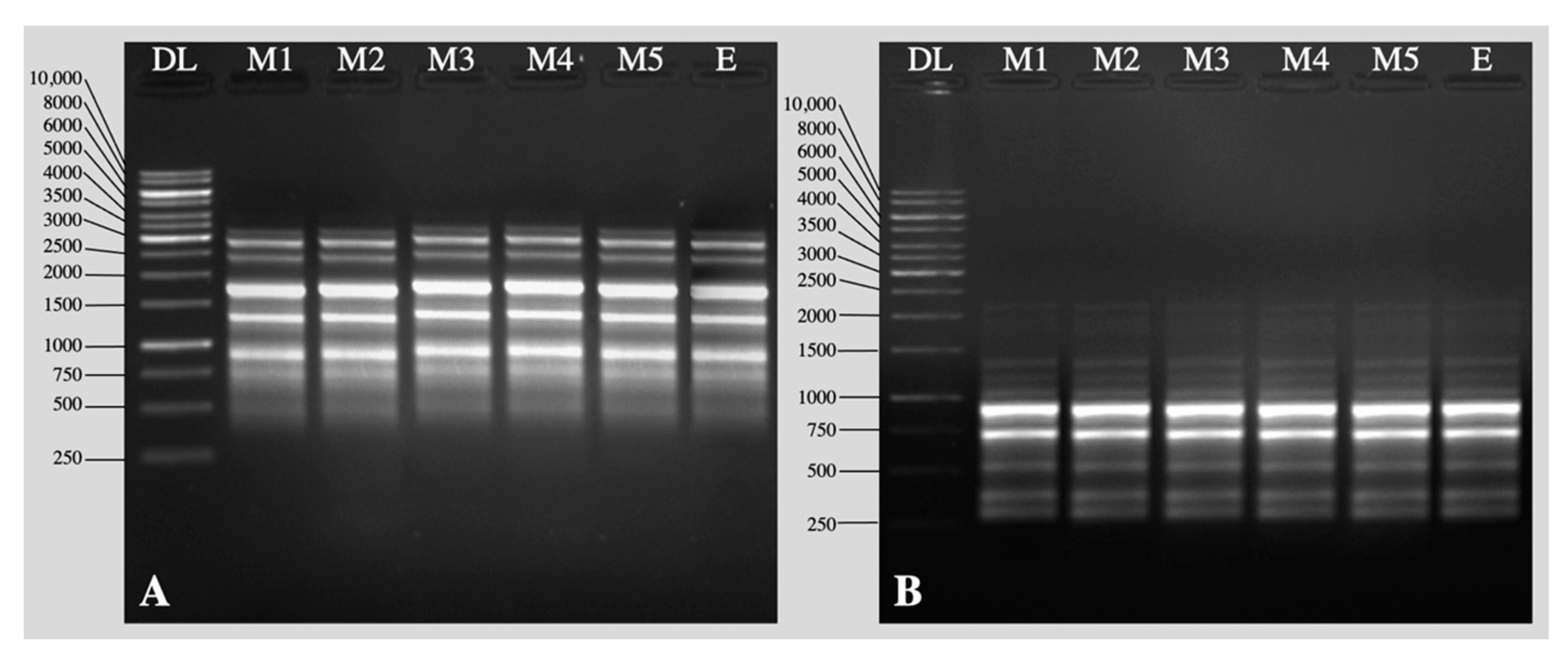
2.2. Acclimation and Assessment of Photosynthetic Pigments, Antioxidative Enzymes and Genetic Fidelity
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Surface Sterilization
4.2. Basal Media and Culture Environment
4.3. Shoot Initiation and Proliferation
4.4. In Vitro Rooting and Plantlet Acclimation
4.5. Photosynthetic Pigments and Net Photosynthetic Rate Assessment
4.6. Extraction and Analysis of Antioxidant Enzymes
4.7. Assessment of Superoxide Dismutase (SOD) Activity
4.8. Catalase (CAT) Activity Assay
4.9. Assessment of Peroxidase (POX) Activity
4.10. Measurement of Lipid Peroxides
4.11. DNA Extraction and Molecular Marker Techniques
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, R.H.; Mai-Hong, T.; Hong, T.D.; Tan, T.T.; Xuan-Chuong, N.D.; Hung, L.Q.; Ngoc-Tam, B.; Le-Tam, V.T. Comparative analysis by protocol and key of seed storage behaviour of sixty Vietnamese tree species. Seed Sci. Technol. 2007, 35, 460–476. [Google Scholar] [CrossRef]
- Sannigrahi, A.K. Biodegradation of leaf litter of tree species in presence of cow dung and earthworms. Indian J. Biotechnol. 2009, 8, 335–338. [Google Scholar]
- Dhakane, M.U.; Yadav, S.G. Pride of India (Lagerstroemia speciosa (L.) Pers.) forming a silver bullet for 21st century. Int. J. For. Crop Improv. 2015, 6, 79–82. [Google Scholar] [CrossRef]
- Deshpande, A.M.; Shirsat, M.K.; Jeyabalan, G. A review of Lagerstroemia speciosa: Pride of India. Int. J. Contemp. Res. Rev. 2018, 9, 20181–20185. [Google Scholar] [CrossRef]
- Hayashi, T.; Maruyama, H.; Kasai, R.; Hattori, K.; Takasuga, S.; Hazeki, O.; Yamasaki, K.; Tanaka, T. Ellagitannins from Lagerstroemia speciosa as activators of glucose transport in fat cells. Planta Med. 2002, 68, 173–175. [Google Scholar] [CrossRef]
- Kotnala, M.; Chakraborthy, G.; Mazumder, A. Lagerstroemia species: A current review. Int. J. PharmTech Res. 2013, 5, 906–909. [Google Scholar]
- Esmail, A.S.A. Medicinal value of Lagerstroemia speciosa: An updated review. Int. J. Curr. Pharm. Res. 2019, 11, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Yamada, K.; Yoshikawa, N.; Nakamura, K.; Haginaka, J.; Kunitomo, M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006, 79, 2474–2479. [Google Scholar] [CrossRef]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.-J.; Ahn, Y.-J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef] [Green Version]
- Laruan, L.M.V.; Balangod, T.; Balangcod, K.; Patacsil, M.; Apastol, O.; Manuel, J.; Cortez, S.; Vallejn, V. Phytochemical and antibacterial study of Lagerstroemia (L.) Pers and its ethno-medicinal importance to indigenous communities of Benquet provinence, Philippines. Indian J. Od Tradit. Knowl. 2013, 12, 379–383. [Google Scholar]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V.; Kumawat, T. In vitro antioxidant studies of Lagerstroemia speciosa leaves. Pharmacogn. J. 2010, 2, 357–360. [Google Scholar] [CrossRef]
