Abstract
Usnea genus (Parmeliaceae, lichenized Ascomycetes) is a potent phytomedicine, due to phenolic secondary metabolites, with various pharmacological effects. Therefore, our study aimed to explore the antioxidant, cytotoxic, and rheological properties of Usnea barbata (L.) Weber ex F.H. Wigg (U. barbata) extract in canola oil (UBO) compared to cold-pressed canola seed oil (CNO), as a green solvent used for lichen extraction, which has phytoconstituents. The antiradical activity (AA) of UBO and CNO was investigated using UV-Vis spectrophotometry. Their cytotoxicity was examined in vivo through a brine shrimp lethality (BSL) test after Artemia salina (A. salina) larvae exposure for 6 h to previously emulsified UBO and CNO. The rheological properties of both oil samples (flow behavior, thixotropy, and temperature-dependent viscosity variation) were comparatively analyzed. The obtained results showed that UBO (IC50 = 0.942 ± 0.004 mg/mL) had a higher 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity than CNO (IC50 = 1.361 ± 0.008 mg/mL). Both UBO and CNO emulsions induced different and progressive morphological changes to A. salina larvae, incompatible with their survival; UBO cytotoxicity was higher than that of CNO. Finally, in the temperature range of 32–37 °C, the UBO and CNO viscosity and viscoelastic behavior indicated a clear weakening of the intermolecular bond when temperature increases, leading to a more liquid state, appropriate for possible pharmaceutical formulations. All quantified parameters were highly intercorrelated. Moreover, their significant correlation with trace/heavy minerals and phenolic compounds can be observed. All data obtained also suggest a possible synergism between lichen secondary metabolites, minerals, and canola oil phytoconstituents.
1. Introduction
In the plant world, the significant interest in lichens (lichenized fungi), symbionts between a fungus and one or more photosynthetic organisms (algae or cyanobacteria), is due to their specific secondary metabolites [1] that protect them against abiotic stress [2] and biological attack [3]. Numerous pharmacological studies proved their significant properties [4], the reason for lichen use in traditional medicine for thousands of years [5]. Nowadays, they are considered potential alternative remedies for modern diseases [6,7]. The most relevant lichen secondary metabolites are phenolic compounds; their reactive hydroxyl groups confer various bioactivities [8] through different mechanisms [4]. In addition to phenolic acids and tannins, lichens contain specific phenolic constituents, classified as depsides, depsidones, dibenzofurans, and pulvinic acid derivatives [9].
According to Prateeska et al. (2016) [10], in the lichen world, the genus Usnea (Parmeliaceae, lichenized Ascomycetes) can be considered a potent phytomedicine, with various pharmacological effects. Using UHPLC, high-resolution mass spectrometry (HRMS), and MS2 analysis, Salgado et al. (2018) [11] analyzed the secondary metabolites of Usnea lichen species. Thus, in a methanol extract of U. barbata (one of the best known representatives of Usnea genus) they identified usnic acid (UA) as a dibenzofurans representative, thirteen depsides, and eight depsidones [11]. Our previous studies proved that U. barbata ethanol extract also contains phenolic acids (caffeic acid, ellagic acid, p-coumaric acid, chlorogenic acid, cinnamic acid, and gallic acid) [12] and tannins [13].
Usnic acid (UA) is a well-known and abundant lichen secondary metabolite and can be isolated from U. barbata [14] and other Usnea sp. [10]. Various lichen bioactivities are due to this compound, such as gastroprotective [15], cardioprotective [16], neuroprotective [17], cytoprotective [18], cytotoxic [19], anticancer [20], antimicrobial [21], antidiabetic [22], analgesic-antipyretic [23], and anti-inflammatory [24] properties, mainly through its antioxidant action, reducing oxidative damage [25]. Some of the other phenolic metabolites previously mentioned also display different biological effects. Thus, from the depsides group, atranorin shows antimicrobial, anti-proliferative, cytoprotective, anti-inflammatory, and antioxidant activities [26]; lecanoric acid has antimicrobial [27], anti-proliferative [28], and antioxidant [29] effects; diffractaic acid shows analgesic-antipyretic [23], hepatoprotective [30], antimycobacterial [31], cytotoxic, and anti-proliferative action [32]. Depsidone’s representatives (norstictic, connorstictic, stictic, salazinic, and lobaric acid) have proven antioxidant, antimicrobial, and antitumor properties [33]. It has been reported that major U. barbata secondary metabolites have antioxidant potential [34]. Moreover, Rabelo et al. (2012) [35] proved that usnic acid displays a dual redox dose-dependent behavior; it protects the normal cells through an antioxidant effect and, at the same time, it induces tumor cell apoptosis by enhancing reactive oxygen species (ROS) levels.
Numerous studies have explored the bioactivities of lichen extracts in chemical solvents, such as acetone [36], ethanol [37], methanol [38], ethyl acetate [13], supercritical CO2 [39], chloroform [40], diethyl-ether, and petroleum ether [41]; only a few studies have described lichen extracts in natural solvents. Nowadays, the solvents with petroleum origin are restricted worldwide; therefore, the research on bio-based and renewable green solvents for extracting, purifying, and formulating natural products for the food and pharmaceutical industry has significantly increased [42]. According to the green chemistry concept, vegetable oils may become more competitive because of economic, food safety, and eco-friendliness concerns [43]. As solvents, vegetable oils are rich in bioactive compounds; there is a relationship between the solubilizing capacity of non-polar and polar bioactive components with the function of fatty acids and/or lipid classes and other minor components [41,42]. Yara-Varón et al. (2017) reported that various edible oils, such as soybean, olive, sunflower, corn, grapeseed, and canola oils, could extract carotenoids and antioxidants (astaxanthin), aromatic, and phenolic compounds [43]. From these common edible oils, one valuable vegetable oil containing various bioactive compounds is canola oil, the second most abundantly produced edible oil in the world, after soybean oil [44].
As cold-pressed rapeseed (Brassica napus) oil, canola oil (CNO) has a low erucic acid and glucosinolate content [45]. Triacylglycerols represent the major components (97–99%) in CNO, while other constituents (polyphenols, phytosterols, tocopherols, carotenoids, chlorophylls, phospholipids, monoglycerides, diglycerides, and free fatty acids) were quantified in a minor amount (1–3%) [46]. Canola oil shows a unique fatty acids profile: it is rich in monounsaturated fatty acids (MUFA, 68.6%) and polyunsaturated fatty acids (PUFA); CNO also significantly contains oleic acid (63.7%), linoleic acid (17.4%), and γ-linolenic acid (6.8%) [47]. Stearic acid, eicosenoic acid, erucic acid, arachidic acid, behenic, and palmitoleic acid are found in minor concentrations [47]. The saturated fatty acids content (SFA, 7.2%) in CNO is lower than in sunflower oil (11%) and soybean oil (15%) [48]. The bioactive constituents of canola oil are phenolic compounds, tocopherols, phytosterols, and carotenoids [47]. The phenolic compounds in CNO are mainly phenolic acids: sinapic acid, cinnamic acid, syringic acid, ferulic acid, 4-hydroxybenzoic acid, vanillic acid, and p-coumaric acid [49]. The heat treatment prior to rapeseed cold-pressing for the CNO obtaining process induces sinapic acid decarboxylation, resulting in canolol [50]; an oil-soluble bioactive phenolic compound that is easier to extract into CNO. It has significant antioxidant, antimutagenic, and anticarcinogenic effects [51]. Tocopherols of canola oil are γ-tocopherol and α-tocopherol, as the major tocopherols, while δ-tocopherol, plastochromanol-8 (PC-8), and β-tocopherol are the minor tocopherols [51]. The main CNO phytosterols are cholesterol, brassicasterol (specific for rapeseed oil), stigmasterol, campesterol, β-sitosterol, and Δ5 -avenasterol [51]. Finally, β-carotene, zeaxanthin, and lutein are the CNO carotenoids [46]. The hydroxyl group (–OH) of phenolic compounds, tocopherols, and phytosterols can scavenge the free radicals [47], with CNO being known for its considerable antioxidant properties.
In their previous study, Bassiouni et al. (2020) [52] prepared sunflower oil extract from U. barbata originated from Mexico; they determined UA content by 1H-NMR spectroscopy and compared its value with the one of a commercial U. barbata supercritical CO2 extract dissolved in sunflower oil. In addition, they comparatively investigated the antibacterial effects (on bacterial strains isolated from poultry) and the cytotoxic potential (on human tumor cell lines) of U. barbata sunflower oil extract and its previously obtained zinc salt precipitate.
Our research was performed on U. barbata, native to a peat bog zone in Romania’s highest volcanic mountains, the Călimani mountains [53]. The content of bioactive compounds represented the criterion in choosing the vegetable oil for the lichen extraction. Of the common edible oils, canola oil has a high content of phenolic compounds (especially phenolic acids) and significant antiradical activity (higher than soybean, corn, and sunflower oils). All studied properties were analyzed by comparing UBO with CNO to explore the impact of CNO-specific bioactive constituents’ interaction with lichen metabolites. This complex research, in fact, represents our study’s novelty. We aimed to investigate whether UBO could be a valuable bioactive extract due to a possible synergism between lichen metabolites and CNO phytoconstituents. Thus, using ICP-MS analysis for 23 metals, from thirteen elements quantified in dried U. barbata lichen (dUB), only six elements were detected in UBO. Five metals were common to both oil samples analyzed; chromium and nickel were over the permissible limits for edible oils and lower than those for plant medicines. The differences between UBO and CNO phytoconstituents were initially evidenced in an overlay of both FT-IR spectra; higher absorbances were observed in some peaks for UBO, suggesting lichen metabolites extracted by CNO. Then, using UHPLC’s considerable precision and power, an appreciable amount of usnic acid was quantified in U. barbata extract in canola oil. This developed UHPLC method was validated by evaluating the specificity, accuracy, precision, linearity, LOD, and LOQ and successfully used in UA determination. Both oil samples displayed a significant phenolic content, higher in UBO than in CNO, due to U. barbata’s phenolic metabolites. UBO also recorded the highest capacity for DPPH radical scavenging of all the autochthonous U. barbata dry extracts previously examined.
Moreover, we prepared emulsions from UBO and CNO for in vivo cytotoxicity evaluation on A. salina larvae. The obtained results proved that both oil emulsions have cytotoxic effects, inducing different progressive morphological changes, incompatible with larvae survival. After 6 h of brine shrimp nauplii exposure, UBO showed the highest cytotoxicity, significantly correlated with UA, phenolic content, heavy/trace metals levels, antiradical activity, and thixotropy (r > 0.984, p < 0.05). Nowadays, U. barbata is used as a homeopathic remedy, SBL Usnea barbata (CH 6, 30, 200, 1M, 10M) tincture (SBL Pvt. Ltd. Jaipur, Rajasthan, India); and as a dietary supplement, Usnea Liquid Extract, Usnea barbata Dried Thallus Tincture (Hawaii Pharm LLC, Honolulu HI, USA). Examining UBO and CNO rheological properties, our study aimed to investigate whether the extract in canola oil of U. barbata from the Călimani Mountains, Romania, could be an appropriate starting point for possible pharmaceutical formulations.
2. Results
2.1. Lichen Sample
Dried U. barbata lichen had a grey-green color, a fresh smell, and a spicy taste [13]. The obtained loss of drying value was 10.94 ± 0.94% for the dried lichen [13].
2.2. Mineral Analysis
The metal contents (µg/g) of the analyzed samples (UBO and CNO) are registered in Table 1.

Table 1.
The metals contents in dried U. barbata lichen (dUB), U. barbata extract in canola oil (UBO) and canola oil (CNO).
