Synergistic Modulation of Seed Metabolites and Enzymatic Antioxidants Tweaks Moisture Stress Tolerance in Non-Cultivated Traditional Rice Genotypes during Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Germination Rate (GR) and Vigor index (VI)
2.3. Relative Water Content (RWC)
2.4. Seed Carbohydrates and Protein
2.4.1. Starch
2.4.2. Protein
2.4.3. Total Free Amino Acids
2.5. Quantitative Assay of Hydrolytic Enzymes
2.5.1. Alpha-Amylase
2.5.2. Protease
2.5.3. Lipase
2.6. Determination of Endogenous Phytohormones
2.6.1. Indole Acetic Acid (IAA)
2.6.2. Giberellic Acid (GA)
2.6.3. Quantification of IAA, GA, ABA and Cytokinin, Using HPLC
2.7. Estimation of Seed Proline Content
2.8. Determination of Antioxidant Enzymes
2.9. Statistical Analysis
3. Results
3.1. Physiological Changes under Moisture Stress
3.2. Remobilization of Seed Macromolecules in the Germinated Seeds under Moisture Stress
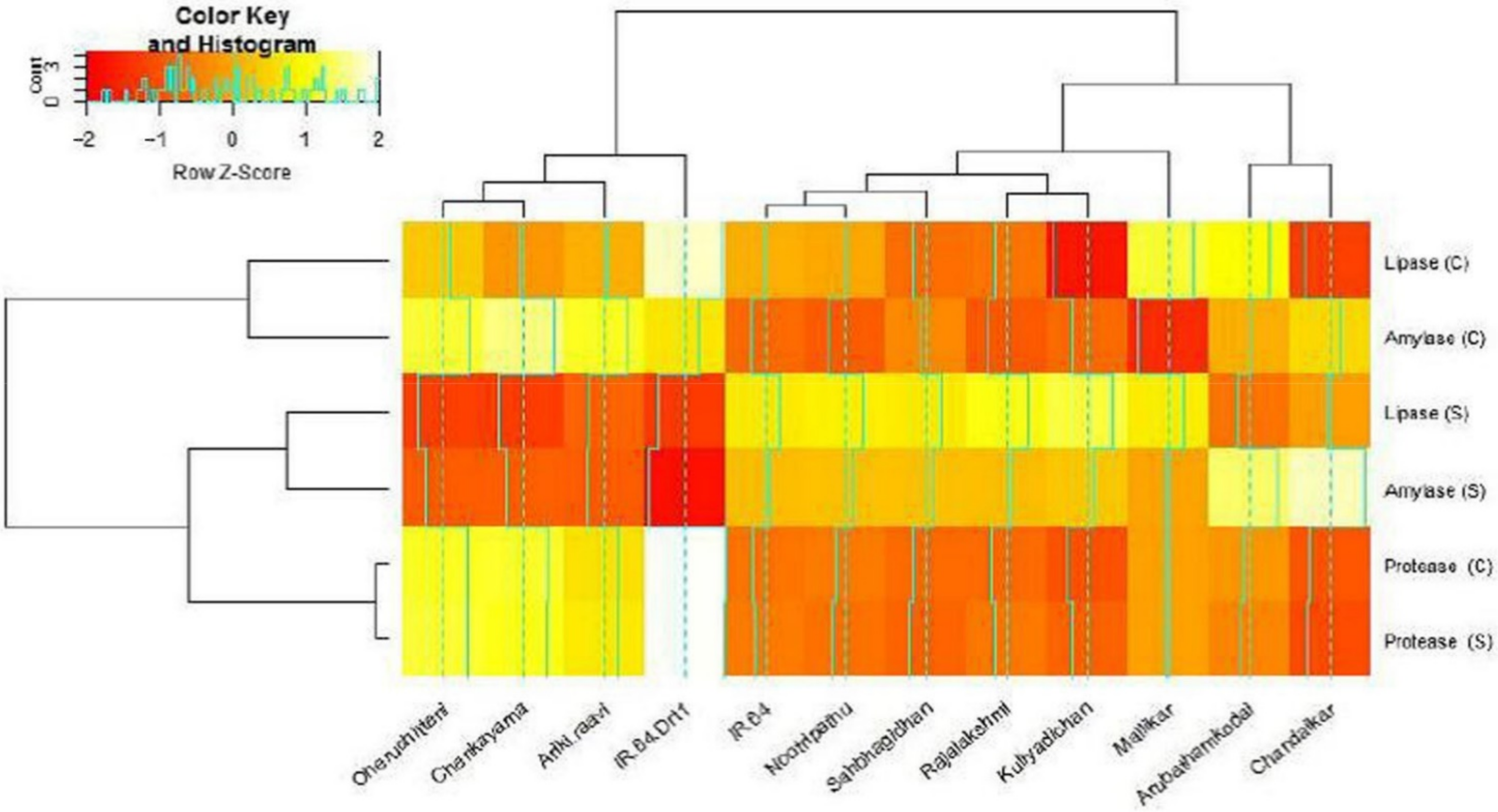
3.3. Hydrolytic Enzyme Activities under Moisture Stress
3.4. Endogenous Hormone Modulation in the Germinated Seeds under Moisture Stress
3.5. Antioxidant Enzymes Triggered under Moisture Stress
3.6. Remodeling Osmolytes during Germination under Moisture Stress
3.7. Principle Component Analysis for Physiological and Metabolic Responses in Rice Genotypes during Germination Influenced by Varying Levels of Moisture Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO News Archive. 2011. Available online: http://www.fao.org/news/archive/news-by-date/2011/en/ (accessed on 12 June 2021).
- Ahmadi, N.; Audebert, A.; Bennett, M.J.; Bishopp, A.; de Oliveira, A.C.; Courtois, B.; Diedhiou, A.; Diévart, A.; Gantet, P.; Ghesquière, A. The roots of future rice harvests. Rice 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of moisture stress and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [PubMed]
- Basnayake, J.; Fukai, S.; Ouk, M. Contribution of Potential Yield, Moisture Stress Tolerance and Escape to Adaptation of 15 Rice Varieties in Rainfed Lowlands in Cambodia, Proc of the Australian Agron Conf; Australian Society of Agronomy: Birsbane, Australia, 2006. [Google Scholar]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root Response to moisture stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Ali, A.S.E.A. Metabolic processes during seed germination. In Advances in Seed Biology; IntechOpen: London, UK, 2017; pp. 141–166. [Google Scholar]
- Krasensky, J.; Jonak, C. Moisture stress, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Mayer, A.M.; Poljakoff-Mayber, A. The Germination of Seeds; Pergamon Press: Oxford, UK; New York, NY, USA, 1989. [Google Scholar]
- Salisbury, F.; Ross, C. Mineral nutrition. Plant Physiol. 1991, 1, 116–135. [Google Scholar]
- Kohno, A.; Nanmori, T. Changes in α and β-Amylase Activities during Germination of Seeds of Alfalfa (Medicago sativa L.). Plant Cell Physiol. 1991, 32, 459–466. [Google Scholar]
- Van der Hoorn, R.A. Plant proteases: From phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef]
- Martinez, M.; Cambra, I.; Carrillo, L.; Diaz-Mendoza, M.; Diaz, I. Characterization of the entire cystatin gene family in barley and their target cathepsin L-like cysteine-proteases, partners in the hordein mobilization during seed germination. Plant Physiol. 2009, 151, 1531–1545. [Google Scholar] [CrossRef]
- Zhang, N.; Jones, B.L. Development of proteolytic activities during barley malting and their localization in the green malt kernel. J. Cereal Sci. 1995, 22, 147–155. [Google Scholar] [CrossRef]