- Unno, T.; Sugimoto, A.; Kakuda, T. Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L.) Pers. J. Ethnopharmacol. 2004, 93, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Schumacher, H.R. Gout: An evidence-based review. J. Clin. Rheumatol. 2008, 14, S55–S62. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, W.; Zhou, Y.-Y.; Zhang, Y.-N.; Li, J.-Y.; Hu, L.-H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008, 584, 21–29. [Google Scholar] [CrossRef]
- Judy, W.V.; Hari, S.P.; Stogsdill, W.W.; Judy, J.S.; Naguib, Y.M.A.; Passwater, R. Antidiabetic activity of a standardized extract (Glucosol™) from Lagerstroemia speciosa leaves in Type II diabetics: A dose-dependence study. J. Ethnopharmacol. 2003, 87, 115–117. [Google Scholar] [CrossRef]
- Vijayan, A.; Padmesh Pillai, P.; Hemanthakumar, A.S.; Krishnan, P.N. Improved in vitro propagation, genetic stability and analysis of corosolic acid synthesis in regenerants of Lagerstroemia speciosa (L.) Pers. by HPLC and gene expression profiles. Plant Cell Tissue Organ Cult. 2015, 120, 1209–1214. [Google Scholar] [CrossRef]
- Sivadas, D.; Pandurangan, A.G. Non-viable seed set in Lagerstroemia speciosa (Lythraceae) populations in the Western Ghats, India. Trop. Ecol. 2022, 63, 141–144. [Google Scholar] [CrossRef]
- Hadiuzzaman, S. In vitro cloning from seedling explants of Lagerstroemia speciosa Pers. and L. thorellii Gagnep. Bangladesh J. Bot. 1992, 21, 59–64. [Google Scholar]
- Zobayed, S.M.A. In vitro propagation of Lagerstroemia spp. from nodal explants and gaseous composition in the culture headspace. Environ. Control Biol. 2000, 38, 1–11. [Google Scholar] [CrossRef]
- Lim-Ho, C.L.; Lee, S.K. Micropropagation of Lagerstroemia speciosa (L.) Pers. (Lythraceae). Gard. Bull. Singap. 1985, 38, 175–184. [Google Scholar]
- Strnad, M.; Hanuš, J.; Vaněk, T.; Kamínek, M.; Ballantine, J.A.; Fussell, B.; Hanke, D.E. Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus × canadensis Moench., cv. Robusta). Phytochemistry 1997, 45, 213–218. [Google Scholar] [CrossRef]
- Werbrouck, S.P.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Anis, M.; Alatar, A.A. Meta-topolin improved micropropagation in Syzygium cumini and acclimatization to ex vitro conditions. Biol. Plant. 2019, 63, 174–182. [Google Scholar] [CrossRef]
- Teklehaymanot, T.; Wannakrairoj, S.; Pipattanawong, N. Meta-topolin for pineapple shoot multiplication under three in vitro systems. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 157–162. [Google Scholar]