The data from Table 1 show that five metals (Al, Ca, Cr, Mg, Ni) were quantified in CNO in different concentrations and lower than in UBO. Generally, the differences between the registered values are statistically significant (p < 0.05), excepting the calcium (Ca) and magnesium (Mg) content in both oil samples (p > 0.5). Thus, Ca and Mg contents had the most similar values (p > 0.5): 76.818 ± 14.289 µg/g and 6.951 ± 0.177 µg/g, respectively, in UBO; and 74.711 ± 4.048 µg/g and 6.852 ± 0.099 µg/g, respectively, in CNO. Aluminum (Al) showed a significant content in UBO (7.688 ± 0.086 µg/g), around 8-fold higher than CNO (0.975 ± 0.049 µg/g). In decreasing order, both trace metals nickel (Ni) and chromium (Cr) contents values were 0.713 ± 0.005 µg/g and 0.195 ± 0.005 µg/g, respectively, in UBO; and 0.339 ± 0.004 µg/g and 0.158 ± 0.002 µg/g, respectively, in CNO. Moreover, copper (Cu) was only found in UBO (0.155 ± 0.002 µg/g), being undetected in CNO (< 0.100 µg/g).
Our previous study quantified thirteen minerals in dried lichen (dUB) [21], of which only six elements (Table 1) were found in UBO, with considerably diminished content. Macro-elements were extracted in an insignificant amount in UBO. Therefore, from the 979.766 ± 12.285 µg/g Ca, 172.721 ± 0.647 µg/g Mg, and 101.425 ± 1.423 µg/g Mn quantified in dUB, in UBO were only found around 2.107 µg/g Ca and 0.99 µg/g Mg, with Mn being undetected. (Table 1). Aluminum decreased from 87.879 ± 1.152 µg/g in dUB, to 7.688 ± 0.086 in UBO; the Fe content of dUB was substantial (52.561 ± 2.582 µg/g); however, in UBO it was undetected. Three heavy/trace metals from dUB, Cu (1.523 ± 0.013 µg/g), Cr (1.002 ± 0.008 µg/g), and Ni (0.449 ± 0.011 µg/g), were also quantified in UBO: Cu and Cr with lower contents (0.155 ± 0.002 µg/g Cu and 0.047 µg/g Cr, respectively), with Ni in higher content (0.713 µg/g). It can be observed that the nickel content value in UBO is around the sum of dUB and CNO.
Only for Al, Cr, and Ni were the registered values (µg/g) in UBO significantly different than in CNO (p < 0.05). The differences in mineral content between UBO and CNO can be attributed to the metals extracted from the lichen; significant for aluminum, chromium, copper, and nickel (Table 1).
2.3. FT-IR Spectra
Fourier transform infrared (FT-IR) spectroscopy was performed to obtain the infrared spectra of both oil samples, and the absorption, emission, and photoconductivity, detecting specific functional groups in UBO compared to CNO. The overlay of FT-IR spectra of UBO and CNO, displayed in Figure 1, highlights the presence of U. barbata lichen compounds in UBO compared to CNO.
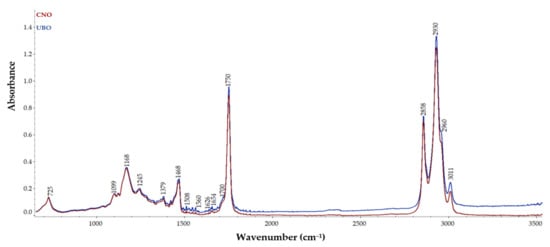
Figure 1.
FT-IR spectra of U. barbata extract in canola oil (UBO) and canola oil (CNO).
As can be seen, no appreciable differences between peaks of UBO and CNO were observed, apart from some distinctions in absorbances values. Both samples exhibited absorption bands at various wavenumbers, as follows: 725 cm−1, which could be assigned to the CH2 group vibration and the out-of-plane vibration of cis –HC=CH– group of disubstituted olefins [54]; 1099, 1168, 1245 cm−1, which could be related to the stretching vibration of the C–O ester groups [55]; 1379 cm−1, due to the bending symmetric vibration of C–H linkages of CH2 groups [56]; 1468 cm−1, from the bending vibration of C–H of CH2 and CH3 aliphatic groups [56]; 1654 cm−1, attributed to the C=C stretching vibration of cis-olefins [54,56]; about 1750 cm−1, due to the stretching vibration of the ester carbonyl functional groups of the triglycerides [54]; 2858 cm−1, probably from the symmetric stretching vibration of C–H of aliphatic CH2 group and 2930 cm−1, due to their asymmetric stretching vibration [55]; 2960 cm−1, attributed to the asymmetric stretching vibration of C–H of the aliphatic CH3 group [54]; 3011 cm−1, due to the C–H stretching symmetric vibration of the cis double bonds, =CH [56].
The peak observed at 2960 cm−1 is related to the C–H vibration of CH2 and CH3 groups, while the band at 1626 cm−1 led to the double C=C bonds found in the aromatic nucleus [20,57]. U. barbata extract in canola oil showed higher absorbances for the peaks at the 1468, 1654, 1700, 1750, 2858, 2930, 2960, and 3011 cm−1 wavenumbers.
2.4. UHPLC Determination of Usnic Acid Content in Usnea barbata Extract in Canola Oil
2.4.1. Specificity
The usnic acid (UA) peak identity was confirmed by matching the analyte peak spectra and retention times extracted from chromatograms concerning the substance. Spectra analysis confirmed the identity of UA from UBO with the usnic acid standard. A peak purity index was obtained by spectral reprocessing using Chromera software, in a 240–700 nm range at a 15% peak height (Figure S1, Supplementary Material). The obtained value of the peak purity index was 1.48.
The obtained chromatograms of usnic acid standard in acetone, UBO in acetone, and two blanks (CNO in acetone and acetone) are represented in Figure 2 and Figure 3.
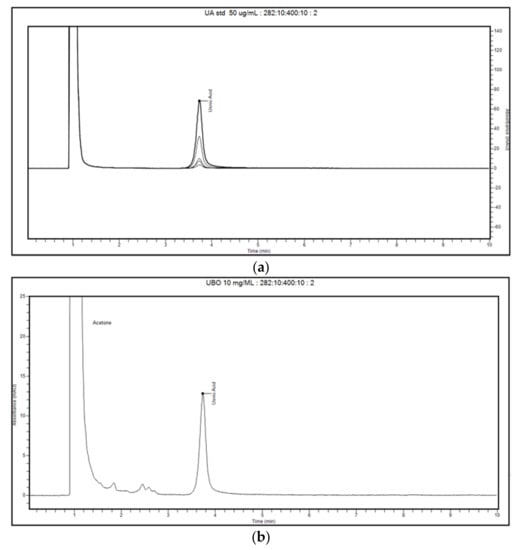
Figure 2.
Chromatograms of usnic acid (UA) standards, an overlay of five peaks in a 2.5–50 μg/mL range (a), and U. barbata extract in canola oil (UBO) 10 mg/mL dissolved in acetone (b) at 282 nm.
Figure 3.
Chromatograms of blank solutions: canola oil 10 mg/mL in acetone (a) and acetone (b).
The retention time (RT) value for the usnic acid standard (50 μg/mL) at 282 nm was around 3.735 min, with the RT relative standard deviation (RSD%) value being about 0.115% (Figure 2a).
In UBO 10 mg/mL chromatograms (six injections), the usnic acid peak was revealed in RT range 3.731–3.736 min, proving the compound identity (Figure 3b and Supplementary Material). Other unknown peaks were recorded at RT 1.838–1.848 min, 2.444–2.452 min, and 2.573–2.585 min, similar to those of canola oil 10 mg/mL (Figure 2b and Figure 3a). Both blank solutions (canola oil and acetone) in the zone of the usnic acid peak, in the RT range of 3.7–3.9 min, did not show any signals (Figure 3a,b). Finally, in all chromatograms, the peak of acetone (the solvent for all standard solutions, control quality solutions (QC), blank, and samples) was reported at RT around 1 min ((Figure 3a,b) and Supplementary Material). Given all these data, our method could discriminate the studied compound (usnic acid) from other unknown constituents of the CNO matrix.
2.4.2. Accuracy
Accuracy % was determined after six injections with QC1 (usnic acid 7.5 µg/mL in acetone) and calculated using the following formula (Equation (1)):
where CcQC1 is the concentration of the injected QC1 solution; CTQC1 is the theoretical concentration of the QC1 solution. More details are presented in the Supplementary Material.
The data are shown in Table 2, and the obtained accuracy % value expressed as mean (n = 6 injections) ± SD was 96.614 ± 1.411%. This accuracy % value is included in the admissible limit range of 100 ± 10%.

Table 2.
Accuracy % calculation after six injections with 7.5 µg/mL UA QC1 solution.
The calculated spike recovery (%) value, as mean (n = 4 injections) ± SD, was 104.179 ± 1.373% (Table 3). The spike recovery (%) value is also included in the range of allowable limits, 100 ± 10% (Figure S2, Supplementary Material).

Table 3.
Spike recovery % calculation, after four injections with 10 µg/mL usnic acid QC2 solution (spike solution).
2.4.3. Precision
Regarding the repeatability at the same concentration level, precision was measured using six injections of QC1 solutions (7.5 μg/mL UA in acetone) and another six injections with sample solution (UBO 10 mg/mL in acetone). The results are displayed as relative standard deviations (RSD%): UA RDS% = 1.631% and UBO RDS% = 3.104%. RDS% values meet the condition of acceptability, being lower than 5%. All these data are detailed in the Supplementary Material.
2.4.4. Linearity
A five-point calibration curve (2.5; 5; 10; 25 and 50) was plotted in the range of 2.5 μg/mL to 50 μg/mL UA standard solutions from five repetitions for each UA concentration (Figure S3, Supplementary Material).
Furthermore, the coefficient of determination value (R2 = 0.995) proved the calibration curve’s admissibility condition (R2 > 0.99). The calibration curve is represented in Figure S2 in the Supplementary Material; the linear equation was the following (Equation (2)):
2.4.5. Detection Limit (LOD) and Quantification Limit (LOQ)
The obtained data are registered in Table 4 and detailed in the Supplementary Material. This UHPLC method was validated for a LOD of 0.300 ug/mL and a LOQ of 1.250 ug/mL, given a S/N ratio of 6.00 and 19.18 μg/mL, respectively.

Table 4.
Calculation of detection limit (LOD) and quantification limit (LOQ) values (μg/mL).
UHPLC determination was performed in triplicate, and the UA content (UAC) value is represented as the mean (n = 3) ± standard deviation (SD); UAC = 0.915 ± 0.018 mg/g UBO.
2.5. Total Phenolic Content
The absorbance values for both oil samples were read at 760 nm, with water as a blank (because the reagents were prepared using water as a solvent). The total phenolic content (TPC) values extracted in ethanol 96% were 2.592 ± 0.097 mg PyE/g in UBO and 2.243 ± 0.049 mg PyE/g in CNO. The TPC values obtained using acetone were 2.277 ± 0.057 mg PyE/g in UBO and 1.769 ± 0.039 mg PyE/g in CNO (Table 5).

Table 5.
Total phenolic content in UBO and CNO.
2.6. Antioxidant Activity
The calculated % DPPH-radical scavenging value for UBO (82.182 ± 0.595%) was significantly different (p < 0.05) from that reported for CNO (64.806 ± 0.399%). Usnic acid 0.8 mg/mL in acetone had the lowest AA: 4.687 ± 0.025% DPPH-radical scavenging, significantly different compared to both oil samples (p < 0.05). The data displayed in Table 6 prove that UBO had a higher antioxidant activity than canola oil; the DPPH IC50 value for UBO is 0.942 ± 0.004 mg/mL and for CNO is 1.361 ± 0.008 mg/mL.

Table 6.
Antioxidant activity of UBO and CNO, and correlation between TPC and AA in both oil samples.
The correlation between TPC and AA (expressed as % DPPH-radical scavenger) was evaluated using linear trendlines, linear equations, and correlation coefficients (R2) for UBO and CNO (Table 6). The R2 values for UBO and CNO showed that their antioxidant activity is highly correlated with phenolic content (R2 > 0.900).
2.7. Cytotoxic Activity
Both emulsions examined under a microscope with different magnifications (40×, 100×, 400×) at 20 °C are displayed in Figure 4. It can be observed that the CNO emulsion is more uniform than that of UBO.