- Cejudo, F.J.; Gonzalez, M.C.; Serrato, A.J.; Sanchez, R.; Dominguez, F. Function and hormonal control of proteases in cereal grains. In Recent Research Development in Plant Physiology; Nature: London, UK, 2001; pp. 57–65. [Google Scholar]
- Vijayakumar, K.; Gowda, L.R. Temporal expression profiling of lipase during germination and rice caryopsis development. Plant Physiol. Biochem. 2012, 57, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Nie, L.; Chen, Y.; Wu, C.; Xiong, D.; Saud, S.; Hongyan, L.; Cui, K.; Huang, J. Crop plant hormones and environmental stress. In Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2015; pp. 371–400. [Google Scholar]
- Donga, S.; Becklesa, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Voß, U.; Bishopp, A.; Farcot, E.; Bennett, M.J. Modelling hormonal response and development. Trends Plant Sci. 2014, 19, 311–319. [Google Scholar] [CrossRef]
- Debeaujon, I.; Koornneef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Kumar, A.; Vijayalakshmi, C.; Vijayalakshmi, D. Osmolyte accumulation, membrane stability and ABA profiles in rice genotypes exposed to heat and moisture stress. Int. J. Bio-Resour. Stress Manag. 2015, 6, 117–122. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and moisture stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 6. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Clegg, K. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1956, 7, 40–44. [Google Scholar] [CrossRef]
- Yemn, E.; Willis, A. The estimation of carbohydrate in plant extracts by anthrone. Biochem. J. 1954, 57, 504–514. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced moisture stress on seed germination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Harvey, B.; Oaks, A. Characteristics of an acid protease from maize endosperm. Plant Physiol. 1974, 53, 449–452. [Google Scholar] [CrossRef][Green Version]
- Malik, S.V.; Kalia, V.; Pundir, C. Immobilization of porcine pancreas lipase on zirconia coated alkylamine glass using glutaraldehyde. Indian J. Chem. Technol. 2000, 7, 64–67. [Google Scholar]
- Andreae, W.; Van Ysselstein, M. Studies on 3-indoleacetic acid metabolism. III. The uptake of 3-indoleacetic acid by pea epicotyls and its conversion to 3-indoleacetylaspartic acid. Plant Physiol. 1956, 31, 235. [Google Scholar] [CrossRef]
- Almeida, T.M.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithöfer, A. Validated method for phytohormone quantification in plants. Front. Plant Sci. 2014, 5, 417. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 2019, 77, 265–278. [Google Scholar] [CrossRef]
- Nagar, P. Changes in absicisic acid, phenols and indole acetic acid in bulbs of tuberose (Polianthes tuberosa L.) during dormancy and sprouting. Sci. Horti. 1995, 63, 77–82. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Azevedo, R.; Alas, R.; Smith, R.; Lea, P. Responce of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to moisture stress stress. Flood Res. 2016, 5, 1554. [Google Scholar]
- Soleymani, A.; Shahrajabian, M.H. Study of cold stress on the germination and seedling stage and determination of recovery in rice varieties. Int. J. Biol. 2012, 4, 23. [Google Scholar] [CrossRef]
- Gampala, S.; Singh, V.J.; Chakraborti, S.; Vishwajith, K.; Manjunath, G. Genotypic differences against poly ethylene glycol (PEG) simulated moisture stress stress in rice. Green Farm. 2015, 6, 117–121. [Google Scholar]