- Westhuizen, A.V.D. Use of Meta-topolin as an alternative cytokinin in the tissue culture of Eucalyptus species. Acta Hortic. 2014, 1055, 25–28. [Google Scholar] [CrossRef]
- Gentile, A.; Frattarelli, A.; Nota, P.; Condello, E.; Caboni, E. The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell Tissue Organ Cult. 2017, 128, 693–703. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Taylor, N.J. Meta-topolin stimulates de novo shoot organogenesis and plant regeneration in cassava. Plant Cell Tissue Organ Cult. 2018, 132, 219–224. [Google Scholar] [CrossRef]
- Ahmad, A.; Anis, M.; Khanam, M.N.; Alatar, A.A. Direct shoot organogenesis from shoot tip explants of a highly medicinal valued tree Pterocarpus marsupium Roxb. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 670–681. [Google Scholar] [CrossRef]
- Elayaraja, D.; Subramanyam, K.; Vasudevan, V.; Sathish, S.; Kasthurirengan, S.; Ganapathi, A.; Manickavasagam, M. Meta-Topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum (L.). Biocatal. Agric. Biotechnol. 2019, 21, 101320. [Google Scholar] [CrossRef]
- Jayaprakash, K.; Manokari, M.; Badhepuri, M.K.; Raj, M.C.; Dey, A.; Shekhawat, M.S. Influence of meta-topolin on in vitro propagation and foliar micro-morpho-anatomical developments of Oxystelma esculentum (L.f.)Sm. Plant Cell Tissue Organ Cult. 2021, 147, 325–337. [Google Scholar] [CrossRef]
- Manokari, M.; Priyadharshini, S.; Jogam, P.; Dey, A.; Shekhawat, M.S. Meta-topolin and liquid medium mediated enhanced micropropagation via ex vitro rooting in Vanilla planifolia Jacks. ex Andrews. Plant Cell Tissue Organ Cult. 2021, 146, 69–82. [Google Scholar] [CrossRef]
- Kucharska, D.; Orlikowska, T.; Maciorowski, R.; Kunka, M.; Wojcik, D.; Pluta, S. Application of meta-Topolin for improving micropropagation of gooseberry (Ribes grossularia). Sci. Hortic. 2020, 272, 109529. [Google Scholar] [CrossRef]
- Erisen, S.; Kurt-Gur, G.; Servi, H. In vitro propagation of Salvia sclarea L. by meta-Topolin, and assessment of genetic stability and secondary metabolite profiling of micropropagated plants. Ind. Crops Prod. 2020, 157, 112892. [Google Scholar] [CrossRef]
- Saeiahagh, H.; Mousavi, M.; Wiedow, C.; Bassett, H.B.; Pathirana, R. Effect of cytokinins and sucrose concentration on the efficiency of micropropagation of ‘Zes006′ Actinidia chinensis var. chinensis, a red-fleshed kiwifruit cultivar. Plant Cell Tissue Organ Cult. 2019, 138, 1–10. [Google Scholar] [CrossRef]
- Debenham, M.; Pathirana, R. Establishment and management of an in vitro repository of kiwifruit (Actinidia spp.) germplasm. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Ahmad, N., Strnad, M., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 279–291. [Google Scholar]