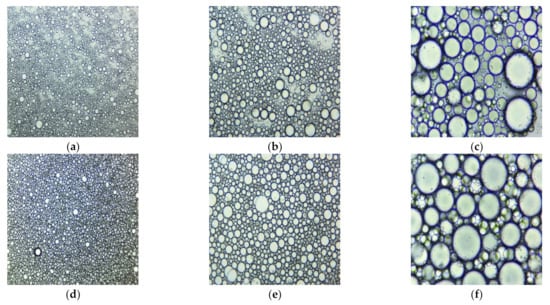
Figure 4.
Microscopical images at 40×, 100×, and 400× magnification of UBO (a–c) and CNO (d–f), emulsions: 40× (a,d), 100× (b,e), and 400× (c,f) (20 °C).
After 24 and 30 h, the viability of A. salina larvae exposed to negative controls (saline solution 0.3% and Poloxamer 407 5%) was 100%. The control solutions from the experimental pots had a regular aspect according to the second larval stage, swimming and showing the normally visible movements.
However, microscopic examination with magnitudes of 100× and 400× evidenced some slight changes in the digestive tract of the larvae exposed to Poloxamer 407 (Figure 5). These modifications consisted of an enlargement of the digestive tract and the presence of more lipid particles, with increased spaces between them (Figure 5g–i), compared to brine shrimp nauplii from the saline solution (Figure 5d–f).
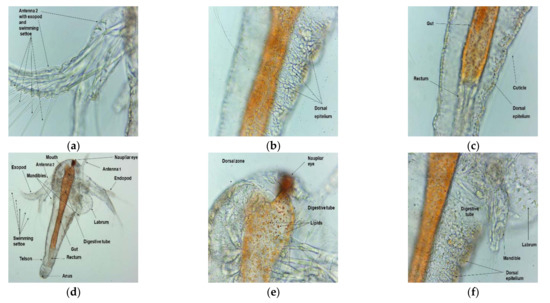
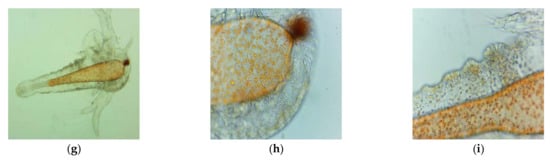
Figure 5.
Microscopic images of A. salina nauplii morphology after 30 h (summing both steps times of BSL-assay, 24 h + 6 h) of exposure in saline solution 0.3% (a–f) and Poloxamer 407 at 3:1 dilution (g–i) with different magnifications: 100× (d,g) and 400× (a–c,e,f,h,i).
After 24 h of exposure at the first dilutions (1:1, 2:1, 3:1) of UBO and CNO stock emulsions, all brine shrimp larvae were considered dead, because they did not show visible movements in experimental pots. By microscopical examination with 400× magnification, the coexistence of actually dead larvae and ones with fine antennae and peristaltic movements was only observed at UBO 1:1 and CNO 1:1 and 2:1 dilutions. Different and significant morphological changes, highlighted by microscopy at 100× and 400× magnifications and correlated with the dilution values, are shown in Figure 6. In Figure 6, it can be seen, first of all, that the larvae swallowed both UBO and CNO emulsions; second, it is evident that both emulsions caused a blockage in the digestive zone. The cytotoxic effect of CNO and UBO, leading to the death of A. salina larvae after 24 h, resulted from their penetration into the larvae’s body through the digestive tract. At 3:1 dilution, the tissue destruction phenomena were significantly increased in both samples.
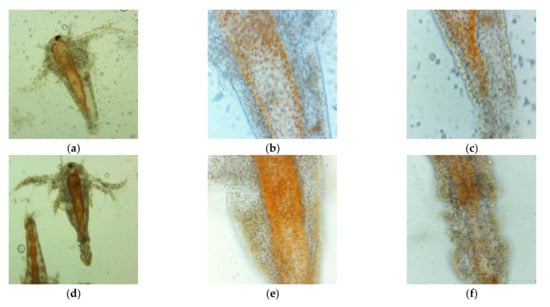
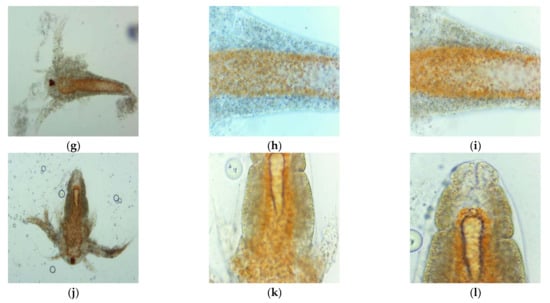
Figure 6.
Morphological changes induced by UBO (a–c,g–i) and CNO (d–f,j–l): 1:1 (a–f) and 2:1 (g–l) on A. salina larvae after 24-h exposure, in microscopical images at different magnifications: 100× (a,d,g,j) and 400× (b,c,e,f,h,i,k,l).
The UBO-induced intestinal blockage weakened the digestive membranes, with epithelium structure disorganization in the terminal abdominal area, tissue destruction, expulsion of intestinal contents (at high concentrations), and finally, death of brine shrimp nauplii (Figure 6a–c,g–i). CNO caused a progressive digestion blockage, followed by a progressive invasion of the surrounding tissues with lipids. The A. salina larvae gradually died due to the increased body structure alterations induced by the aggressive emulsified lipid accumulation (Figure 6d–f,j–l). The lipid emulsification from both oil samples facilitates their passage through biological membranes and cell penetration. Thus, it is justified that the cytotoxicity phenomena were manifested relatively quickly, profoundly affecting the A. salina larvae in 24 h. Moreover, usnic acid and other cytotoxic lichen phenolic metabolites in UBO accelerated tissue damage, and larval death occurred faster than in the case of CNO. Summing the lipid accumulation with the canolol cytotoxic action also explains CNO’s intense effects on brine shrimp nauplii after 24 h.
All these observations led to the next step of the BSL assay, diminishing the larvae’s exposure time to only 6 h and simultaneously increasing the dilutions of both emulsions in the range 1:1–1:4. The results are displayed in Table 7 and Figure 7.

Table 7.
The results obtained from the BSL assay after 6 h of A. salina larvae exposure, at different dilutions of UBO and CNO emulsions.
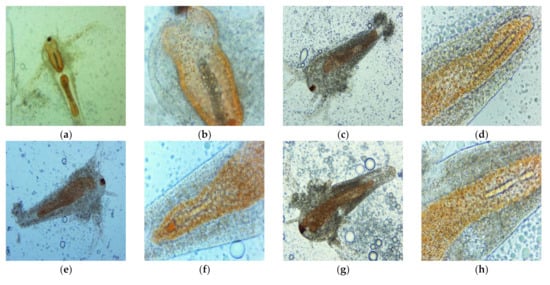
Figure 7.
Morphological changes induced by UBO (a,b,e,f,i,j,m,n) and CNO (c,d,g,h,k,l,o,p) with different dilutions: 1:1 (a–d), 1:2 (e–h), 1:3 (i–l), 1:4 (m–p) on A. salina larvae after 6 h exposure, in microscopical images at different magnifications: 100× (a,c,e,g,i,k,m,o) and 400× (b,d,f,h,j,l,n,p).
Data from Table 7 show the highest cytotoxicity (76.000 ± 5.354 %) for the UBO emulsion at 1:1 dilution (15% UBO). Thereby, the UBO cytotoxic action, in this case, is highly correlated (r > 0.984, p < 0.05) with UA, phenolic secondary metabolites, and heavy/trace minerals (Cr, Cu, Ni, Al) content. All these organic and mineral constituents are known for their cytotoxicity. Usnic acid, lichen phenolic metabolites, and copper are only found in UBO; Al, Ni, and Cr were quantified in UBO in higher content than in CNO. They could act synergistically with the CNO’s phytoconstituents and generate a significant cytotoxicity. Their penetration into the larvae cells was facilitated by the lipid emulsification of the U. barbata oil extract. The data from Table 7 show that UBO cytotoxicity did not vary proportionally with UBO concentration when the sample’s dilution increased. At 1:2 (10% UBO), larvae mortality significantly decreased to 44.583 ± 4.125%. Furthermore, higher UBO dilutions (1:3 and 1:4) induced similar mortality values as for CNO (24.912 ± 1.464 and 21.025 ± 1.450%, versus 21.801 ± 2.800 and 20.134 ± 1.652%).
The UBO cytotoxicity recorded approximately similar values, in the range 20–30%, with progressively diminished differences when the dilution was increased. Table 7 shows that 29.444 ± 3.425% was the highest mortality induced by CNO. The following values were fairly similar: 22.692 ± 2.059, 21.801 ± 2.800, and 20.134 ± 1.652 %, respectively.
2.8. Color Evaluation
The color parameters of oil samples and the refractive indexes are displayed in Table 8. The UBO sample luminosity (L*) was 46.597 ± 0.058 and shows a significant difference (p < 0.05) compared to CNO, which had a value of 49.293 ± 0.072. The green nuance described by the negative values of the a* parameter was lower for UBO (−3.213 ± 0.006) than CNO (−3.950 ± 0.026). In contrast, the yellow nuance indicated by the positive values of b* was more pronounced in CNO (29.040 ± 0.062) than in UBO (26.843 ± 0.038).

Table 8.
Color evaluation and rheological properties of UBO and CNO.
Hue angle (hab) did not show significant differences (p > 0.05) among oil samples. Both samples were classified as greenish-yellow (>90°) and green color (<180°), according to the diagram with the sequence of colors according to hue angle [58]. On the other hand, Chroma (C*) varied significantly, from 27.035 ± 0.038 for UBO, to 29.307 ± 0.065 for CNO. The color difference (ΔE) between UBO and CNO was 3.556 ± 0.095, which is considered a clear difference [49]. The refractive index did not highlight significant differences between the canola oil and U. barbata extract in canola oil.
2.9. Rheological Properties
The flow behavior of the oil samples was determined by steady shear measurement, as shown in Figure 8.
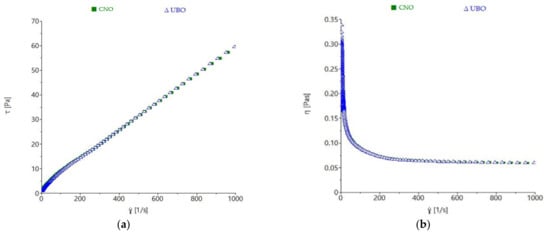
Figure 8.
Influence of shear rate on (a) shear stress and (b) viscosity of UBO (U. barbata extract in canola oil) and CNO (canola oil).
A proportional increase of shear stress (τ) with shear rate (γ) was observed in both oils. Their viscosity decreased to about 250 s−1 shear rate, showing a non-Newtonian behavior, and remained constant after these values, exhibiting a Newtonian-like liquid character from these points onward. The shear stress and viscosity values were similar for UBO and CNO, with no noticeable differences observed. Power-law model parameters also showed that no significant differences (p > 0.05) for consistency index (K) and flow index (n) between CNO and UBO were obtained (Table 7).
Thixotropy is a time-dependent shear thinning characteristic and is defined as ‘the continuous reduction of viscosity with time when shear stress is applied to a sample that has been previously at rest, and the subsequent recovery of viscosity in time when the shear stress is discontinued’ [59]. Both oil samples a exhibited time-dependent behavior, which was more pronounced in UBO than CNO (Figure 9a).
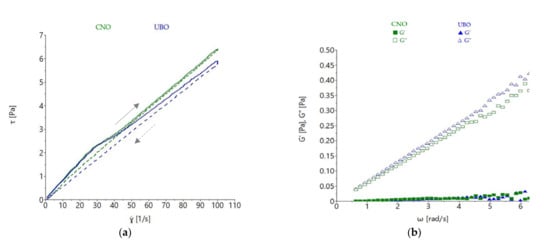
Figure 9.