- Mishra, S.S.; Panda, D. Leaf traits and antioxidant defense for moisture stress tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Casanas, F.; Simo, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Moisture stress stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Govindaraj, M.; Shanmugasundaram, P.; Sumathi, P.; Muthiah, A. Simple, rapid and cost effective screening method for moisture stress resistant breeding in pearl millet. Electron. J. Plant Breed. 2010, 1, 590–599. [Google Scholar]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Moisture stress stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Swapna, S.; Shylaraj, K.S. Screening for osmotic stress responses in rice varieties under moisture stress condition. Rice Sci. 2017, 24, 253–263. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell. 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Rook, F.; Hadingham, S.A.; Li, Y.; Bevan, M.W. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 2006, 29, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cruz, J.; Pastenes, C. Water-stress-induced thermotolerance of photosynthesis in bean (Phaseolus vulgaris L.) plants: The possible involvement of lipid composition and xanthophyll cycle pigments. Environ. Exp. Bot. 2012, 77, 127–140. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to moisture stress and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Tawfik, K.; Abd El-Gawad, Z. Tolerance of seven faba bean varieties to moisture stress and salt stresses. Res. J. Agric. Biol. Sci. 2008, 4, 175–186. [Google Scholar]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Liang, H. Effect on plumular axis on the amylase activity in cotyledons of germinating pea seeds. Acta Phytophysiol. Sin. 1988, 14, 244–249. [Google Scholar]
- Kondhare, K.; Farrell, A.; Kettlewell, P.; Hedden, P.; Monaghan, J. Pre-maturity α-amylase in wheat: The role of abscisic acid and gibberellin. J. Cereal Sci. 2015, 63, 95–108. [Google Scholar] [CrossRef]
- Grudkowska, M.; Zagdańska, B. Multifunctional role of plant cysteine proteinases. Acta Biochim. Pol. 2004, 51, 609–624. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects moisture stress and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved moisture stress stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Carrera, E.; Holman, T.; Medhurst, A.; Dietrich, D.; Footitt, S.; Theodoulou, F.L.; Holdsworth, M.J. Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J. 2008, 53, 214–224. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Schopfer, P.; Plachy, C. Control of seed germination by Abscisic Acid: III. Effect on embryo growth potential (Minimum Turgor Pressure) and growth coefficient (Cell Wall Extensibility) in Brassica napus L. Plant Physiol. 1985, 77, 676–686. [Google Scholar] [CrossRef]
- Bhaskar, R.V.; Mohanty, B.; Verma, V.; Wijaya, E.; Kumar, P.P. A hormone-responsive C1-domain-containing protein At5g17960 mediates stress response in Arabidopsis thaliana. PLoS ONE 2015, 10, e0115418. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. CR Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Cembrowska-Lech, D.; Koprowski, M.; Kępczyński, J. Germination induction of dormant Avena fatua caryopses by KAR1 and GA3 involving the control of reactive oxygen species (H2O2 and O2−) and enzymatic antioxidants (superoxide dismutase and catalase) both in the embryo and the aleurone layers. J. Plant Physiol. 