- Nowakowska, K.; Pacholczak, A. Comparison of the effect of meta-Topolin and benzyladenine during Daphne mezereum L. micropropagation. Agronomy 2020, 10, 1994. [Google Scholar] [CrossRef]
- Plíhal, O.; Szüčová, L.; Galuszka, P. N9-substituted aromatic cytokinins with negligible side effects on root development are an emerging tool for in vitro culturing. Plant Signal. Behav. 2013, 8, e24392. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, M.S.; Priyadharshini, S.; Jogam, P.; Kumar, V.; Manokari, M. Meta-topolin and liquid medium enhanced in vitro regeneration in Scaevola taccada (Gaertn.) Roxb. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 296–306. [Google Scholar] [CrossRef]
- Pramanik, B.; Sarkar, S.; Bhattacharyya, S.; Gantait, S. meta-Topolin-induced enhanced biomass production via direct and indirect regeneration, synthetic seed production, and genetic fidelity assessment of Bacopa monnieri (L.) Pennell, a memory-booster plant. Acta Physiol. Plant. 2021, 43, 107. [Google Scholar] [CrossRef]
- Gladfelter, H.J.; Johnston, J.; Wilde, H.D.; Merkle, S.A. Adventitious shoot-based propagation of Franklinia alatamaha for commercial horticulture and restoration. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 857–864. [Google Scholar] [CrossRef]
- Gentile, A.; Gutierrez, M.J.; Martinez, J.; Frattarelli, A.; Nota, P.; Caboni, E. Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell Tissue Organ Cult. 2014, 118, 373–381. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Valero-Aracama, C.; Kane, M.E.; Wilson, S.B.; Philman, N.L. Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul. 2009, 60, 43–49. [Google Scholar] [CrossRef]
- Podlesakova, K.; Zalabak, D.; Cudejkova, M.; Plihal, O.; Szucova, L.; Dolezal, K.; Spichal, L.; Strnad, M.; Galuszka, P. Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS ONE 2012, 7, e39293. [Google Scholar] [CrossRef]
- Vylíčilová, H.; Bryksová, M.; Matušková, V.; Doležal, K.; Plíhalová, L.; Strnad, M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules 2020, 10, 832. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Dolezal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Cassells, A.C.; Curry, R.F. Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: Implications for micropropagators and genetic engineers. Plant Cell Tissue Organ Cult. 2001, 64, 145–157. [Google Scholar] [CrossRef]
- Şen, A. Oxidative stress studies in plant tissue culture. Antioxid. Enzym. 2012, 3, 59–88. [Google Scholar]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult. 2020, 140, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Benson, E.E. Do free radicals have a role in plant tissue culture recalcitrance? Vitr. Cell. Dev. Biol. Plant 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hou, P.; Su, X.; Zhao, P.; Zhao, H.; Liu, S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul. 2014, 73, 289–297. [Google Scholar] [CrossRef]