(a) Thixotropy loops of UBO—U. barbata extract in canola oil and CNO—canola oil, (b) variation of storage (G′) and loss (G″) moduli with frequency: UBO—U. barbata extract in canola oil, CNO—canola oil.
UBO presented a higher thixotropy area (32.763 ± 1.975 Pa·s) than CNO (17.430 ± 0.990 Pa·s), as shown in Table 7.
The measurements conducted in oscillatory mode showed that the CNO and UBO samples presented higher loss modulus values (G″) than storage modulus (G′), which was expected; with a proportional increase of both moduli with frequency increase being observed (Figure 9b). The storage modulus measures the energy stored by the sample, while the loss moduli expresses the energy lost during deformation; a liquid material exhibiting values of G′ close to zero [60]. As expected, the G′ values of both oil samples were close to 0, with G″ values being much higher than G′, which indicated that the energy required to deform the sample was dissipated viscously. The rheological behavior was characteristic of a liquid [60]. The differences regarding G′ and G″ among samples were not appreciable, the values obtained at more than 5 rad/s being characteristic of a disorganized state [49].
The variations of apparent viscosity with temperature are shown in Figure 10.
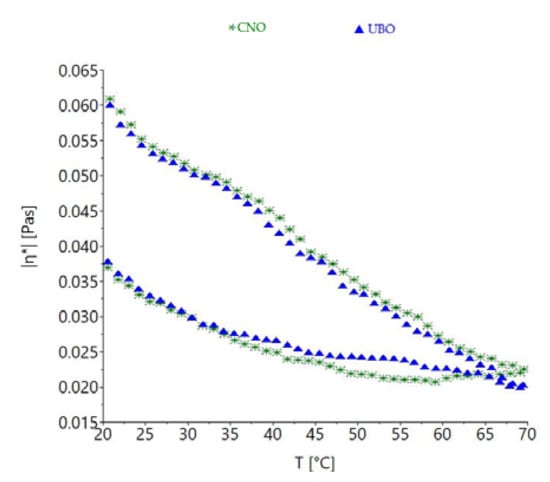
Figure 10.
Variation of apparent viscosity (η*) with temperature: UBO—U. barbata extract in canola oil; CNO—canola oil.
As expected, both UBO and CNO viscosity values were strongly reduced with temperature increase, with the curve of apparent viscosity being higher for the region corresponding to the temperature increase of 20–70 °C. Minor differences among the oil samples’ viscosity behavior with temperature were observed.
2.10. Relationships between Characteristics
The similarities and differences among the considered characteristics are presented in Figure 11 and detailed in Supplementary Material.
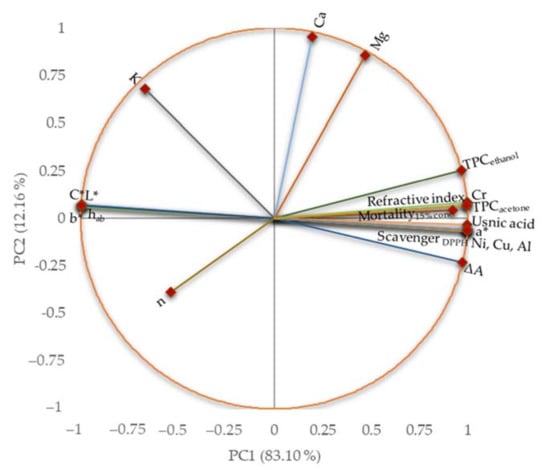
Figure 11.
Principal component analysis (PCA) plot for UBO and CNO characteristics: L*—luminosity, a* and b*—color indicator parameters, hab—hue angle, C*—chroma (color intensity indicator), K—consistency coefficient, n—flow index, ΔA—thixotropy area, Al—aluminum, Ca—calcium, Cr—chromium, Cu—copper, Mg—magnesium, Ni—nickel, Mortality 15% conc.—mortality of A. salina larvae at 15% oil sample concentration, TPC—total phenolic compounds.
The first principal component (PC1) explained 83.10% of the data variance, while the second one (PC2) explained 12.16% of the total variance, which quantified 95.26%. The total polyphenols contents, color parameters, usnic acid content, thixotropy, and some metal contents (Ni, Cu, Al, and Cr) were associated with PC1. In contrast, Ca, Mg, and the consistency index were associated with PC2. There was an opposition between L*, C*, b*, and hab color parameters and usnic acid content and TPC.
The most significant correlations (r > 0.878, p < 0.05) between the studied characteristics are presented in the Supplementary Material. Thus, a considerably positive correlation (r > 0.947, p < 0.05) was obtained between a* parameter and DPPH radical scavenging activity, TPC, usnic acid, and Cr, Cu, Ni, and Al content. In contrast, all other color parameters were negatively correlated (r < −0.945, p < 0.05) with all the characteristics previously mentioned. The refractive index was positively correlated (r > 0.895, p < 0.05) with DPPH radical scavenging activity, TPC, usnic acid, Cr, Cu, Al, and Ni contents, and negatively correlated with all color parameters (r < −0.901, p < 0.05). The thixotropy was positively correlated (r > 0.878, p < 0.05) with DPPH radical scavenging activity, TPC, usnic acid, Cr, Cu, Ni, and Al contents. As expected, both TPC values were remarkably correlated (r > 0.948, p < 0.05) with DPPH radical scavenging activity and usnic acid content. Strongly positive correlations (r > 0.947, p < 0.05) were observed between TPC, usnic acid, Al, Cr, Cu, and Ni content. Finally, A. salina larvae mortality at 15% oil sample concentration showed the highest correlation (r > 0.984, p < 0.05) with TPC, usnic acid, Cr, Cu, Ni, Al contents, DPPH radical scavenging, and thixotropy.
3. Discussion
Based on green chemistry and green engineering principles, vegetable oils could become an ideal alternative solvent to extract various compounds [43]. They can be obtained from renewable resources at acceptable prices and be easily recycled. Goula et al. (2017) performed a green ultrasound-assisted extraction of carotenoids from pomegranate wastes using sunflower and soybean oils [61]; thereby, they obtained oils enriched with antioxidants. Li et al. (2014) [42] described a green oleo-extraction of natural volatile and non-volatile bioactive compounds from rosemary leaves with 12 refined vegetable oils (soybean, grapeseed, rapeseed, peanut, sunflower, olive, avocado, almond, apricot, corn, wheat germ, and hazelnut oils) and optimized them [62]. The U. barbata oil extraction was adapted from the simple and low-cost method presented by Bassiouni et al. [52]. They used 1000 mL sunflower oil; however, we opted for only 500 mL cold-pressed canola seed oil to approximately 20 g ground lichen.
Wickramasuriya et al. (2015) [63] showed that canola meal is a quality source of essential minerals; it contains Ca (0.68%), Mg (0.64%), Cu (10 µg/g), Fe (159 µg/g), Mn (54 µg/g), and Zn (71 µg/g). Of these six minerals, only Ca (74.711 ± 4.048 µg/g) and Mg (6.852 ± 0.099 µg/g) were quantified in canola oil; moreover, three other metals (Table 2) were found in low contents: Al (0.975 ± 0.049 µg/g), Cr (0.158 ± 0.002 µg/g), and Ni (0.339 ± 0.004 µg/g). These elements could have been accumulated due to the different technologies applied and vessels used in the canola oil obtaining process. According to international requirements for edible oils, the copper and nickel contents (0.713 ± 0.005 µg/g and 0.195 ± 0.005 µg/g, respectively) were higher than the permissible limits (0.1 µg/g Cu and 0.2 µg/g Ni [62]) in UBO; with only nickel (0.339 ± 0.004 µg/g) in CNO. Generally, when heavy metals are found in edible oils, the vegetable seed oil needs refining, to remove part of these for safe use [64]. Moreover, the admissible heavy metal levels of Cu and Ni in herbal medicines are 150 µg/g and 2.14 µg/g, respectively [65], higher than the Cu and Ni contents in UBO and CNO.
The FT-IR spectra evaluation showed that UBO exhibited higher absorbances in some bands. Thus, the appearance of a peak at 1626 cm−1 assigned to the C=C bonds from the aromatic nucleus and the increased absorbance in 2930 cm−1 wavenumber in UBO could be explained by the presence of usnic and placodiolic acids from U. barbata [11]. The absorption band at 1700 cm−1 in UBO, attributed to the carbonyl C=O group, also supports this hypothesis. The intense peaks observed in UBO at 2930, 2960, and 3011 cm−1 could also be related to the O–H stretching vibration of alcohols, polyphenols, carbohydrates, peroxides, and polysaccharides [66] extracted from the U. barbata dried lichen.
The usnic acid content of 0.915 mg/g UBO obtained using the UHPLC method corresponds to 2.162 mg% usnic acid in dried U. barbata lichen (Table 8). In another previous study, usnic acid was quantified in U. barbata acetone extract, and a similar UA concentration (2.115 mg %) was obtained [5]. Furthermore, Cansaran et al. (2006) [67] prepared six acetone extracts of Usnea sp of Anatolia (U. florida, U. barbata, U. longissima, U. rigida, U. hirta, and U. subflorida) by extracting 0.05 g of ground air-dried lichens with 10 mL acetone at room temperature. Using an HPLC method, they quantified usnic acid, obtaining 2.160 mg% UA content in U. barbata dried lichen (Table 8). In our research on the native lichen species U. barbata from the Călimani Mountains, Romania, we performed comparative studies on five dry extracts in different ‘preferable’ solvents, according to the green chemistry concept (acetone, ethyl acetate, ethanol, and methanol) [13]. They were obtained using Soxhlet extraction for 8 h, evaporating the solvent, and drying the extract in a niche for 12 h. In U. barbata dry extracts in ethanol, methanol, acetone, and ethyl acetate, the usnic acid content range was 127.21–376.73 mg/g (Table 8), notably higher than in UBO (0.915 mg/g). Finally, Zizovic et al. (2012) [68] and Ivanovic et al. (2013) [69] highlighted the advantage of high-pressure processing and supercritical fluid extraction (SFE) with carbon dioxide for usnic acid extraction from U. barbata. Using the same pressure (30 MPa) and different temperature values (40 °C and 60 °C, respectively), Zizovic et al. [68] obtained U. barbata extracts with minimal yields (0.60% and 0.38%, respectively) with significant usnic acid content (364.9 mg/g and 594.8 mg/g extract, respectively) in the Autoclave Engineers Screening System. The essential comparative data are displayed in Table 9.

Table 9.
Various extraction processes with different conditions correlated with usnic acid content expressed as mg/g extract and % UA in dried U. barbata lichen.
Examining the data from Table 9, it can be seen that UBO shows 2.162 mg usnic acid/ 100 mg dried lichen, similar to UB-SFE obtained at 30 MPa, and 40 °C and 60 °C (2.190 and 2.226% UA), and higher than UB-SFE prepared at 50 MPa and 40 °C (1.243% UA). Moreover, U. barbata dry extract in ethyl acetate (obtained in our previous study) has a higher yield (6.27%), usnic acid content as mg/g extract (376.73 mg/g), and mg% in dried lichen (2.262%) than U. barbata obtained using SFE with CO2 at 40 °C (0.60% yield, UA = 364.9 mg/g UB-SFE and 2.190% UA) and a significantly lower cost price. Finally, UB-SFE obtained at 30 MPa and 60 °C recorded the highest UA content as mg/g extract (594.80 mg/g) but the lowest yield (0.38%).
Ivanovic et al. (2013) [69] performed various lichen thallus pre-treatments (in various mills: roller mill, ultra-centrifugal mill, and cutting mill ± rapid gas decompensation) and with different SFE conditions (pressure, temperature, CO2 pressure), aiming to obtain UB-SFE extracts with significant UA contents (Table 10).

Table 10.
The influence of lichen thalli pre-treatment and extraction conditions on usnic acid (UA) content and extraction yield in UB-SFE [69].