2015, 176, 169–179. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Y.; Li, X.; Han, W.Y.; Chen, S. Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J. Plant Growth Regul. 2018, 37, 1349–1356. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Aoki, N.; Kasa, S.; Sakamoto, M.; Kai, K.; Tomokiyo, R.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M. The interrelationship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination. Front. Plant Sci. 2017, 8, 275. [Google Scholar] [CrossRef]
- Kai, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M.; Ishibashi, Y. Role of reactive oxygen species produced by NADPH oxidase in gibberellin biosynthesis during barley seed germination. Plant Signal. Behav. 2016, 11, e1180492. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Zhang, Y.; Lin, C.; Gong, D.; Guan, Y.; Hu, J. Reactive oxygen species and gibberellin acid mutual induction to regulate tobacco seed germination. Front. Plant Sci. 2018, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yokoya, S. Enhanced tolerance to moisture stress stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17. 7. Plant Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sengar, K.; Sengar, R. Gene regulation and biotechnology of moisture stress tolerance in rice. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 547–552. [Google Scholar]
- Mishra, S.S.; Behera, P.K.; Panda, D. Genotypic variability for moisture stress tolerance-related morpho-physiological traits among indigenous rice landraces of Jeypore tract of Odisha, India. J. Crop Improv. 2019, 33, 254–278. [Google Scholar] [CrossRef]
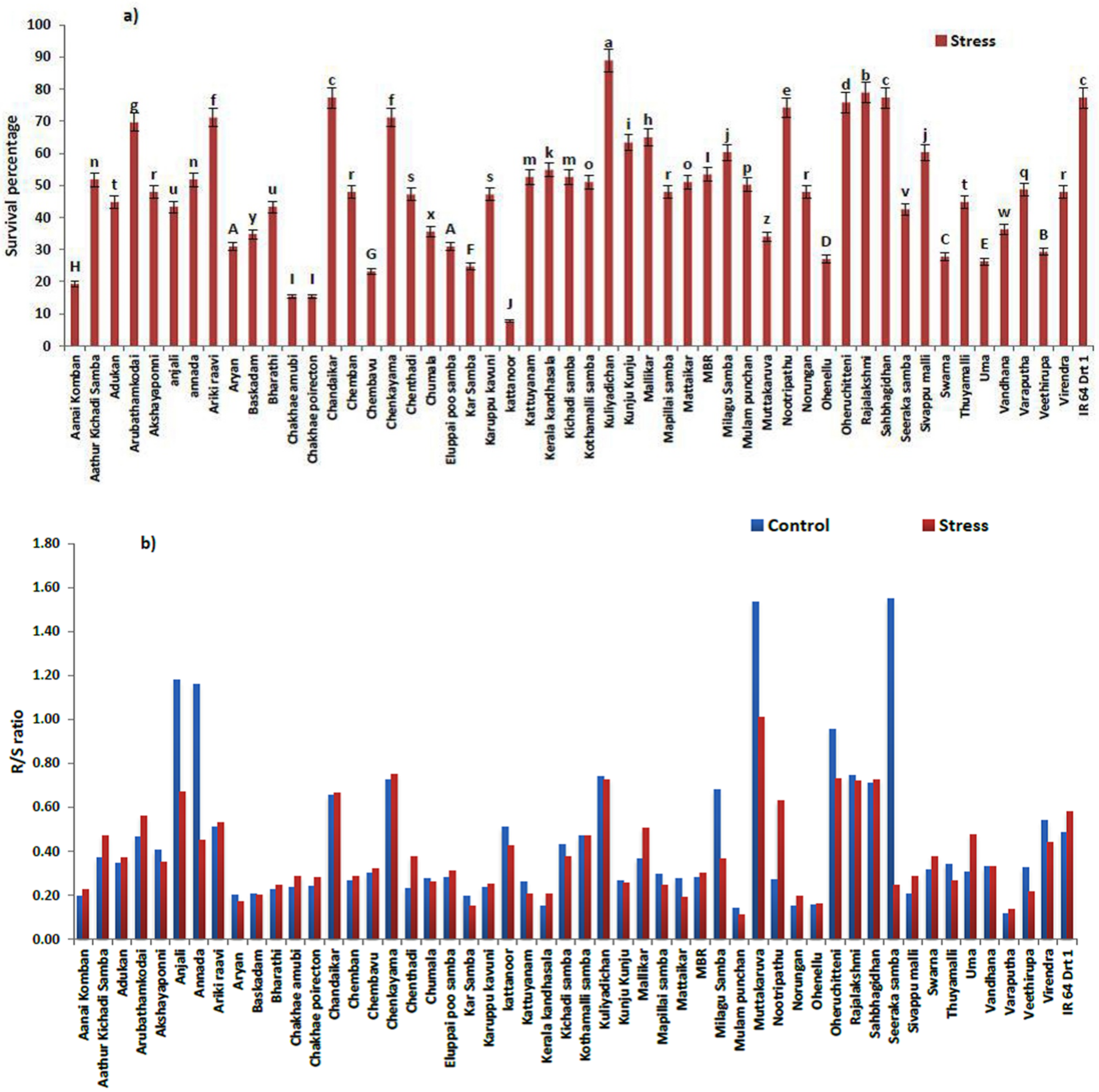
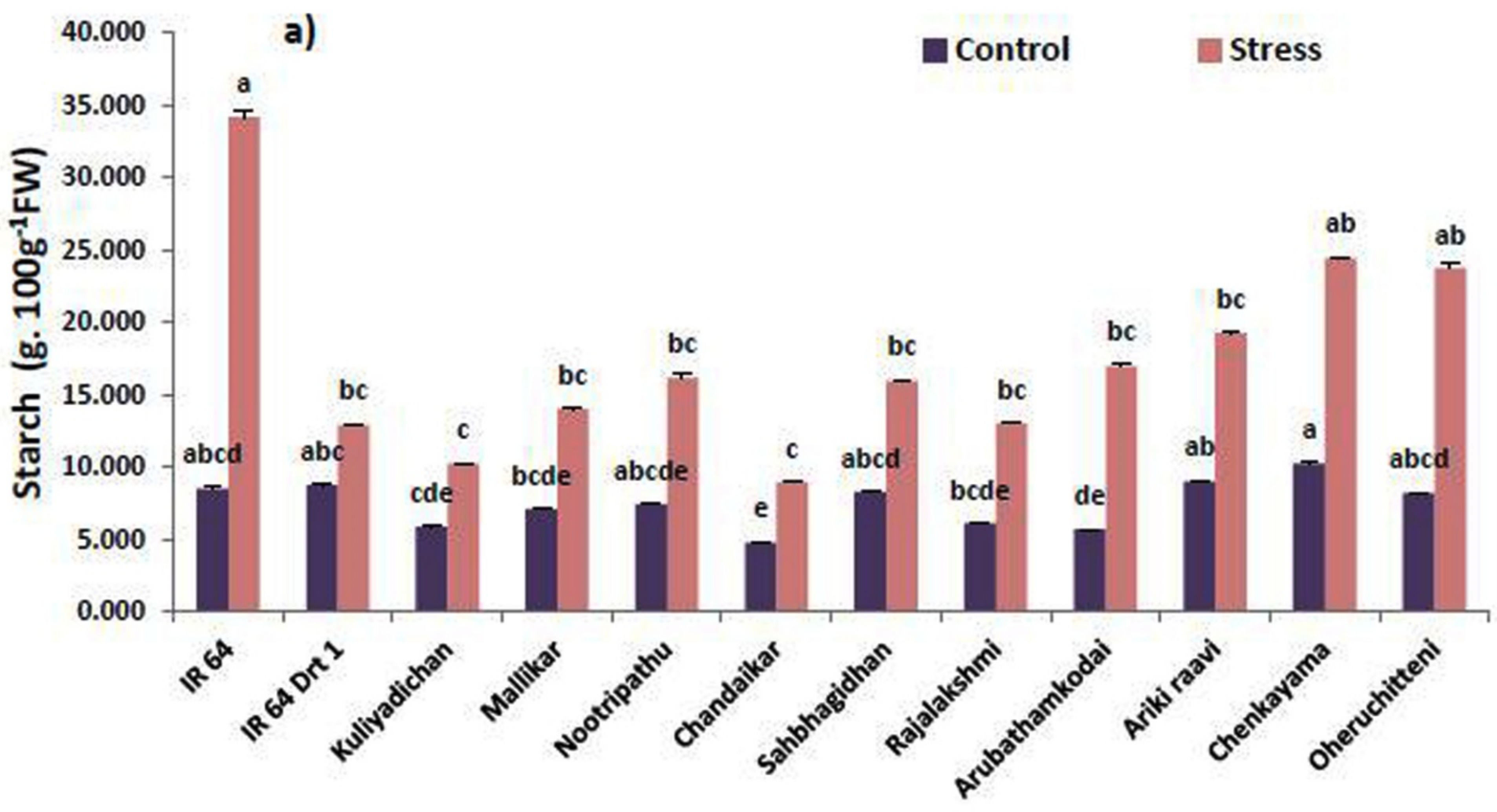
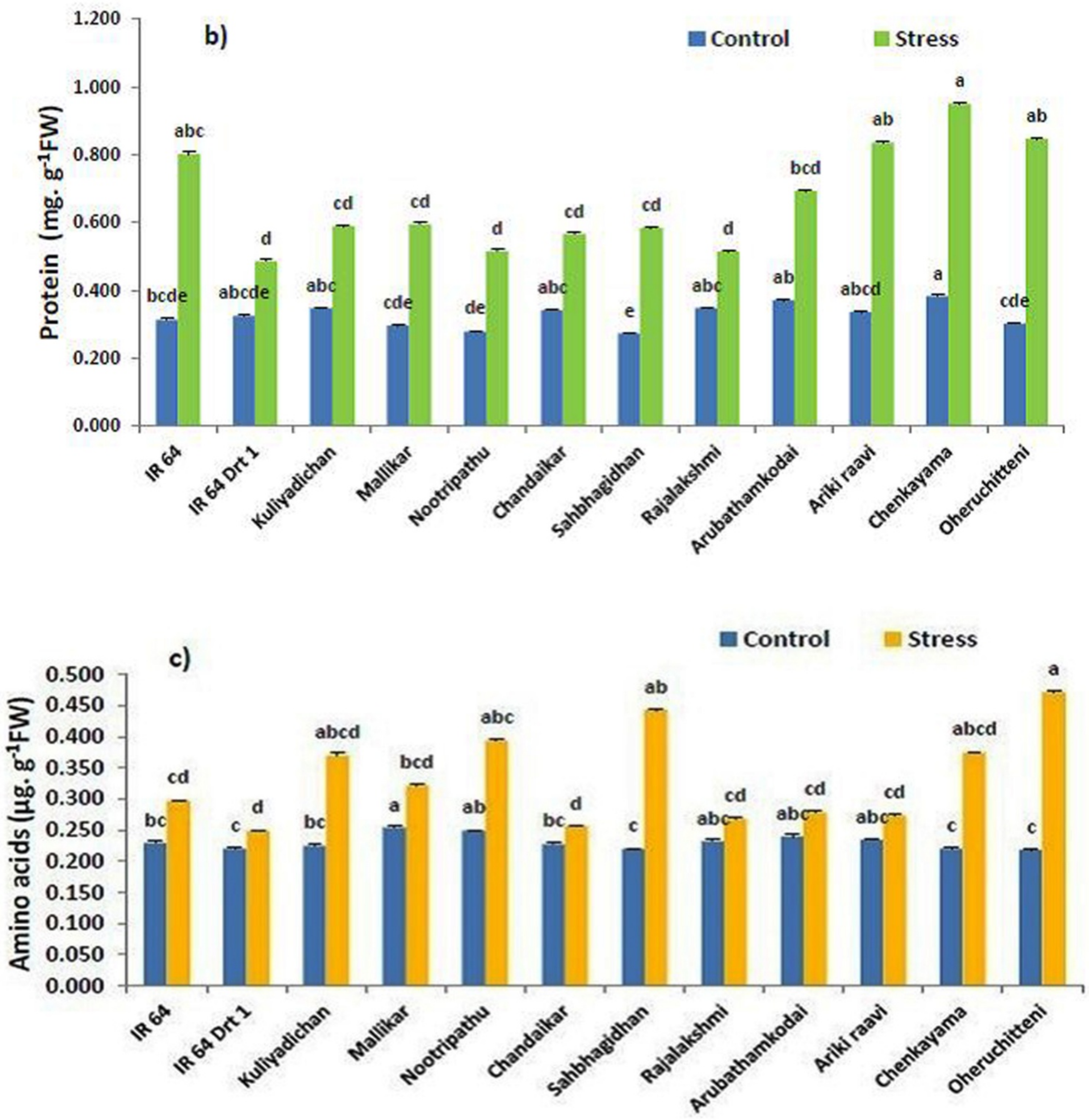



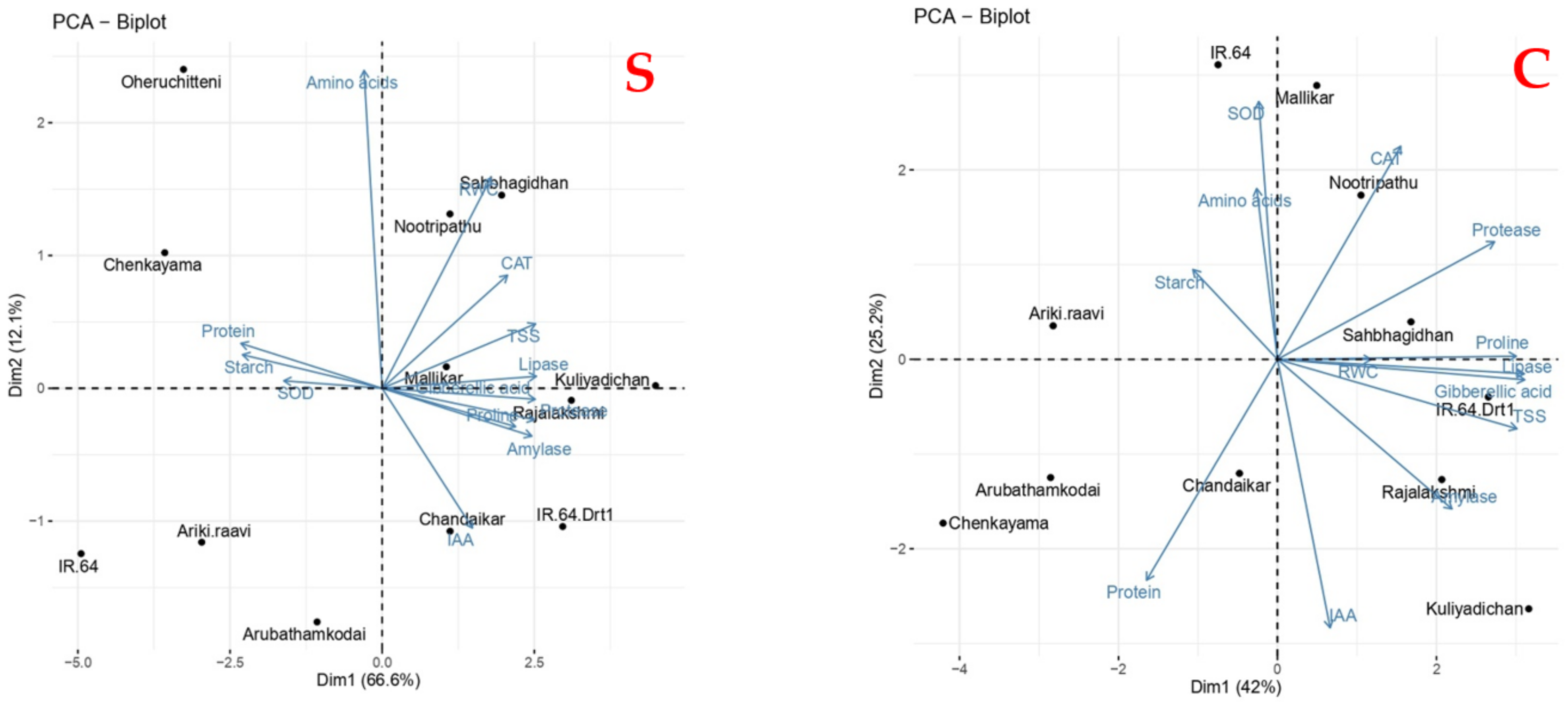
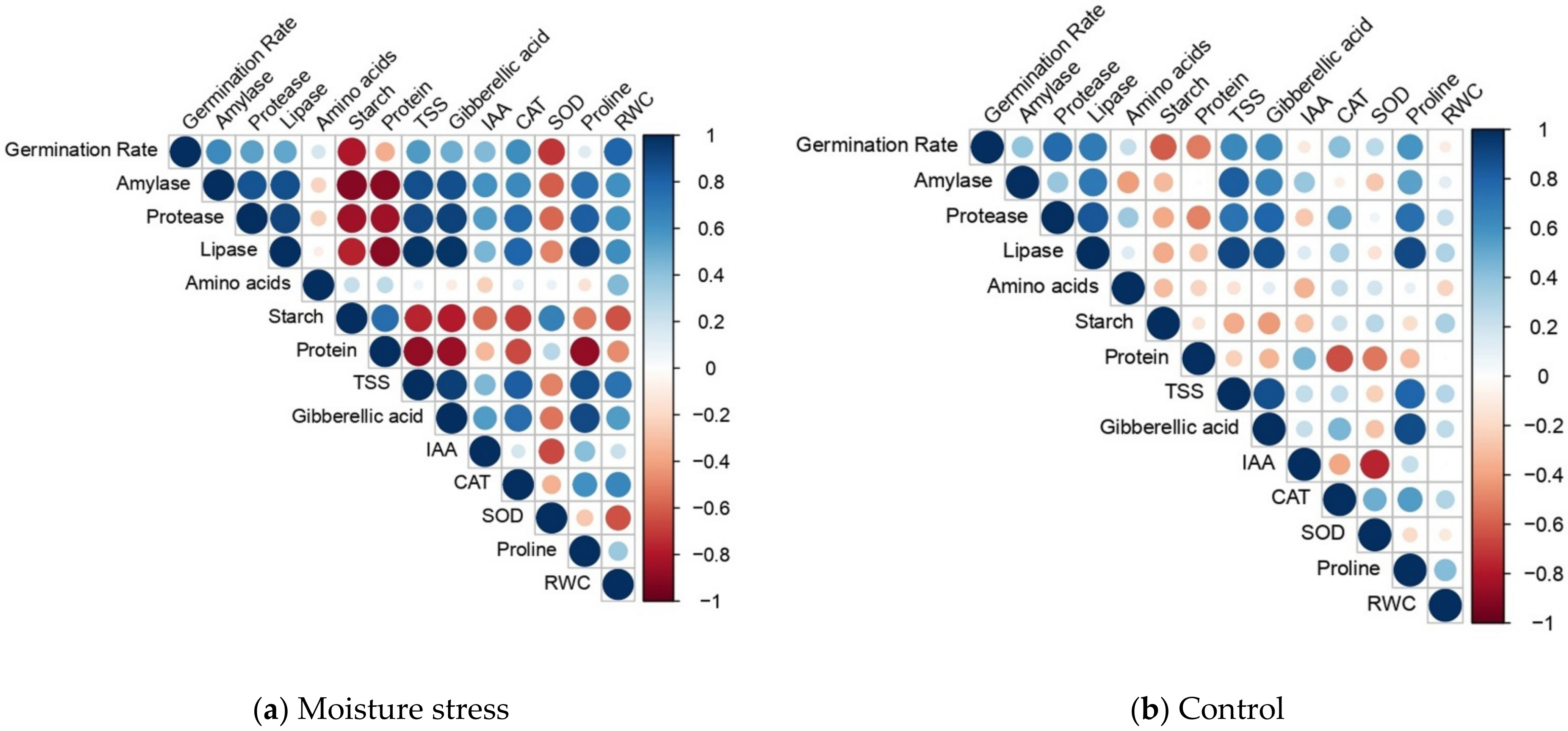
| Genotypes | Stress Intensity | Germination Rate | Root Length | Shoot Length | RS | Vigor Index | RWC |
|---|---|---|---|---|---|---|---|
| Aanai Komban | C | 3.08 ± 0.03 | 4.66 ± 0.08 | 24.02 ± 0.44 | 0.194 ± 0.001 | 2334.44 ± 40.00 | 38.13 ± 0.08 |
| −1.5 MPa | 2.76 ± 0.11 | 4.00 ± 0.07 | 17.66 ± 0.32 | 0.227 ± 0.001 | 423.56 ± 18.59 | 23.15 ± 0.10 | |
| Aathur Kichadi Samba | C | 3.67 ± 0.03 | 12.67 ± 0.21 | 34.39 ± 0.63 | 0.368 ± 0.002 | 4554.98 ± 78.05 | 33.54 ± 0.07 |
| −1.5 MPa | 7.40 ± 0.30 | 10.64 ± 0.18 | 22.68 ± 0.41 | 0.469 ± 0.002 | 1743.52 ± 76.52 | 22.64 ± 0.11 | |
| Adukan | C | 3.62 ± 0.03 | 8.31 ± 0.14 | 24.22 ± 0.45 | 0.343 ± 0.002 | 3106.13 ± 53.22 | 38.13 ± 0.08 |
| −1.5 MPa | 6.41 ± 0.26 | 5.47 ± 0.09 | 14.85 ± 0.27 | 0.369 ± 0.001 | 921.09 ± 40.42 | 23.15 ± 0.10 | |
| Arubathamkodai | C | 3.63 ± 0.03 | 7.60 ± 0.13 | 16.25 ± 0.30 | 0.468 ± 0.002 | 2282.62 ± 39.11 | 34.50 ± 0.08 |
| −1.5 MPa | 9.94 ± 0.41 | 6.18 ± 0.10 | 11.04 ± 0.20 | 0.560 ± 0.002 | 1209.94 ± 53.10 | 20.50 ± 0.11 | |
| Akshayaponni | C | 3.67 ± 0.03 | 10.13 ± 0.17 | 25.02 ± 0.46 | 0.405 ± 0.002 | 3401.70 ± 58.29 | 44.15 ± 0.06 |
| −1.5 MPa | 6.85 ± 0.28 | 7.30 ± 0.12 | 20.87 ± 0.38 | 0.350 ± 0.001 | 1364.81 ± 59.90 | 22.13 ± 0.13 | |
| Anjali | C | 3.63 ± 0.03 | 9.69 ± 0.16 | 8.22 ± 0.15 | 1.179 ± 0.006 | 1707.52 ± 29.26 | 34.35 ± 0.10 |
| −1.5 MPa | 6.19 ± 0.25 | 5.98 ± 0.10 | 8.93 ± 0.16 | 0.670 ± 0.002 | 651.59 ± 28.60 | 12.69 ± 0.19 | |
| Annada | C | 3.67 ± 0.03 | 14.06 ± 0.24 | 12.14 ± 0.22 | 1.159 ± 0.006 | 2525.09 ± 43.27 | 38.13 ± 0.08 |