- Jeon, M.-W.; Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of photon flux density on the morphology, photosynthesis and growth of a CAM orchid, Doritaenopsis during post-micropropagation acclimatization. Plant Growth Regul. 2005, 45, 139–147. [Google Scholar] [CrossRef]
- Goncalves, S.; Martins, N.; Romano, A. Physiological traits and oxidative stress markers during acclimatization of micropropagated plants from two endangered Plantago species: P. algarbiensis Samp. and P. almogravensis Franco. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 249–255. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shahzad, A.; Sharma, S.; Parveen, S. Ex vitro rescue, physiochemical evaluation, secondary metabolite production and assessment of genetic stability using DNA based molecular markers in regenerated plants of Decalepis salicifolia (Bedd. ex Hook.f.) Venter. Plant Cell Tissue Organ Cult. 2018, 132, 497–510. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szucova, L.; Dolezal, K.; Finnie, J.F.; Van Staden, J. Genetic fidelity in tissue-cultured ‘Williams’ bananas-The effect of high concentration of topolins and benzyladenine. Sci. Hortic. 2013, 161, 324–327. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Doležal, K.; van Staden, J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ (Musa spp. AAA). Plant Cell Tissue Organ Cult. 2008, 95, 373–379. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Anis, M.; Ahmad, I. An improved in vitro encapsulation protocol, biochemical analysis and genetic integrity using DNA based molecular markers in regenerated plants of Withania somnifera L. Ind. Crops Prod. 2013, 50, 468–477. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crops Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: A potential medicinal plant. Agrofor. Syst. 2017, 91, 637–647. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mackinney, G.R. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Wienheim, Germany, 1974; Volume 1, pp. 481–482. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Ed.; NATO AS1 Series; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar]


| meta-Topolin Concentration (µM) | 4 Weeks | 8 Weeks | 12 Weeks | |||
|---|---|---|---|---|---|---|
| Number of Shoots | Shoot Length (cm) | Number of Shoots | Shoot Length (cm) | Number of Shoots | Shoot Length (cm) | |
| 0.0 | 0.00 ± 0.00 j | 0.00 ± 0.00 g | 0.00 ± 0.00 i | 0.00 ± 0.00 f | 0.00 ± 0.00 j | 0.00 ± 0.00 f |
| 0.1 | 2.04 ± 0.04 i | 0.88 ± 0.02 f | 5.20 ± 0.00 h | 0.92 ± 0.16 e | 6.85 ± 0.03 i | 1.60 ± 0.10 e |