Generally, it can be observed that the concentration of usnic acid in supercritical extract varies inversely in proportion with the extraction yield. The highest usnic acid content (648 mg/g UB-SFE) was obtained with ultracentrifugation and shearing as pre-treatments, and 40 °C, 50 MPa, and 992 m3/kg CO2 extraction conditions (Table 10). The UA content was significant, varying between 423–648 mm/g extract; the yield was very low (in the range 0.78–2.28%) and %UA/dried lichen of 0.479–1.243%.
The TPC values were 2.592 ± 0.097 mg PyE/g in UBO, and 2.243 ± 0.049 mg PyE/g in canola oil; two phenolic acids (cinnamic acid and p-coumaric acid) found in CNO were identified in U. barbata in another previous study [5]. In the sunflower extract of U. barbata (originated from Mexico), Basiouni et al. (2020) [29] recorded a phenolic content (4.4 mg ± 1.4 mg/mL) higher than the TPC determined in UBO. All the U. barbata dry extracts previously mentioned [13] displayed a total phenolic content in the range of 42.40–101.09 mg PyE/g, significantly higher than UBO (2.592 ± 0.097 mg PyE/g). However, their antioxidant activity was considerably lower (DPPH IC50 = 3.300–7.701 mg/mL) than that of UBO (DPPH IC50 = 0.942 ± 0.004 mg/mL). The remarkable antioxidant activity of UBO is due to the coexistence of the lichen phenolic secondary metabolites and canola oil bioactive constituents (phenolic acids, tocopherols, phytosterols, and carotenoids). The dried lichen extraction in canola oil could synergize cold-pressed rapeseed oil phytoconstituents with lichen secondary metabolites. Canola oil is rich in PUFA; in this case, the antioxidant activity could be defined as limiting or inhibiting nutrient oxidation (especially lipids and proteins) by restraining oxidative chain reactions [70]. Lichen phenolic compounds could delay the canola oil rancidity, avoiding lipid autooxidation; a fact also confirmed by the strong correlations obtained between scavenging DPPH activity, and TPC and usnic acid contents (r > 0.956, p < 0.05); they could break one of the phases of initiation or propagation of lipids autooxidation by hydrogen donation or electron transfer [71]. The dual redox behavior of usnic acid also justifies the high correlation (r > 0.984, p < 0.05) between antiradical activity and the cytotoxic effect of UBO.
Moreover, lichen phenolic metabolites in UBO and their interaction with CNO phytoconstituents significantly influenced the properties of the corresponding emulsions and the morphological changes in A. salina larvae, exposed to diluted samples for both 12 and 6 h. Simultaneously, the lipid emulsification from both oil samples shortened the time of passage through cell membranes and accelerated the penetration of usnic acid and other cytotoxic phenolic metabolites into the cells. This process explains how the cytotoxic phenomena with morphological changes and tissue damage, and ending with the death of A. salina larvae, were installed so quickly. Initially, based on the results of our previous studies [13], the sample dilutions were made referring to the usnic acid content estimated in each diluted sample. The exposure time was 24 h, as we utilized in previous studies on the other U. barbata extracts [13,72]. However, due to the mechanisms mentioned above, we did not quantify the cytotoxicity assay results gradually (dose-dependent) because the resulting mortality was 100% for all sample dilutions. Only by decreasing the exposure time from 24 to 6 h and the concentration of both extracts we managed to see different cytotoxic effects at dilutions with successively decreased usnic acid concentration. At the lowest dilution, 1:1, with the highest % UBO (15%), the death rate obtained showed the highest correlation (r > 0.984, p < 0.05) with oil extract mineral constituents, with four heavy/trace metals (Cr, Cu, Ni, and Al). This strong correlation could also have been the consequence of using the oil extract as an emulsion. With the accumulation of emulsified lipids, they penetrated and accumulated in the cells; thus, these metals synergistically act with lichen metabolites, inducing oxidative stress [73,74,75,76,77] and accelerating cell damage.
The color and rheological parameters of UBO and CNO are directly correlated to their constituents. Color parameters showed significant differences (p < 0.05) between UBO and CNO in luminosity, green nuance, yellow nuance, and chroma. The increase of darkness and green nuance, the decrease of color purity (described by chroma), and the diminishing of yellow nuance in UBO could be related to the colored constituents of U. barbata dried lichen (chlorophyll and phenolic compounds); a fact also supported by the significant correlations obtained in this study. The color parameters of various oil extracts in CIELab colorimetric systems have been previously studied [78]. The color difference (ΔE) between UBO and CNO was 3.556 ± 0.095; Buhalova et al. (2014) [79] obtained higher color differences when performing extraction of pine cones, oregano, and thyme with sunflower oil. They prepared oil extracts in a 1:5 ratio (herb: sunflower oil), keeping them in refrigerated conditions (0–4 °C) for six months. ΔE decreased in the following order compared to the control (sunflower oil): pinecone oil extract (21.6), thyme oil extract (13.0), and oregano oil extract (10.9). The refractive index did not register significant differences (p > 0.05) among both oil samples, because the specific gravity, molecular weight, and polarity of constituents in UBO did not change significantly compared to CNO; our results were close to those reported by Önal and Ergin for canola oil [80]. In the previously mentioned study, the pinecone extract had a decreased brightness and increased color difference compared to the control [79]; in our study, UBO showed diminished luminosity and recorded color differences compared to CNO as the control.
Rheological measurements are valuable analyses for oil extract flow behavior. Rheological property determination has an essential role in describing heat transfer, or in designing, evaluating, and modeling various treatments, with many applications in pharmaceutical [60] and food sciences [81]. Viscosity is the most important parameter evaluated by the rheological methods applied for various fluids, to identify their texture [82]. The rheological characteristics of oil extracts are influenced by many factors, such as temperature, shear rate, time, concentration, pressure, physicochemical properties, and phytoconstituents [83]; however, this type of flow is determined by temperature variation. Both UBO and CNO showed a proportional increase of shear stress with shear rate, and a decrease in viscosity with share rate increase up to 250 s−1, it being thought that this behavior could be due to the CNO long-chain molecules [60]. Flow index values closer to 1 indicate a viscous material, while values close to 0 are specific to elastic ones [84]; in our study, UBO and CNO displayed flow index values around 1.20, proving a viscous character. Aksoy et al. (2010) [85] reported a decrease of canola oil viscosity with temperature increase. Hojjatoleslamya et al. (2005) [86] studied the viscosity of some oils (soybean oil, sunflower oil, and canola oil) as a function of the shear rate; they also examined shear stress as a function of shear rate at different temperatures. The results obtained revealed that during heating, oils with a higher unsaturated fatty acid content (as soybean oil and canola oil) displayed a more rapid viscosity change with temperature than oils containing lower amounts of the previously mentioned acids (sunflower oil), due to their loosely packed structure [86]. Studying 12 vegetable oils (almond, canola, corn, grapeseed, hazelnut, olive, peanut, safflower, sesame, soybean, sunflower, and walnut), Fasina et al. (2006) [87] reported that their viscosities were positively correlated with MUFA content and negatively correlated with the amount of PUFA. Moreover, they stated that the viscosity of vegetable oils could be predicted, by knowing PUFA and/or MUFA contents. This aspect can be used in the pharmaceutical domain, in the selection and design of equipment and processes for using and storing vegetable oils and oil extracts.
A time-dependent viscosity change is the most desirable property in pharmaceutical formulations, due to their requirement of flexibility in drug delivery [88]. When the rheological manifestation of viscosity-producing structural changes is reversible and time-dependent, the effect is called thixotropy [89]. According to obtained data, the UBO thixotropy area (ΔA = 32.763 ± 1.975 Pa·s) being higher than CNO (17.430 ± 0.990 Pa·s) was due to the phenolic lichen metabolites quantified in UBO; their antioxidant activity could limit the incidence of irreversible structural changes in canola oil. These facts are also supported by the positive correlations between thixotropy and TPC and usnic acid content (r > 0.878, p < 0.05). Rheological pattern is also a valuable quality control procedure in pharmaceutical research, regarding drug formulation development for optimal delivery. Thus, this can justify the strong correlation (r > 0.984, p < 0.05) between cytotoxicity and thixotropy in oil extracts. While a 32 ± 0.5 °C value represents a commonly adopted parameter for the simulation of the stratum corneum conditions for topical pharmaceutical formulations, in the case of oral ones, a 37 ± 0.5 °C value is relevant for predicting the oils in vivo behavior [90]. The profile registered on the hysteresis curve is significant in the 32–37 °C range; UBO and CNO viscosity and viscoelastic behavior indicate a clear weakening of the intermolecular bond when the temperature increases, leading to a more liquid state, useful for an enhanced spreadability (in case of topical application) and flowability (in case of oral intake). Due to the change in activation energy, a linear relationship between viscosity and temperature can be seen. For oral pharmaceutical formulations, flowability is essential for ensuring a controlled deformation after intake; favorable for constituent release and increased compliance.
4. Materials and Methods
4.1. Materials
All chemicals, reagents, and standards used in our study were of analytical grade. Usnic acid standard 98.1% purity, DPPH, and Poloxamer 407 were purchased from Sigma-(Sigma-Aldrich Chemie GmbH., Taufkirchen, Germany); 65% HNO3, 30% H2O2, Folin-Ciocâlteu reagent, Pyrogallol, acetone, and ethanol were supplied by Merck (Merck KGaA, Darmstadt, Germany).
Artemia Brine Shrimp Eggs and Artemia salt (Dohse Aquaristik GmbH & Co. Gelsdorf, Germany) were bought online from https://www.aquaristikshop.com/ (accessed on 5 February 2022). Canola oil (TAF PRESOIL SRL, Cluj, Romania) was purchased from the manufacturer’s distribution units.
4.2. Lichen Extract Preparation
U. barbata thalli were harvested one by one from the branches of conifers in the Călimani Mountains (47°29′ N, 25°12′ E, and 900 m altitude) [53]—the highest Romanian volcanic mountains—in March 2020. The freshly collected lichen was separated from impurities; then, it was dried at 18–25 °C in a herbal room, protected from sunlight. Dried lichen preservation for an extended period was performed in similar conditions (Figure S4a, Supplementary Material). The Department of Pharmaceutical Botany of the Faculty of Pharmacy, Ovidius University of Constanta, accomplished U. barbata identification using the standard methods [68]. It is preserved in the Herbarium of Pharmacognosy Department, Faculty of Pharmacy, Ovidius University of Constanta (Popovici 2/2020, Ph-UOC) [91].
To determine loss on drying for the lichen sample, a weighing ampoule was brought to a constant weight together with the lichen sample was kept in the oven at 105 °C for two hours; then cooled in a desiccator and weighed. The drying process continued in the oven for one hour [13], then cooled and weighed until a constant weight was achieved [92].
The extract of U. barbata in canola oil (UBO) was obtained using a method adapted from that described by Basiouni et al. (2020) [29]; from 20.2235 g dried and ground lichen (Figure S4b, Supplementary Material) and 500 mL cold-pressed canola seed oil (TAF PRESOIL SRL, Cluj, Romania), in a dark place, at room temperature (21–22 °C). The brown container with both components was daily shaken manually for three months; after this period, UBO was filtered in a brown vessel with a sealed plug and preserved in a plant room, sheltered from sun rays. Both oil samples had a pH = 4.
4.3. Mineral Analysis
Both samples, CNO and UBO, were used for ICP-MS mineral analysis, according to European Pharmacopoeia 10.0 [70]; 23 metals were analyzed: Ca, Fe, Mg, Mn, Zn, Al, Ag, Ba, Co, Cr, Cu, Li, Ni, Tl, V, Mo, Pd, Pt, Sb, As, Pb, Cd, and Hg, using the ICP ability to generate charged ions from the metal species within the lichen sample [93]; thus, they were guided into a mass spectrometer and separated according to their mass-to-charge ratio (m/z). This ICP-MS method was detailed in our previous study [21].