| −1.5 MPa | 7.40 ± 0.30 | 5.67 ± 0.10 | 12.64 ± 0.23 | 0.449 ± 0.002 | 958.67 ± 42.07 | 23.15 ± 0.10 | |
| Arikiraavi | C | 3.08 ± 0.03 | 11.35 ± 0.19 | 22.23 ± 0.41 | 0.511 ± 0.003 | 2724.87 ± 46.69 | 34.14 ± 0.09 |
| −1.5 MPa | 10.17 ± 0.41 | 9.97 ± 0.17 | 18.86 ± 0.35 | 0.529 ± 0.002 | 2071.50 ± 90.91 | 14.59 ± 0.12 | |
| Aryan | C | 3.08 ± 0.03 | 8.71 ± 0.15 | 43.06 ± 0.79 | 0.202 ± 0.001 | 4213.39 ± 72.20 | 32.25 ± 0.10 |
| −1.5 MPa | 4.42 ± 0.18 | 4.76 ± 0.08 | 27.99 ± 0.51 | 0.170 ± 0.001 | 1025.18 ± 44.99 | 20.24 ± 0.14 | |
| Baskadam | C | 3.67 ± 0.03 | 5.07 ± 0.09 | 24.92 ± 0.46 | 0.203 ± 0.001 | 2907.44 ± 49.82 | 25.31 ± 0.09 |
| −1.5 MPa | 4.97 ± 0.20 | 3.55 ± 0.06 | 17.76 ± 0.32 | 0.200 ± 0.001 | 750.02 ± 32.92 | 10.22 ± 0.09 | |
| Bharathi | C | 3.08 ± 0.03 | 5.98 ± 0.10 | 26.41 ± 0.49 | 0.226 ± 0.001 | 2635.40 ± 45.16 | 38.13 ± 0.08 |
| −1.5 MPa | 6.19 ± 0.25 | 3.95 ± 0.07 | 16.15 ± 0.30 | 0.245 ± 0.001 | 880.53 ± 38.64 | 23.15 ± 0.10 | |
| Chakhaeamubi | C | 3.08 ± 0.03 | 8.61 ± 0.15 | 36.18 ± 0.67 | 0.238 ± 0.001 | 3644.01 ± 62.44 | 33.78 ± 0.10 |
| −1.5 MPa | 2.21 ± 0.09 | 6.49 ± 0.11 | 22.88 ± 0.42 | 0.284 ± 0.001 | 459.13 ± 20.15 | 23.16 ± 0.11 | |
| Chakhaepoirecton | C | 3.08 ± 0.03 | 8.31 ± 0.14 | 34.78 ± 0.64 | 0.239 ± 0.001 | 3505.73 ± 60.07 | 31.49 ± 0.12 |
| −1.5 MPa | 2.21 ± 0.09 | 6.69 ± 0.11 | 23.98 ± 0.44 | 0.279 ± 0.001 | 479.57 ± 21.05 | 21.86 ± 0.12 | |
| Chandaikar | C | 3.68 ± 0.03 | 16.52 ± 0.28 | 25.22 ± 0.46 | 0.655 ± 0.003 | 4045.60 ± 69.32 | 38.13 ± 0.08 |
| −1.5 MPa | 11.05 ± 0.45 | 12.67 ± 0.21 | 19.06 ± 0.35 | 0.665 ± 0.002 | 2476.48 ± 108.69 | 23.15 ± 0.10 | |
| Chemban | C | 3.08 ± 0.03 | 10.34 ± 0.17 | 38.67 ± 0.71 | 0.267 ± 0.001 | 3985.64 ± 68.29 | 34.50 ± 0.08 |
| −1.5 MPa | 6.85 ± 0.28 | 6.59 ± 0.11 | 22.88 ± 0.42 | 0.288 ± 0.001 | 1428.18 ± 62.68 | 20.50 ± 0.11 | |
| Chembavu | C | 3.08 ± 0.03 | 9.93 ± 0.17 | 33.29 ± 0.61 | 0.298 ± 0.002 | 3513.87 ± 60.21 | 33.54 ± 0.07 |
| −1.5 MPa | 3.31 ± 0.14 | 6.49 ± 0.11 | 20.17 ± 0.37 | 0.322 ± 0.001 | 625.02 ± 27.43 | 22.64 ± 0.11 | |
| Chenkayama | C | 2.31 ± 0.02 | 18.54 ± 0.31 | 25.61 ± 0.47 | 0.724 ± 0.004 | 2684.20 ± 45.99 | 54.55 ± 0.67 |
| −1.5 MPa | 10.17 ± 0.41 | 17.02 ± 0.29 | 22.68 ± 0.41 | 0.751 ± 0.003 | 2849.76 ± 125.07 | 24.13 ± 0.66 | |
| Chenthadi | C | 3.08 ± 0.03 | 8.51 ± 0.14 | 37.08 ± 0.68 | 0.230 ± 0.001 | 3709.08 ± 63.55 | 38.13 ± 0.08 |
| −1.5 MPa | 6.74 ± 0.27 | 7.50 ± 0.13 | 19.97 ± 0.37 | 0.376 ± 0.001 | 1309.23 ± 57.46 | 23.15 ± 0.10 | |
| Chumala | C | 2.31 ± 0.02 | 7.50 ± 0.13 | 27.11 ± 0.50 | 0.277 ± 0.001 | 2110.76 ± 36.17 | 30.18 ± 0.07 |
| −1.5 MPa | 5.08 ± 0.21 | 5.27 ± 0.09 | 20.37 ± 0.37 | 0.259 ± 0.001 | 922.19 ± 40.47 | 17.35 ± 0.12 | |
| Eluppai Poo Samba | C | 3.08 ± 0.03 | 10.64 ± 0.18 | 37.87 ± 0.70 | 0.281 ± 0.001 | 3944.97 ± 67.60 | 28.69 ± 0.07 |
| −1.5 MPa | 4.42 ± 0.18 | 7.50 ± 0.13 | 24.28 ± 0.44 | 0.309 ± 0.001 | 993.74 ± 43.61 | 8.44 ± 0.11 | |
| Kar Samba | C | 2.31 ± 0.02 | 6.59 ± 0.11 | 33.29 ± 0.61 | 0.198 ± 0.001 | 2434.08 ± 41.71 | 46.99 ± 0.09 |
| −1.5 MPa | 3.54 ± 0.14 | 3.14 ± 0.05 | 20.77 ± 0.38 | 0.151 ± 0.001 | 598.76 ± 26.28 | 13.97 ± 0.15 | |
| Karuppukavuni | C | 3.77 ± 0.03 | 9.83 ± 0.17 | 41.86 ± 0.77 | 0.235 ± 0.001 | 5152.54 ± 88.29 | 47.20 ± 0.07 |
| −1.5 MPa | 6.74 ± 0.27 | 7.19 ± 0.12 | 28.90 ± 0.53 | 0.249 ± 0.001 | 1721.66 ± 75.56 | 10.93 ± 0.11 | |
| Kattanoor | C | 3.77 ± 0.03 | 6.08 ± 0.10 | 11.94 ± 0.22 | 0.509 ± 0.003 | 1788.61 ± 30.65 | 47.20 ± 0.07 |