| 0.5 | 3.20 ± 0.20 h | 1.32± 0.08 e | 7.62 ± 0.17 e | 1.62 ± 0.18 d | 10.2 ± 0.18 h | 3.36 ± 0.16 d |
| 1.0 | 4.36 ± 0.36 g | 2.50 ± 0.00 c | 12.8 ±0.04 c | 2.70 ± 0.33 bc | 14.1 ± 0.10 f | 3.86 ± 0.02 e |
| 2.5 | 8.10 ± 0.10 d | 3.20 ± 0.20 b | 14.4 ± 0.18 b | 3.08 ± 0.50 b | 18.5 ± 0.15 c | 4.60 ± 0.00 b |
| 5.0 | 12.0 ± 0.02 a | 3.76 ± 0.06 a | 16.4± 0.12 a | 4.10 ± 0.10 a | 22.4 ± 0.02 a | 5.22 ± 0.00 a |
| 7.5 | 10.0 ± 0.00 b | 2.52 ± 0.11 c | 11.5 ± 0.15 d | 3.12 ± 0.09 b | 20.2 ± 0.20 b | 4.84 ± 0.08 b |
| 10.0 | 9.16 ± 0.04 c | 2.00 ± 0.00 d | 6.50 ± 0.00 f | 2.30 ± 0.08 cd | 15.6 ± 0.10 d | 4.40 ± 0.04 b |
| 12.5 | 7.10 ± 0.10 e | 1.20 ± 0.00 e | 5.60 ± 0.17 g | 1.50 ± 0.00 de | 14.7 ± 0.06 e | 3.80 ± 0.40 c |
| 15.0 | 5.00 ± 0.00 f | 0.80 ± 0.00 f | 5.00 ± 0.00 h | 1.20 ± 0.20 de | 11.4 ± 0.04 g | 2.96 ± 0.06 d |
| Plant Growth Regulators (µM) | % Regeneration | Number of Shoots/Explant | Mean Shoot Length (cm) | ||
|---|---|---|---|---|---|
| IAA | IBA | NAA | |||
| 0.1 | 70 | 12.5 ± 0.19 gh | 4.40 ± 0.40 ef | ||
| 0.5 | 75 | 14.4 ± 0.40 e | 5.00 ± 0.00 cd | ||
| 1.0 | 60 | 8.70 ± 0.12 j | 4.92 ± 0.02 cd | ||
| 1.5 | 70 | 8.00 ± 0.00 k | 4.20 ± 0.00 efg | ||
| 2.0 | 65 | 7.48 ± 0.12 l | 3.92 ± 0.02 g | ||
| 0.1 | 80 | 13.6 ± 0.08 f | 4.68 ± 0.08 de | ||
| 0.5 | 85 | 16.0 ± 0.00 d | 5.22 ± 0.02 bc | ||
| 1.0 | 75 | 14.6 ± 0.16 e | 4.34 ± 0.04 ef | ||
| 1.5 | 60 | 12.9 ± 0.31 g | 3.84 ± 0.05 g | ||
| 2.0 | 60 | 10.0 ± 0.00 i | 2.60 ± 0.10 h | ||
| 0.1 | 85 | 17.5 ± 0.36 c | 5.16 ± 0.05 bc | ||
| 0.5 | 95 | 24.3 ± 0.19 a | 5.74 ± 0.06 a | ||
| 1.0 | 85 | 18.2 ± 0.20 b | 5.50 ± 0.00 ab | ||
| 1.5 | 80 | 15.8 ± 0.30 d | 5.20 ± 0.20 bc | ||
| 2.0 | 75 | 12.0 ± 0.00 h | 5.04 ± 0.24 cd | ||
| Plant Growth Regulators (µM) | % Shoots with Roots | Number of Roots Per Microshoot | Root Length (cm) | ||
|---|---|---|---|---|---|
| IAA | IBA | NAA | |||
| 0.0 | 00 | 0.00 ± 0.00 k | 0.00 ± 0.00 h | ||
| 0.5 | 85 | 3.44 ± 0.14 g | 2.46 ± 0.06 b | ||
| 1.0 | 85 | 4.36 ± 0.14 f | 2.51 ± 0.22 b | ||
| 1.5 | 70 | 3.84 ± 0.04 gh | 2.02 ± 0.12 d | ||
| 2.0 | 65 | 3.24 ± 0.06 h | 1.98 ± 0.12 cd | ||
| 2.5 | 60 | 2.60 ± 0.10 ij | 1.60 ± 0.10 ef | ||
| 0.5 | 65 | 7.36 ± 0.16 c | 2.20 ± 0.20 bc | ||
| 1.0 | 93 | 10.3 ± 0.12 a | 3.56 ± 0.05 a | ||
| 1.5 | 70 | 8.20 ± 0.20 b | 1.76 ± 0.06 de | ||
| 2.0 | 60 | 6.52 ± 0.21 d | 1.54 ± 0.04 ef | ||
| 2.5 | 50 | 3.20 ± 0.20 h | 1.36 ± 0.16 f | ||
| 0.5 | 70 | 5.69 ± 0.22 e | 1.78 ± 0.12 de | ||
| 1.0 | 75 | 7.24 ± 0.26 c | 2.24 ± 0.07 bc | ||
| 1.5 | 65 | 6.10 ± 0.04 de | 2.08 ± 0.20 cd | ||
| 2.0 | 65 | 2.72 ± 0.13 i | 1.54 ± 0.05 ef | ||
| 2.5 | 55 | 2.20 ± 0.00 j | 0.84 ± 0.04 g | ||
| S. No. | Kit A | ||
|---|---|---|---|
| Primers | Primers Sequences (5′-3′) | No. of Bands | |