The quadrupole inductively coupled plasma mass spectrometer was a NexION™ 300S (PerkinElmer, Inc., Hopkinton, MA, USA) with a triple cone interface and a four-stage vacuum system. This ICP-MS system is equipped with a universal cell with two gas lines (helium, ammonia, methane), which allows operation in collision mode (helium) and reaction mode (ammonia/methane) [21]. It is also equipped with a recirculating chiller (Perkin Elmer Shelton) and a peristaltic pumping system with acid-resistant tubing; 0.38-mm interior diameter (id) tubing for sample introduction and 1.3-mm id for drain exclusive. The samples were digested in mineralization Teflon vessels using Rotor 16HF100 in a PROSOLV microwave digestion system (Anton Paar GmbH, Graz, Austria), using a pressure-activated-venting concept. The Directed Multimode Cavity (DMC) enables highly efficient turbo heating with one magnetron in a compact system combined with a turbo cooling system, for rapid cooling from 180 °C to 70 °C [21]. The ICP-MS mineral analysis was performed using the kinetic energy discrimination (KED) method, measuring unit = counts per second (CPS). The peristaltic pumping system was washed with each sample (35 s), followed by a read delay (15 s) and the analytical phase. Finally, the peristaltic pump was washed with ultrapure deionized water (45 s). All processes involved an operation speed = 20–24 rotations / minute (rpm) [21].
The data obtained for mineral content were processed with Syngistix Software (PerkinElmer, Inc., Hopkinton, MA, USA) version 2.3 for ICP-MS. Mineral analysis was performed in triplicate, and the results are expressed as mean (n = 3) ± SD.
4.4. FT-IR Spectra Acquisition
FT-IR spectra of both oil samples were recorded in triplicate from 650 to 4000 cm−1 wavenumbers on a Thermo Scientific Nicolet iS20 (Waltham, MA, USA) spectrometer. The average spectra were used for interpretation. The resolution value was 4 cm−1. The data were processed with OMNIC software (9.9.549 version, Thermo Fisher Scientific, Waltham, MA, USA), and the characteristic bands were identified according to previous literature [52,53,62].
4.5. UHPLC Determination of Usnic Acid Content in Usnea barbata Extract in Canola Oil
In order to assess the concentration of usnic acid extracted in canola oil, the UHPLC method developed and validated in our previous studies [28] was adapted for this new purpose.
4.5.1. Equipment and Chromatographic Conditions
In brief, the method for usnic acid quantification entailed analyzing the U. barbata extract in canola oil using a PerkinElmer® Flexar® FX-15 UHPLC system (PerkinElmer, Shelton, CT, USA) fitted with a Flexar FX PDA-Plus photodiode array detector (PDA). The UHPLC-PDA system is equipped with a Brownlee Analytical C18 column, having a length of 150 mm, an inner diameter of 4.6 mm, and filled with 5 µm porous particles (150 mm/4.6 mm, v5 µm). As a mobile phase, an isocratic methanol/water/glacial acetic acid (80:15:5) was used for 10 min per run, with a flow rate of 1.5 mL/min and an injection volume of 10 μL. The oven temperature was set to 25 °C, and the detection was made at 282 nm. All data analysis, peak purity, and processing were achieved using PerkinElmer Chromera® CDS software (PerkinElmer, Inc, Hopkinton, MA, USA).
4.5.2. Sample, Blank, Standard, and Quality Control (QC) Solutions Preparation
All requested solutions were prepared using acetone (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) as a solvent; injection volume was lowered to 10 μL to avoid peak distortion.
The sample (UBO) and blank (CNO) solutions were made by diluting around 1 g UBO or CNO 100-fold in acetone (the concentration of obtained solutions was 10 μg/mL). Another blank solution was acetone; the solvent used for all solutions.
Five standard solutions were prepared using usnic acid at 98.1% purity (Sigma-Aldrich, St. Louis, MO, USA) by serial dilution in acetone: 50, 25, 10, 5, 2.5, 1.25, 0.612, 0.3 μg/mL.
Two quality control (QC) solutions, QC1 and QC2, were prepared. The first, QC1, consisted of usnic acid standard 7.5 μg/mL in acetone. QC2 was obtained by dissolving around 1 mg usnic acid in 1 mL CNO, followed by 100-fold dilution in acetone (the QC2 final concentration was 10 μg/mL, similarly to the sample and blank solutions).
4.5.3. Validation of UHPLC Method
According to International Conference on Harmonization (ICH) guidelines (ICH Q2A 1994) [71], due to significant changes (sample matrix, sample preparation, injection volume, range of calibration curve), the UHPLC method parameters were revalidated for: specificity, precision, accuracy, linearity, the limit of detection (LOD), and limit of quantification (LOQ) [72].
4.5.4. Specificity
Specificity was evaluated by injecting one acetone blank solution, one canola oil blank solution (the same oil that was used for sample extraction) diluted 100-fold in acetone to resemble the sample, one standard solution (50 μg/mL), and one unknown sample solution for which peak purity was determined in a 240–700 nm range at 15% peak height.
4.5.5. Accuracy
Accuracy was estimated as the closeness of the experimental value to the actual amount by injecting six QC1 solutions of known concentration (7.5 µg/mL usnic acid in acetone). Moreover, the accuracy was expressed as spike recovery % by injecting four spike solutions prepared by dissolving usnic acid reference in canola oil (1 mg/mL) and diluting 100-fold (QC2). Results were expressed as the percentage accuracy by comparing the practical concentration to the theoretical one, considering the standard usnic acid purity of 98.1%. The accuracy, expressed as a percent of the spike recovery, was considered admissible when its value was 100 ± 10%.
4.5.6. Precision
For the area repeatability at the same concentration level, precision was measured by injecting six control quality solutions 7.5 μg/mL (QC1) and 6 UBO solutions at 10 mg/mL concentration (100-fold dilution). This was expressed as relative standard deviation (RSD%), with an RDS% ≤ 5% acceptance criterion.
4.5.7. Linearity
The linearity of the method was determined in the range 2.5–50 μg/mL, by calculating the coefficient of determination (R2) of the calibration curve (Figure S3, Supplementary Material) constructed from 5 repetitions for each point (Supplementary Material). An R2 value higher than 0.99 was considered an admissible criterion of linearity.
4.5.8. Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD (S/N ≥ 3) and LOQ (S/N ≥ 10) were determined by injecting successively different UA standard solutions with low concentrations in decreasing order (1.250, 0.612, and 0.300 μg/mL) and calculated using the following formula (Equation (3)):
where S—signal, N—noise, H—usnic acid peak height, and h—noise height in blank solution (acetone).
4.5.9. Data Processing
Data analysis, peak purity determination, and processing were realized using PerkinElmer Chromera® CDS software.
4.6. Total Phenolic Content
According to a previously described method, the total phenolic content was determined using Folin–Ciocâlteu reagent [13]. Pyrogallol was used as standard, the TPC values being calculated as µg of Pyrogallol equivalents (PyE) per g UBO (CNO). For this analysis, in two volumetric flasks of 25 mL, 5 mL of each CNO and UBO (A1 and A2) was added, completing up to the sign with ethanol 96%; B1 and B2 solutions were obtained. In two volumetric flasks of 25 mL, 2 mL of B1 and B2 solution was added. Then, 1 mL of Folin–Ciocâlteu reagent, 10 mL water, and 12 mL of 290 g/L of Na2CO3 solution were added up to the mark; in each volumetric flask, a blue coloration appeared. After 30 min of reaction in a dark place at room temperature, the absorbance values (each value being A1 in the calculation formula) were read at 760 nm, using a Jasco V630 UV-Vis Spectrophotometer (JASCO Corporation, Tokyo, Japan) with Spectra Manager™ software. A similar determination of phenolic contents was performed by dissolving UBO and CNO in acetone. The total polyphenols content (TPC) determination was performed in triplicate, and the obtained data were expressed as means (n = 3) ± SD.
4.7. Antioxidant Activity
The antioxidant activity of UBO and CNO was determined on a Jasco V630 UV-Vis Spectrophotometer (JASCO Corporation, Tokyo, Japan) using a DPPH free radical scavenging assay [28]. The DPPH solution was obtained by dissolution of DPPH (Sigma Aldrich, St. Louis, MO, USA) in methanol, to assess the absorbance value of 0.8 ± 0.02; then, 3.9 mL of DPPH solution with 0.1 mL of each oil sample were vortexed for 30 s. Their reaction time in a dark place at room temperature was 30 min; finally, the absorbances values at 515 nm were registered. The DPPH solution in methanol with no added extract was used as a standard, methanol as a blank, and canola oil and 0.800 mg/mL of usnic acid (0.02 g in 25 mL volumetric flask and up to the mark with acetone) as positive controls. Two dilutions in acetone were obtained (1:2 and 1:4) for UBO and CNO, and the scavenger activity was calculated according to Equation (4).
A control and A sample are the absorbencies values at 515 nm for DPPH and sample solutions. This determination was performed in triplicate; the obtained data are expressed as mean (n = 3) ± SD.
4.8. Cytotoxic Activity
4.8.1. Preparation of CNO and UBO L/H Emulsions
Poloxamer 407 (Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)) is a non-ionic and water-soluble surfactant that is made up of a hydrophobic residue of polyoxypropylene (POP) between the two hydrophilic units of polyoxyethylene (POE) [94]. The FDA guide mentions Poloxamer 407 as an inactive ingredient for different pharmaceutical preparations (intravenous fluids, oral emulsions, suspension, ophthalmic, or topical formulations) [95]. It was selected for the present study due to its low toxicity and compatibility with cells, body fluids, and a wide range of chemicals [96].
Two L/H emulsions containing the same 30% w/w concentration of UBO and CNO, as oil phase fractions, were prepared. In the first step, the selected emulsifier agent, Poloxamer 407 (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) at 5% w/w concentration, was dissolved into the amount of water required for obtaining 100 g of each emulsion. The dissolution was performed using a Heidolph MR 3001 K magnetic stirrer (Heidolph Instruments, Schwabach, Germany) at a low 300 rpm speed and room temperature. The oily phase was added slowly, the speed was increased to 1200 rpm, and the emulsion was stirred for 30 min until a homogenous binary system was achieved.
These emulsions were stored at 3–8 °C until use. Both emulsions had pH = 5.5.
4.8.2. BSL Assay
Brine shrimp larvae were obtained by introducing the cysts of A. salina for 24–48 h, in a saline solution of 0,35%, under conditions of continuous light and aeration, at a temperature of 20 °C. After hatching brine shrimp in the first larval stage (instar I), they were separated and introduced into experimental pots (with a volume of 1 mL) in 0.3% saline solutions [13]. To obtain accurate results regarding the UBO cytotoxic effect, the analysis of the UBO emulsion was performed compared to the CNO one. A. salina larvae were not fed during the test, so as to not interfere with the tested extracts. This bioassay was valid for 24 h, during which the larvae had embryonic energy reserves as lipids.
Brine shrimp larvae were initially exposed to different UBO and CNO emulsions dilutions (emulsion: saline water) for 24 h (Table 11). As negative controls, the Poloxamer 407 aqueous solution 5% w/w concentration and saline aqueous solution 0.3% (pH > 7) were used.

Table 11.
The samples and control dilutions and/or concentrations used in BSL assay for 24 h.
After 24 h of exposure, the death rate (the larvae without visible movements in their experimental pots were considered dead) was the measurable parameter for quantifying larvae response to the various concentrations of CNO and UBO. These effects were also evaluated using microscopy with different magnifications and compared with negative controls.
The second step of the BSL assay consisted of lower dilutions preparation: 1:1, 1:2, 1:3, 1:4 from stock UBO and CNO emulsions in 0.3% saline aqueous solution (Figure S5, Supplementary Material). The brine shrimp larvae were exposed for only 6 h at these new dilutions; then, the cytotoxic effects were evaluated by counting the A. salina larvae without visible movements in their pots and examining with the microscope with different magnifications, for observing the presence or absence of fine movements (antennae and peristaltic movements) and morphological changes.