| −1.5 MPa | 1.10 ± 0.05 | 4.35 ± 0.07 | 10.23 ± 0.19 | 0.425 ± 0.001 | 113.89 ± 5.00 | 10.93 ± 0.11 | |
| Kattuyanam | C | 3.77 ± 0.03 | 5.17 ± 0.09 | 19.83 ± 0.37 | 0.261 ± 0.001 | 2487.18 ± 42.62 | 38.13 ± 0.08 |
| −1.5 MPa | 7.51 ± 0.31 | 3.45 ± 0.06 | 16.86 ± 0.31 | 0.204 ± 0.001 | 1079.90 ± 47.39 | 23.15 ± 0.10 | |
| Kerala Kandhasala | C | 3.77 ± 0.03 | 5.27 ± 0.09 | 34.39 ± 0.63 | 0.153 ± 0.001 | 3956.59 ± 67.80 | 24.84 ± 0.09 |
| −1.5 MPa | 7.84 ± 0.32 | 4.36 ± 0.07 | 21.27 ± 0.39 | 0.205 ± 0.001 | 1423.38 ± 62.47 | 12.28 ± 0.08 | |
| Kichadi Samba | C | 3.08 ± 0.03 | 10.84 ± 0.18 | 25.22 ± 0.46 | 0.430 ± 0.002 | 2928.22 ± 50.17 | 38.13 ± 0.08 |
| −1.5 MPa | 7.51 ± 0.31 | 6.79 ± 0.11 | 18.06 ± 0.33 | 0.376 ± 0.001 | 1320.47 ± 57.95 | 23.15 ± 0.10 | |
| Kothamalli Samba | C | 3.08 ± 0.03 | 10.54 ± 0.18 | 22.43 ± 0.41 | 0.470 ± 0.002 | 2676.07 ± 45.85 | 24.12 ± 0.10 |
| −1.5 MPa | 7.29 ± 0.30 | 7.19 ± 0.12 | 15.25 ± 0.28 | 0.472 ± 0.002 | 1157.10 ± 50.78 | 10.96 ± 0.14 | |
| Kuliyadichan | C | 3.82 ± 0.01 | 18.75 ± 0.32 | 25.42 ± 0.47 | 0.738 ± 0.004 | 4440.41 ± 54.40 | 51.70 ± 0.38 |
| −1.5 MPa | 12.71 ± 0.52 | 16.01 ± 0.27 | 22.07 ± 0.40 | 0.725 ± 0.003 | 3417.54 ± 149.99 | 28.05 ± 0.49 | |
| Kunju Kunju | C | 3.78 ± 0.03 | 10.74 ± 0.18 | 40.46 ± 0.75 | 0.266 ± 0.001 | 5116.55 ± 87.67 | 28.69 ± 0.07 |
| −1.5 MPa | 9.06 ± 0.37 | 7.30 ± 0.12 | 28.49 ± 0.52 | 0.256 ± 0.001 | 2295.03 ± 100.72 | 8.44 ± 0.11 | |
| Mallikar | C | 3.78 ± 0.03 | 7.50 ± 0.13 | 20.43 ± 0.38 | 0.367 ± 0.002 | 2744.17 ± 26.88 | 47.20 ± 0.07 |
| −1.5 MPa | 9.28 ± 0.38 | 6.59 ± 0.11 | 13.04 ± 0.24 | 0.505 ± 0.002 | 1277.11 ± 63.99 | 25.00 ± 0.73 | |
| Mapillai Samba | C | 3.58 ± 0.03 | 10.44 ± 0.18 | 35.38 ± 0.65 | 0.295 ± 0.002 | 4331.17 ± 74.21 | 24.84 ± 0.09 |
| −1.5 MPa | 6.85 ± 0.28 | 6.49 ± 0.11 | 26.49 ± 0.48 | 0.245 ± 0.001 | 1598.78 ± 70.17 | 12.28 ± 0.08 | |
| Mattaikar | C | 3.74 ± 0.03 | 7.50 ± 0.13 | 27.01 ± 0.50 | 0.278 ± 0.001 | 3412.97 ± 58.48 | 42.67 ± 0.07 |
| −1.5 MPa | 7.29 ± 0.30 | 3.85 ± 0.07 | 20.17 ± 0.37 | 0.191 ± 0.001 | 1240.13 ± 54.43 | 10.63 ± 0.17 | |
| MBR | C | 3.58 ± 0.03 | 9.22 ± 0.16 | 32.69 ± 0.60 | 0.282 ± 0.001 | 3962.36 ± 67.89 | 24.84 ± 0.09 |
| −1.5 MPa | 7.62 ± 0.31 | 6.59 ± 0.11 | 21.97 ± 0.40 | 0.300 ± 0.001 | 1540.60 ± 67.61 | 12.28 ± 0.08 | |
| Milagu Samba | C | 3.78 ± 0.03 | 15.71 ± 0.27 | 23.12 ± 0.43 | 0.679 ± 0.004 | 3867.39 ± 66.27 | 25.98 ± 0.07 |
| −1.5 MPa | 8.62 ± 0.35 | 8.01 ± 0.14 | 21.97 ± 0.40 | 0.364 ± 0.001 | 1827.40 ± 80.20 | 8.06 ± 0.08 | |
| Mulampunchan | C | 3.58 ± 0.03 | 3.37 ± 0.06 | 23.71 ± 0.44 | 0.142 ± 0.001 | 578.26 ± 9.91 | 28.46 ± 0.06 |
| −1.5 MPa | 7.18 ± 0.29 | 2.43 ± 0.04 | 21.87 ± 0.40 | 0.111 ± 0.000 | 1236.67 ± 54.27 | 10.57 ± 0.09 | |
| Muttakaruva | C | 3.77 ± 0.03 | 12.95 ± 0.22 | 8.44 ± 0.16 | 1.535 ± 0.008 | 2113.39 ± 36.21 | 24.84 ± 0.09 |
| −1.5 MPa | 4.86 ± 0.20 | 6.89 ± 0.12 | 6.82 ± 0.12 | 1.011 ± 0.004 | 470.23 ± 20.64 | 12.28 ± 0.08 | |
| Nootripathu | C | 3.67 ± 0.03 | 5.88 ± 0.10 | 21.63 ± 0.40 | 0.272 ± 0.001 | 2620.77 ± 26.16 | 47.20 ± 0.07 |
| −1.5 MPa | 10.61 ± 0.43 | 5.88 ± 0.10 | 9.33 ± 0.17 | 0.630 ± 0.002 | 1130.82 ± 56.72 | 25.90 ± 0.21 | |
| Norungan | C | 3.77 ± 0.03 | 4.76 ± 0.08 | 31.40 ± 0.58 | 0.152 ± 0.001 | 3601.44 ± 61.71 | 24.84 ± 0.09 |
| −1.5 MPa | 6.85 ± 0.28 | 3.45 ± 0.06 | 17.66 ± 0.32 | 0.195 ± 0.001 | 1023.61 ± 44.92 | 12.28 ± 0.08 | |
| Ohenellu | C | 3.77 ± 0.03 | 4.97 ± 0.08 | 31.69 ± 0.58 | 0.157 ± 0.001 | 3651.18 ± 62.56 | 38.13 ± 0.08 |