| 1 | OPA 01 | GTTTCGCTCG | 9 |
| 2 | OPA 02 | TGATCCCTGG | 11 |
| 3 | OPA 03 | CATCCCCCTG | 8 |
| 4 | OPA 04 | GGACTGGAGT | 10 |
| 5 | OPA 05 | TGCGCCCTTC | 6 |
| 6 | OPA 06 | TGCTCTGCCC | 3 |
| 7 | OPA 07 | GGTGACGCAG | 10 |
| 8 | OPA 08 | GTCCACACGG | 11 |
| 9 | OPA 09 | TGGGGGACTC | 9 |
| 10 | OPA 10 | CTGCTGGGAC | 9 |
| 11 | OPA 11 | GTAGACCCGT | 8 |
| 12 | OPA 12 | CCTTGACGCA | 5 |
| 13 | OPA 13 | TTCCCCCGCT | 3 |
| 14 | OPA 14 | TCCGCTCTGG | Nil |
| 15 | OPA 15 | GGAGGGTGTT | 3 |
| 16 | OPA 16 | TTTGCCCGGA | 3 |
| 17 | OPA 17 | AGGGAACGAG | 9 |
| 18 | OPA 18 | CCACAGCAGT | 7 |
| 19 | OPA 19 | ACCCCCGAAG | 3 |
| 20 | OPA 20 | GGACCCTTAC | Nil |
| S. No. | Primers | Primers Sequences (5′-3′) | Annealing Temperature (°C) | No. of Bands |
|---|---|---|---|---|
| 1 | UBC-801 | (AT)8T | 35.0 | 3 |
| 2 | UBC-811 | (GA)8C | 49.0 | 5 |
| 3 | UBC-825 | (AC)8T | 45.7 | Nil |
| 4 | UBC-827 | (AC)8G | 49.0 | Nil |
| 5 | UBC-834 | (AG)8YT | 49.0 | 9 |
| 6 | UBC-841 | (GA)8YC | 49.0 | Nil |
| 7 | UBC-855 | (AC)8YT | 49.0 | 6 |
| 8 | UBC-866 | (CTC)6 | 55.4 | Nil |
| 9 | UBC-868 | (GAA)6 | 45.7 | 2 |
| 10 | UBC-880 | (GGGGT)3G | 49.0 | 12 |
| 11 | UBC-889 | DBDA(CA)6C | 45.7 | 13 |
| 12 | UBC-891 | HVHT (GT)6G | 45.7 | 10 |
| 13 | UBC-900 | ACTTCCCCACAGGTTAACAC | 58.0 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Fatima, N.; Faisal, M.; Alatar, A.A.; Pathirana, R. Photosynthetic Parameters and Oxidative Stress during Acclimation of Crepe-Myrtle (Lagerstroemia speciosa (L.) Pers.) in a meta-Topolin-Based Micropropagation System and Genetic Fidelity of Regenerated Plants. Plants 2022, 11, 1163. https://doi.org/10.3390/plants11091163
Ahmad N, Fatima N, Faisal M, Alatar AA, Pathirana R. Photosynthetic Parameters and Oxidative Stress during Acclimation of Crepe-Myrtle (Lagerstroemia speciosa (L.) Pers.) in a meta-Topolin-Based Micropropagation System and Genetic Fidelity of Regenerated Plants. Plants. 2022; 11(9):1163. https://doi.org/10.3390/plants11091163
Chicago/Turabian StyleAhmad, Naseem, Nigar Fatima, Mohammad Faisal, Abdulrahman A. Alatar, and Ranjith Pathirana. 2022. "Photosynthetic Parameters and Oxidative Stress during Acclimation of Crepe-Myrtle (Lagerstroemia speciosa (L.) Pers.) in a meta-Topolin-Based Micropropagation System and Genetic Fidelity of Regenerated Plants" Plants 11, no. 9: 1163. https://doi.org/10.3390/plants11091163
APA StyleAhmad, N., Fatima, N., Faisal, M., Alatar, A. A., & Pathirana, R. (2022). Photosynthetic Parameters and Oxidative Stress during Acclimation of Crepe-Myrtle (Lagerstroemia speciosa (L.) Pers.) in a meta-Topolin-Based Micropropagation System and Genetic Fidelity of Regenerated Plants. Plants, 11(9), 1163. https://doi.org/10.3390/plants11091163