4.8.3. Data Processing
The microscopic images were achieved using a VWR microscope VisiScope 300D (VWR International, Radnor, PA, USA) with Visicam X3 camera (VWR International Hilden, Germany) at 40×, 100×, and 400× magnifications and processed with VisiCam Image Analyzer 2.13. All observations were realized in triplicate.
4.9. Color and Refractive Index Evaluation
The color of oil samples was measured by reflectance using a Konica Minolta CR-400 (Konica Minolta, Tokyo, Japan) colorimeter. The luminosity (L*), red (a*+) or green nuance (a*−), and yellow (b*+) or blue nuance (b*−) were recorded in triplicate. The hue angle (hab), chroma (C*), and difference of color (ΔE) against the control sample were calculated by using Equations (5), (6), and (7), respectively. Chroma measures the degree of difference against a grey color with similar lightness for each hue. In contrast, hue angle is the parameter that allows a color to be differentiated concerning a grey color with similar lightness [69].
where hab—hue angle (0°—red, 90°—yellow, 180°—green, and 270°—blue), C*—chroma (0—gray, 100—pure color), L*—luminosity (0 for absolute black, 100 for absolute white), a*—positive describes red and negative green nuance, b*—positive represents yellow and negative blue nuance, ΔE—color difference against control, ΔL* = L*UBO − L*Control, Δa* = a*UBO − a*Control, Δb* = b*UBO − b*Control.
The refractive oil index was measured in triplicate using an Abbe portable refractometer (Kern Optics, Balingen, Germany) at 20 °C.
4.10. Rheological Properties
All rheological measurements were performed using a dynamic rheometer Thermo-HAAKE, MARS 40 (Karlsruhe, Germany) with a Peltier temperature controller, using cone (2°) and plate geometry of 35 mm diameter. The volume of the sample was 0.4 mL.
4.10.1. Steady Shear Test
Measurements were done in the shear rate range of 0.1–1000 s−1 at a temperature of 20 °C, according to the method described by Yalcin et al. [30] with modifications. The oil sample was placed with a micropipette between the cone and plate, and the test was started immediately. The flow curve, shear stress versus shear rate, and viscosity in function of shear rate were obtained by raising the shear rate. The data for shear stress vs. shear rate were fit to the power-law model (Equation (8)):
where τ—shear stress (Pa), γ—shear rate (s−1), K—consistency index (Pa·sn), n—flow index.
4.10.2. Thixotropy Loop
A thixotropic loop was obtained using a rotational rheological test; an increasing shear rate from 0 to 100 s−1 in 100 s was applied to the sample, then it was kept at 100 s−1 for 30 s, after that the shear rate was decreased from 100 to 0 s−1 in 100 s. The measurements were made at 20 °C. The areas under the upstream data points (A↑) and the downstream data points (A↓), and the thixotropy area (ΔA = A↑ − A↓) were calculated using Rheowin Data Manager software.
4.10.3. Frequency Sweep Test
Before evaluating storage (G′) and loss moduli (G″) changes with frequency, the linear viscoelastic region (LVR) of each sample was determined in the 0.1–5.0 Hz frequency range. Then, a frequency sweep test was performed at 0.3 Pa (within the LVR) and 20 °C, in a frequency range of 0.628–6.283 rad/s, according to the protocol described by Yalcin et al. [30].
4.10.4. Apparent Viscosity Variation with Temperature
For evaluating the influence of temperature on oil viscosity, the temperature was increased from 20 to 70 °C and decreased immediately to 20 °C, at a constant shear stress of 0.30 Pa and a frequency of 10 Hz. The heating rate was 5 °C/min, the method being adapted from that presented by Ennouri et al. [48].
4.10.5. Data Processing
The data obtained for the rheological properties were processed using RheoWin software 4.80.0010 version (Thermo Haake, Karlsruhe, Germany).
4.11. Statistical Analysis
The differences among samples were evaluated using the Student t-test and ANOVA with Tukey test, with differences being considered significant at p < 0.05. Principal component analysis (PCA) with Pearson correlations were performed to identify the relationships between the studied characteristics. For this purpose, XLSTAT for Excel 2021 version (Addinsoft, New York, NY, USA) was used.
5. Conclusions
Our study results recommend the canola oil extract of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania, as a valuable lichen extract, which could be a starting point for future pharmaceutical products. Further research could optimize the extraction process, to obtain a high metabolite content in U. barbata oil extract. Future studies could investigate other pharmacological activities due to synergic interaction between canola oil compounds and lichen secondary metabolites. Finally, the potential pharmaceutical applications of autochthonous U. barbata extract in canola oil could be explored, developing optimal formulations for delivery of its bioactive constituents.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants11070854/s1, Figure S1. Spectral reprocessing in 240–700 nm range at 15% of peak height for peak identity confirmation and peak purity determination for usnic acid; Figure S2. UA 10 µg recovery, a. Spike solution 1; b. Spike solution 2; c. Spike solution 3; d. Spike solution 4; Figure S3. The usnic acid standard calibration curve; Figure S4. The dried U. barbata lichen (a) whole; (b) ground, used for UBO preparation; Figure S5. UBO and CNO dilutions in triplicate for 6 h of A. salina larvae exposure.
Author Contributions
Conceptualization, V.P. and M.U.-I.; methodology, L.B., C.E.G., D.R., S.I.C., E.I.C., T.C., M.U.-I., M.O., E.-A.O. and V.S.; software, V.P., L.B., D.R., S.I.C., E.I.C., T.C., M.U.-I., S.M. and V.S.; validation, L.B., D.R., S.I.C., E.I.C., T.C., M.U.-I. and M.O.; formal analysis, V.P. and V.S.; investigation, V.P., E.-A.O., D.L. and A.C.; resources, V.P., L.B., V.S. and E.-A.O.; data curation, C.E.G., S.M., E.-A.O. and A.C.; writing—original draft preparation, V.P., D.R., S.I.C., M.U.-I. and E.-A.O.; writing—review and editing, V.P., C.E.G., L.B., M.U.-I., M.O., E.-A.O., D.L. and S.M.; visualization, L.B., C.E.G., M.O., S.M., V.S., E.-A.O., D.L., A.C. and V.B.; supervision, E.I.C., T.C., L.B., C.E.G., M.O., S.M., V.S., E.-A.O., D.L., A.C. and V.B.; project administration, V.B.; funding acquisition, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project ANTREPRENORDOC, in the framework of Human Resources Development Operational Programme 2014–2020, financed from the European Social Fund under the contract number 36355/23.05.2019 HRD OP/380/6/13—SMIS Code: 123847.
Data Availability Statement
Not available.
Acknowledgments
This study was performed in collaboration with the Research Centre of Instrumental Analysis SCIENT, Tancabesti, Ilfov, Romania; Faculty of Food Engineering and Integrated Center for Research, Development, and Innovation in Advanced Materials, Nanotechnologies and Distributed Systems for Fabrication and Control (MANSiD), Stefan cel Mare University of Suceava, Romania; and Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential. In Reference Series in Phytochemistry; Springer Science and Business Media B.V.: Berlin, Germany, 2020; pp. 175–209. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Gómez-Serranillos, M.P.; Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A. Parmeliaceae family: Phytochemistry, pharmacological potential and phylogenetic features. RSC Adv. 2014, 4, 59017–59047. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B. Studies on Antioxidant Properties of Lichen Secondary Metabolites. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 105–125. [Google Scholar]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Daba, G.M. Lichens, an alternative drugs for modern diseases. Int. J. Res. Pharm. Biosci. 2019, 6, 5–9. [Google Scholar]
- Shrestha, G.; St. Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef]
- Prateeksha; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.N.R.; Nayaka, S.; Joshi, Y.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. and the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gîrd, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of Usnea barbata (L.) F.H. Wigg. dry extracts in different solvents. Plants 2021, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic acid and Usnea barbata (L.) F.H. Wigg. dry extracts promote apoptosis and DNA damage in human blood cells through enhancing ROS levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.H.; Mesquita, T.; Miguel-dos-Santos, R.; de Almeida, G.K.M.; de Sá, L.A.; dos Passos Menezes, P.; de Souza Araujo, A.A.; Lauton-Santos, S. Inclusion complex with β-cyclodextrin is a key determining factor for the cardioprotection induced by usnic acid. Chem. Biol. Interact. 2020, 332, 109297. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: Identification of active compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Maulidiyah, M.; Darmawan, A.; Ahmad, E.; Musdalifah, A.; Wibowo, D.; Salim, L.O.A.; Arham, Z.; Mustapa, F.; Nurdin, I.F.A.; Nurdin, M. Antioxidant activity-guided isolation of usnic acid and diffractaic acid compounds from lichen genus Usnea sp. J. Appl. Pharm. Sci. 2021, 11, 075–083. [Google Scholar] [CrossRef]
- Shi, Q.; Greenhaw, J.; Salminen, W.F. Inhibition of cytochrome P450s enhances (+)-usnic acid cytotoxicity in primary cultured rat hepatocytes. J. Appl. Toxicol. 2014, 34, 835–840. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In vitro anticancer activity and oxidative stress biomarkers status determined by Usnea barbata (L.) F.H. Wigg. dry extracts. Antioxidants 2021, 10, 1141. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef]
- Maulidiyah, M.; Darmawan, A.; Wahyu, W.; Musdalifah, A.; Ode, L.; Salim, A.; Nurdin, M. Potential of Usnic Acid Compound from Lichen Genus Usnea sp. as Antidiabetic Agents. J. Oleo Sci. 2022, 134, 127–134. [Google Scholar] [CrossRef]
- Okuyama, E.; Umeyama, K.; Yamazaki, M.; Kinoshita, Y.; Yamamoto, Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995, 61, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Erfani, S.; Valadbeigi, T.; Aboutaleb, N.; Karimi, N.; Moghimi, A.; Khaksari, M. Usnic acid improves memory impairment after cerebral ischemia/reperfusion injuries by anti-neuroinflammatory, anti-oxidant, and anti-apoptotic properties. Iran. J. Basic Med. Sci. 2020, 23, 1225–1231. [Google Scholar] [PubMed]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.G.D.; dos Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; de Bittencourt Pasquali, M.A.; Rabelo, T.K.; da Rocha, R.F.; Quintans, J.L.; de Souza Araújo, A.A.; da Silva, F.A.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. Vitr. 2011, 25, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Poornima, S.; Ponnusamy, P. Antimicrobial and antiproliferative activities of depside compound isolated from the mycobiont culture of Parmotrema austrosinense (Zahlbr.) hale. J. Pure Appl. Microbiol. 2020, 14, 2525–2541. [Google Scholar] [CrossRef]
- Solár, P.; Hrčková, G.; Koptašíková, L.; Velebný, S.; Solárová, Z.; Bačkor, M. Murine breast carcinoma 4T1 cells are more sensitive to atranorin than normal epithelial NMuMG cells in vitro: Anticancer and hepatoprotective effects of atranorin in vivo. Chem. Biol. Interact. 2016, 250, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yamamoto, Y.; Kim, J.A.; Jung, J.S.; Koh, Y.J.; Hur, J.S. Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar Biol. 2009, 32, 1033–1040. [Google Scholar] [CrossRef]