| −1.5 MPa | 3.87 ± 0.16 | 3.95 ± 0.07 | 24.28 ± 0.44 | 0.163 ± 0.001 | 773.21 ± 33.93 | 23.15 ± 0.10 | |
| Oheruchitteni | C | 3.58 ± 0.03 | 20.17 ± 0.34 | 21.13 ± 0.39 | 0.955 ± 0.005 | 3886.71 ± 66.60 | 45.44 ± 0.62 |
| −1.5 MPa | 10.83 ± 0.44 | 14.09 ± 0.24 | 19.26 ± 0.35 | 0.731 ± 0.003 | 2550.22 ± 111.92 | 26.89 ± 0.28 | |
| Rajalakshmi | C | 3.78 ± 0.03 | 20.98 ± 0.35 | 28.21 ± 0.52 | 0.744 ± 0.004 | 4896.70 ± 83.90 | 49.67 ± 2.04 |
| −1.5 MPa | 11.27 ± 0.46 | 18.75 ± 0.32 | 26.09 ± 0.48 | 0.719 ± 0.003 | 3568.48 ± 156.61 | 27.01 ± 0.20 | |
| Sahbhagi Dhan | C | 3.77 ± 0.03 | 13.68 ± 0.23 | 19.34 ± 0.36 | 0.708 ± 0.004 | 3273.13 ± 56.08 | 41.61 ± 1.33 |
| −1.5 MPa | 11.05 ± 0.45 | 11.65 ± 0.20 | 16.05 ± 0.29 | 0.726 ± 0.003 | 2162.00 ± 94.88 | 25.89 ± 0.33 | |
| Seeraka Samba | C | 3.58 ± 0.03 | 3.55 ± 0.06 | 2.29 ± 0.04 | 1.548 ± 0.008 | 548.49 ± 9.40 | 35.73 ± 0.07 |
| −1.5 MPa | 6.08 ± 0.25 | 2.63 ± 0.04 | 10.64 ± 0.19 | 0.248 ± 0.001 | 570.77 ± 25.05 | 19.95 ± 0.12 | |
| Sivappumalli | C | 3.80 ± 0.03 | 5.57 ± 0.09 | 27.41 ± 0.50 | 0.203 ± 0.001 | 3314.27 ± 56.79 | 39.07 ± 0.06 |
| −1.5 MPa | 8.62 ± 0.35 | 4.36 ± 0.07 | 15.25 ± 0.28 | 0.286 ± 0.001 | 1195.78 ± 52.48 | 12.94 ± 0.09 | |
| Swarna | C | 3.77 ± 0.03 | 9.22 ± 0.16 | 29.20 ± 0.54 | 0.316 ± 0.002 | 3758.32 ± 37.18 | 47.20 ± 0.07 |
| −1.5 MPa | 3.98 ± 0.16 | 8.31 ± 0.14 | 22.07 ± 0.40 | 0.377 ± 0.001 | 847.11 ± 42.38 | 10.93 ± 0.11 | |
| Thuyamalli | C | 3.77 ± 0.03 | 6.99 ± 0.12 | 20.43 ± 0.38 | 0.342 ± 0.002 | 2725.95 ± 46.71 | 39.07 ± 0.06 |
| −1.5 MPa | 6.41 ± 0.26 | 4.36 ± 0.07 | 16.25 ± 0.30 | 0.268 ± 0.001 | 934.77 ± 41.02 | 12.94 ± 0.09 | |
| Uma | C | 3.58 ± 0.03 | 12.57 ± 0.21 | 41.06 ± 0.76 | 0.306 ± 0.002 | 4986.09 ± 49.42 | 41.26 ± 0.09 |
| −1.5 MPa | 3.76 ± 0.15 | 11.96 ± 0.20 | 25.08 ± 0.46 | 0.477 ± 0.002 | 975.40 ± 48.86 | 7.00 ± 0.16 | |
| Vandhana | C | 3.77 ± 0.03 | 6.18 ± 0.10 | 18.84 ± 0.35 | 0.328 ± 0.002 | 2487.18 ± 42.62 | 35.77 ± 0.09 |
| −1.5 MPa | 5.19 ± 0.21 | 4.46 ± 0.08 | 13.55 ± 0.25 | 0.329 ± 0.001 | 661.42 ± 29.03 | 14.91 ± 0.13 | |
| Varaputha | C | 2.31 ± 0.02 | 4.66 ± 0.08 | 39.37 ± 0.73 | 0.118 ± 0.001 | 2690.30 ± 46.10 | 28.95 ± 0.09 |
| −1.5 MPa | 6.96 ± 0.28 | 3.65 ± 0.06 | 26.49 ± 0.48 | 0.138 ± 0.000 | 1485.89 ± 65.21 | 9.91 ± 0.14 | |
| Veethirupa | C | 3.77 ± 0.03 | 7.60 ± 0.13 | 23.42 ± 0.43 | 0.325 ± 0.002 | 3084.10 ± 52.85 | 28.69 ± 0.07 |
| −1.5 MPa | 4.20 ± 0.17 | 4.15 ± 0.07 | 19.46 ± 0.36 | 0.213 ± 0.001 | 702.06 ± 30.81 | 8.44 ± 0.11 | |
| Virendra | C | 3.55 ± 0.03 | 15.61 ± 0.26 | 29.00 ± 0.53 | 0.538 ± 0.003 | 4178.39 ± 71.60 | 36.27 ± 0.08 |
| −1.5 MPa | 6.85 ± 0.28 | 8.51 ± 0.14 | 19.26 ± 0.35 | 0.442 ± 0.002 | 1345.32 ± 59.04 | 19.10 ± 0.12 | |
| IR 64 Drt1 | C | 3.77 ± 0.03 | 10.94 ± 0.18 | 22.62 ± 0.42 | 0.484 ± 0.003 | 3332.80 ± 57.11 | 50.68 ± 2.35 |
| −1.5 MPa | 11.05 ± 0.45 | 9.32 ± 0.16 | 16.05 ± 0.29 | 0.581 ± 0.002 | 1981.18 ± 86.95 | 24.52 ± 0.53 |
| Germplasm | α-Amylase (U.g−1 FW) | Protease (U.g−1 FW) | Lipase (U.g−1 FW) | |||
|---|---|---|---|---|---|---|
| C | S | C | S | C | S | |
| IR 64 | 19.55 bcde | 2.13 d | 1.72 ab | 0.33 f | 29.73 cde | 5.51 d |
| IR 64 Drt1 | 23.72 ab | 12.65 a | 1.87 a | 1.03 abc | 40.07 a | 25.10 ab |
| Kuliyadichan | 24.51 a | 13.57 a | 1.71 ab | 1.19 a | 38.51 ab | 29.21 a |
| Mallikar | 15.33 e | 8.70 abcd | 1.88 a | 0.97 abcd | 31.30 bcde | 18.88 abcd |
| Nootripathu | 20.97 abcd | 11.50 ab | 1.83 a | 0.66 bcdef | 36.33 abc | 23.42 ab |
| Chandaikar | 22.34 abc | 12.95 a | 1.67 ab | 0.88 abcde | 29.20 cdef | 15.20 abcd |
| Sahbhagi Dhan | 21.75 abcd | 11.20 ab | 1.80 ab | 0.81 abcdef | 33.51 abcd | 21.83 abc |
| Rajalakshmi | 22.15 abcd | 12.30 a | 1.75 ab | 1.09 ab | 37.88 ab | 26.51 a |
| Arubathamkodai | 18.43 cde | 10.53 abc | 1.55 bc | 0.55 cdef | 28.42 cdef | 11.20 bcd |
| Ariki raavi | 19.57 bcde | 4.94 bcd | 1.31 d | 0.47 def | 25.33 def | 8.46 cd |
| Chenkayama | 17.53 de | 3.88 cd | 1.27 d | 0.33 f | 21.52 f | 5.33 d |
| Oheruchitteni | 18.26 cde | 4.10 cd | 1.33 cd | 0.45 ef | 23.77 ef | 5.88 d |
| CD (0.05) | 4.12 | 6.39 | 0.22 | 0.44 | 7.28 | 12.74 |
| RWC | Proline | TSS | GA | IAA | CAT | SOD | α-Amylase | Protease | Lipase | |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | 1610.0 *** | 8355.7 *** | 5379.7 *** | 44.246 *** | 4211.1 *** | 12.6370 *** | 3908.9 *** | 648.40 *** | 3.8728 *** | 3569.6 *** |
| Treatment | 9005.9 *** | 28,792.8*** | 16,172.1 *** | 38.500 *** | 3982.8 *** | 1.0015 *** | 8.4 | 2299.44 *** | 14.9331 *** | 4006.9 *** |
| GXT | 835.1 *** | 2225.1 *** | 2565.0 *** | 1.001 **** | 1751.2 *** | 6.0718 *** | 54.4 | 158.35 *** | 0.8296 *** | 257.8 *** |
| Residuals | 268.1 | 75.1 | 29.1 | 0.187 | 13.9 | 0.1110 | 274.7 | 7.06 | 0.0480 | 16.0 |
| Details | SS | MSS | F Value | |||
|---|---|---|---|---|---|---|
| Cytokinin | ABA | Cytokinin | ABA | Cytokinin | ABA | |
| Genotypes | 0.218 | 0.026 | 0.036 | 0.004 | 91.26 *** | 20.686 *** |
| Treatment | 0.076 | 0.041 | 0.079 | 0.041 | 198.46 *** | 196.126 *** |
| GXT | 0.009 | 0.006 | 0.001 | 0.001 | 3.85 ** | 5.059 ** |
| Residuals | 0.011 | 0.0066 | 0.001 | 0.002 | ||
| CV | 6.4 | 12.5 | ||||
| CD(0.05) | 0.013 | 0.008 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binodh, A.K.; Thankappan, S.; Ravichandran, A.; Mitra, D.; Alagarsamy, S.; Panneerselvam, P.; Senapati, A.; Sami, R.; Al-Mushhin, A.A.M.; Aljahani, A.H.; et al. Synergistic Modulation of Seed Metabolites and Enzymatic Antioxidants Tweaks Moisture Stress Tolerance in Non-Cultivated Traditional Rice Genotypes during Germination. Plants 2022, 11, 775. https://doi.org/10.3390/plants11060775
Binodh AK, Thankappan S, Ravichandran A, Mitra D, Alagarsamy S, Panneerselvam P, Senapati A, Sami R, Al-Mushhin AAM, Aljahani AH, et al. Synergistic Modulation of Seed Metabolites and Enzymatic Antioxidants Tweaks Moisture Stress Tolerance in Non-Cultivated Traditional Rice Genotypes during Germination. Plants. 2022; 11(6):775. https://doi.org/10.3390/plants11060775
Chicago/Turabian StyleBinodh, Asish Kanakaraj, Sugitha Thankappan, Anupriya Ravichandran, Debasis Mitra, Senthil Alagarsamy, Periyasamy Panneerselvam, Ansuman Senapati, Rokayya Sami, Amina A. M. Al-Mushhin, Amani H. Aljahani, and et al. 2022. "Synergistic Modulation of Seed Metabolites and Enzymatic Antioxidants Tweaks Moisture Stress Tolerance in Non-Cultivated Traditional Rice Genotypes during Germination" Plants 11, no. 6: 775. https://doi.org/10.3390/plants11060775
APA StyleBinodh, A. K., Thankappan, S., Ravichandran, A., Mitra, D., Alagarsamy, S., Panneerselvam, P., Senapati, A., Sami, R., Al-Mushhin, A. A. M., Aljahani, A. H., Alyamani, A., & Alqurashi, M. (2022). Synergistic Modulation of Seed Metabolites and Enzymatic Antioxidants Tweaks Moisture Stress Tolerance in Non-Cultivated Traditional Rice Genotypes during Germination. Plants, 11(6), 775. https://doi.org/10.3390/plants11060775