- Karagoz, I.D.; Ozaslan, M.; Kilic, I.H.; Guler, I.; Uyar, C.; Tuter, D.; Kazanci, U.; Aslan, A.; Cakir, A.; Gezici, S. Hepatoprotective effect of diffractaic acid on carbon tetrachloride-induced liver damage in rats. Biotechnol. Biotechnol. Equip. 2015, 29, 1011–1016. [Google Scholar] [CrossRef]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.B.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q.F. Antimycobacterial activity of lichen substances. Phytomedicine 2010, 17, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar KC, S.; Müller, K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J. Nat. Prod. 1999, 62, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsidones with Biological Interest. Planta Med. 2021, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A.; Mez-Serranillos, M.P.G. Pharmaceutical Biology Antioxidant potential of lichen species and their secondary metabolites. A systematic review Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; dos Santos, J.P.A.; da Rocha, R.F.; de Bittencourt Pasquali, M.A.; Rybarczyk-Filho, J.L.; Araújo, A.A.S.; Moreira, J.C.F.; Gelain, D.P. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. Vitr. 2012, 26, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, C.P.; Engels, I.H.; Rothbart, A.; Lauber, K.; Renz, A.; Schlosser, S.F.; Schulze-Osthoff, K.; Wesselborg, S. Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ. 2001, 8, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Yeash, E.A.; Letwin, L.; Malek, L.; Suntres, Z.; Knudsen, K.; Christopher, L.P. Biological activities of undescribed North American lichen species. J. Sci. Food Agric. 2017, 97, 4721–4726. [Google Scholar] [CrossRef]
- Tang, J.Y.; Wu, K.H.; Wang, Y.Y.; Farooqi, A.A.; Huang, H.W.; Yuan, S.S.F.; Jian, R.I.; Tsao, L.Y.; Chen, P.A.; Chang, F.R.; et al. Methanol extract of Usnea barbata induces cell killing, apoptosis, and DNA damage against oral cancer cells through oxidative stress. Antioxidants 2020, 9, 694. [Google Scholar] [CrossRef]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of old man’s beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar]
- Çobanoğlu, G.; Sesal, C.; Birkan, A.; Karaltı, İ. Evaluation of antimicrobial activity of the lichens Physcia aipolia, Xanthoria parietina, Usnea florida, Usnea subfloridana and Melanohalea exasperata. Mod. Phytomorphol. 2016, 10, 19–24. [Google Scholar]
- Devi, G.K.; Anantharaman, P.; Kathiresan, K.; Balasubramanian, T. Antimicrobial activities of the lichen Roccella belangeriana (Awasthi) from mangroves of Gulf of Mannar. Indian J. Mar. Sci. 2011, 40, 449–453. [Google Scholar]
- Li, Y.; Fabiano-Tixier, A.S.; Ginies, C.; Chemat, F. Direct green extraction of volatile aroma compounds using vegetable oils as solvents: Theoretical and experimental solubility study. LWT-Food Sci. Technol. 2014, 59, 724–731. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef] [PubMed]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) byproducts: Optimization of extraction parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar]
- Tańska, M.; Mikołajczak, N.; Konopka, I. Comparison of the effect of sinapic and ferulic acids derivatives (4-vinylsyringol vs. 4-vinylguaiacol) as antioxidants of rapeseed, flaxseed, and extra virgin olive oils. Food Chem. 2018, 240, 679–685. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar]
- McDowell, D.; Elliott, C.T.; Koidis, A. Characterization and comparison of UK, Irish, and French cold pressed rapeseed oils with refined rapeseed oils and extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1600327. [Google Scholar] [CrossRef]
- Sobota, A.; Wirkijowska, A.; Zarzycki, P. Application of vegetable concentrates and powders in coloured pasta production. Int. J. Food Sci. Technol. 2020, 55, 2677–2687. [Google Scholar] [CrossRef]
- Kraljić, K.; Škevin, D.; Barišić, L.; Kovačević, M.; Obranović, M.; Jurčević, I. Changes in 4-vinylsyringol and other phenolics during rapeseed oil refining. Food Chem. 2015, 187, 236–242. [Google Scholar] [CrossRef]
- Siger, A.; Kaczmarek, A.; Rudzińska, M. Antioxidant activity and phytochemical content of cold-pressed rapeseed oil obtained from roasted seeds. Eur. J. Lipid Sci. Technol. 2015, 117, 1225–1237. [Google Scholar] [CrossRef]
- Basiouni, S.; Fayed, M.A.A.; Tarabees, R.; El-Sayed, M.; Elkhatam, A.; Töllner, K.R.; Hessel, M.; Geisberger, T.; Huber, C.; Eisenreich, W.; et al. Characterization of sunflower oil extracts from the lichen Usnea barbata. Metabolites 2020, 10, 353. [Google Scholar] [CrossRef]
- Cazacu, B.C.; Buzgar, N.; Iancu, O.G. Geochemical and spatial distribution of heavy metals in forest soils adjacent to the Tinovul Mare Poiana Stampei peat bog. Rev. Chim. 2018, 69, 434–438. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Jovic, O.; Smolic, T.; Jurišic, Z.; Meic, Z.; Hrenar, T. Chemometric analysis of Croatian extra virgin olive oils from Central Dalmatia Region. Croat. Chem. Acta 2013, 86, 335–344. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Dini, M.; Raseira, M.D.; Scariotto, S.; Carrà, B.G.; Abreu, E.S.; Mello-Farias, P.C.; Cantillano, R.F. Color Shade Heritability of Peach Flesh. J. Agric. Sci. 2019, 11, 236. [Google Scholar] [CrossRef]
- Yi, S.; Hao, L.; Li, S.; Song, W. The influence of water content in rice husk bio-oil on the rheological properties of coal bio-oil slurries. Energy 2019, 189, 116307. [Google Scholar] [CrossRef]
- Yalcin, H.; Toker, O.S.; Dogan, M. Effect of oil type and fatty acid composition on dynamic and steady shear rheology of vegetable oils. J. Oleo Sci. 2012, 61, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Li, Y.; Bundeesomchok, K.; Rakotomanomana, N.; Fabiano-Tixier, A.S.; Bott, R.; Wang, Y.; Chemat, F. Towards a zero-waste biorefinery using edible oils as solvents for the green extraction of volatile and non-volatile bioactive compounds from rosemary. Antioxidants 2019, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Wickramasuriya, S.S.; Yi, Y.J.; Yoo, J.; Kang, N.K.; Heo, J.M. A review of canola meal as an alternative feed ingredient for ducks. J. Anim. Sci. Technol. 2015, 57, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Zhang, J.; Ma, T.; Qiu, H.; Ma, S. Determination of heavy metal elements in different vegetable edible oils in China. Asian J. Chem. 2017, 29, 937–939. [Google Scholar] [CrossRef]
- Ssempijja, F.; Iceland Kasozi, K.; Daniel Eze, E.; Tamale, A.; Ewuzie, S.A.; Matama, K.; Ekou, J.; Bogere, P.; Mujinya, R.; Musoke, G.H.; et al. Consumption of Raw Herbal Medicines Is Associated with Major Public Health Risks amongst Ugandans. J. Environ. Public Health 2020, 2020, 516105. [Google Scholar] [CrossRef] [PubMed]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
- Cansaran, D.; Kahya, D.; Yurdakulol, E.; Atakol, O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Z. Naturforsch.-Sect. C J. Biosci. 2006, 61, 773–776. [Google Scholar] [CrossRef]
- Zizovic, I.; Ivanovic, J.; Misic, D.; Stamenic, M.; Djordjevic, S.; Kukic-Markovic, J.; Petrovic, S.D. SFE as a superior technique for isolation of extracts with strong antibacterial activities from lichen Usnea barbata L. J. Supercrit. Fluids 2012, 72, 7–14. [Google Scholar] [CrossRef]
- Ivanovic, J.; Meyer, F.; Misic, D.; Asanin, J.; Jaeger, P.; Zizovic, I.; Eggers, R. Influence of different pre-treatment methods on isolation of extracts with strong antibacterial activity from lichen Usnea barbata using carbon dioxide as a solvent. J. Supercrit. Fluids 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Guclu, G.; Kelebek, H.; Selli, S. Antioxidant activity in olive oils. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Amsterdam, Netherlands, 2021; pp. 313–325. [Google Scholar]
- Boroski, M.; Aguiar, A.C.; Rotta, E.M.; Bonafe, E.G.; Valderrama, P.; Souza, N.E.; Visentainer, J.V. Antioxidant activity of herbs and extracted phenolics from oregano in canola oil. Int. Food Res. J. 2018, 25, 2444–2452. [Google Scholar]
- Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the cytotoxic activity of the Usnea barbata (L.) F. H. Wigg dry extract. Molecules 2020, 25, 1865. [Google Scholar] [CrossRef] [Green Version]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Lele, K.C.; Chikezie, P.C.; Verla, A.W.; Enyoh, C.E. Bioaccumulation and health risk assessment of heavy metals in Musa paradisiaca, Zea mays, Cucumeropsis manii and Manihot esculenta cultivated in Onne, Rivers State, Nigeria. Environ. Health Toxicol. 2020, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2020, 12, 45–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar] [PubMed]
- Merle, U.; Stremmel, W. Copper toxicity in Wilson disease explained in a new way. Hepatology 2011, 54, 358–360. [Google Scholar] [CrossRef]
- Sayyad, R.; Ghomi, M. Evaluation of fatty acid profile, color characteristics, oxidative quality and stability of common Kilka (Clupeonella cultriventris caspia) oil obtained by various extraction techniques. J. Food Sci. Technol. 2017, 54, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Buhalova, D.; Nikolova, K.; Antova, G.; Tomova, I.; Aladjadjiyan, A.; Aleksieva, Y.; Petkova, Z. Comparative characteristics of sunflower oil with supplement of traditional Bulgarian herbs. Bulg. Chem. Commun. 2014, 46, 34–38. [Google Scholar]
- Önal, B.; Ergin, G. Antioxidative effects of α-tocopherol and ascorbyl palmitate on thermal oxidation of canola oil. Nahrung 2002, 46, 420–426. [Google Scholar] [CrossRef]
- Iuga, M.; Boestean, O.; Ghendov-Mosanu, A.; Mironeasa, S. Impact of dairy ingredients on wheat flour dough rheology and bread properties. Foods 2020, 9, 828. [Google Scholar] [CrossRef]
- Capar, T.D.; Kavuncuoglu, H.; Yalcin, H.; Toga, G. Rheological analysis for detection of extra virgin olive oil adulteration with vegetable oils: Predicting oil type by artificial neural network. Qual. Assur. Saf. Crop. Foods 2019, 11, 687–699. [Google Scholar] [CrossRef]
- Paul, A.K.; Borugadda, V.B.; Reshad, A.S.; Bhalerao, M.S.; Tiwari, P.; Goud, V.V. Comparative study of physicochemical and rheological property of waste cooking oil, castor oil, rubber seed oil, their methyl esters and blends with mineral diesel fuel. Mater. Sci. Energy Technol. 2021, 4, 148–155. [Google Scholar] [CrossRef]
- Abdelraziq, I.R.; Nierat, T.H. Rheology Properties of Castor Oil: Temperature and Shear Rate-dependence of Castor Oil Shear Stress. J. Mater. Sci. Eng. 2015, 5, 220. [Google Scholar]
- Aksoy, F.; Baydir, S.A.; Bayrakçeken, H. An investigation on the effect in the viscosity of canola and corn oil biodiesels at a temperature range of 0 to 100 °C. Energy Sources Part A Recover. Util. Environ. Eff. 2010, 32, 157–164. [Google Scholar] [CrossRef]
- Hojjatoleslamya, M.; Dehghannejada, N.; Zahedia, A.; Gharachorloo, M. The Effect of Heating on Rheological Behavior of Vegetable Edible Oils Mohammad Hojjatoleslamy. Curr. Trends Technol. Sci. 2005, 2, 323–326. [Google Scholar]
- Fasina, O.O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 1972–1982. [Google Scholar] [CrossRef]
- Sheth, B. Viscosity measurement and interpretation of viscosity data. J. Texture Stud. 1976, 7, 157–178. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mǎnescu, O.; Lupuleasa, D.; Miron, D.S.; Budura, E.A.; Rǎdulescu, F.Ş. In vitro drug release from topical antifungal pharmaceutical formulations. Farmacia 2011, 59, 15–23. [Google Scholar]
- Stern, W.L.; Chambers, K.L. The Citation of Wood Specimens and Herbarium Vouchers in Anatomical Research. Int. Assoc. Plant Taxon. 2018, 9, 7–13. [Google Scholar] [CrossRef]
- Prasad, S.B.; Sharma, A. Standardisation of convolvulus pluricaulis choisy herbs collected from Jalandhar, Punjab. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1412–1416. [Google Scholar]
- European Pharmacopoeia (Ph. Eur.) 10th Edition, EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 17 November 2021).
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).